

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -53.8 | n/a | n/a | 2.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50241083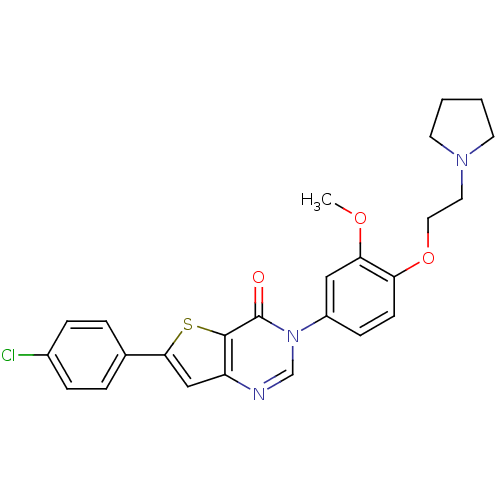 (6-(4-chlorophenyl)-3-(3-methoxy-4-(2-(pyrrolidin-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rat MCHR1 | Bioorg Med Chem Lett 25: 2793-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.008 BindingDB Entry DOI: 10.7270/Q29C7053 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120624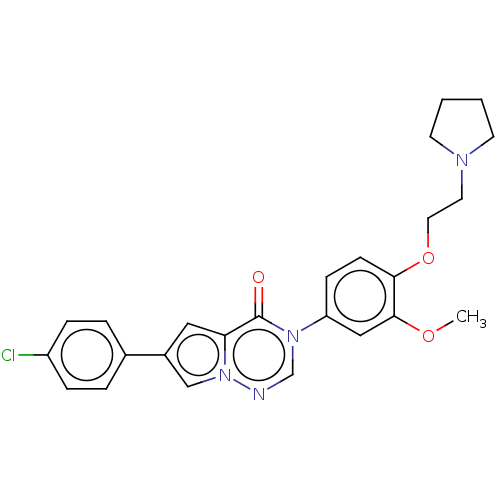 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120635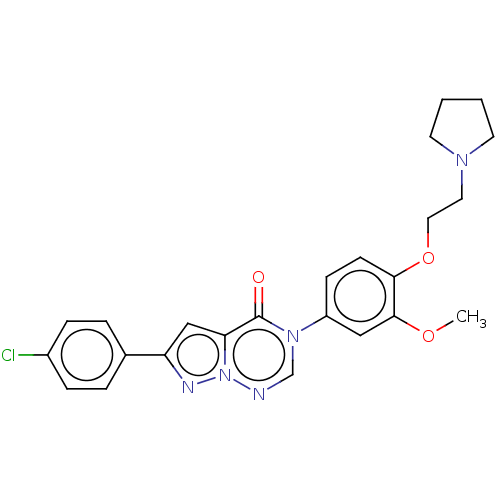 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120624 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11542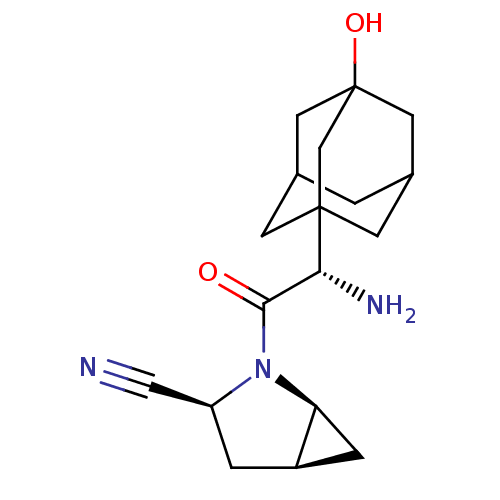 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxyadamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225074 ((1S,3S,5S)-2-[(S)-2-amino-2-(3-hydroxy-adamantan-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 6476-80 (2007) Article DOI: 10.1016/j.bmcl.2007.09.090 BindingDB Entry DOI: 10.7270/Q2B56JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120635 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120628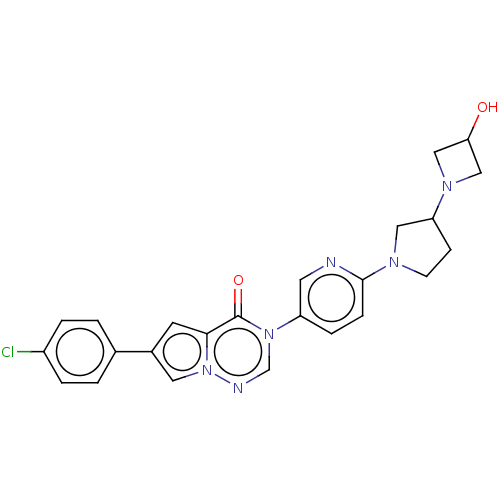 (CHEMBL3618334) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50094880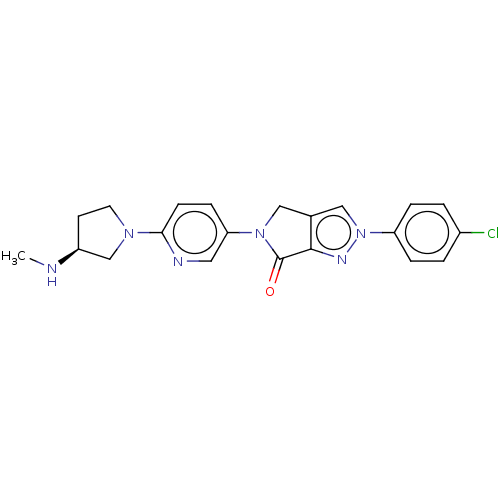 (CHEMBL3589145) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rat MCHR1 | Bioorg Med Chem Lett 25: 2793-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.008 BindingDB Entry DOI: 10.7270/Q29C7053 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50507371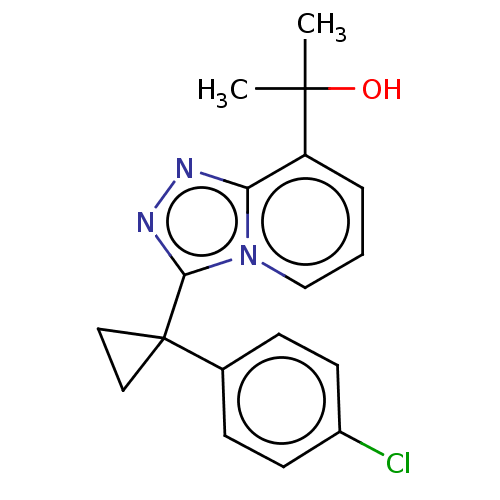 (BMS-823778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human 11beta-HSD1 expressed in HEK293 cell microsomes using [3H]cortisone as substrate after 4 hrs by homogeneous immuno-ra... | ACS Med Chem Lett 9: 1170-1174 (2018) Article DOI: 10.1021/acsmedchemlett.8b00307 BindingDB Entry DOI: 10.7270/Q20R9SP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11541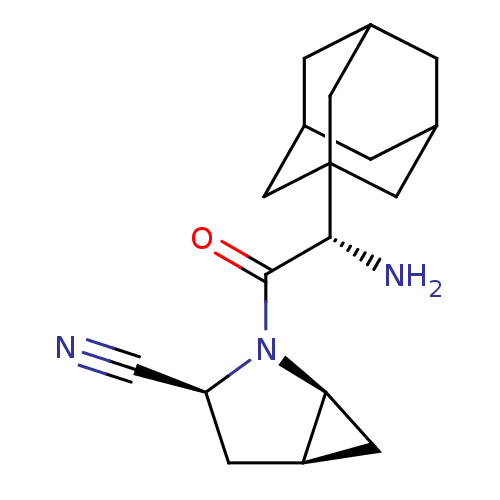 ((1S,3S,5S)-2-[(2S)-2-(adamantan-1-yl)-2-aminoacety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18162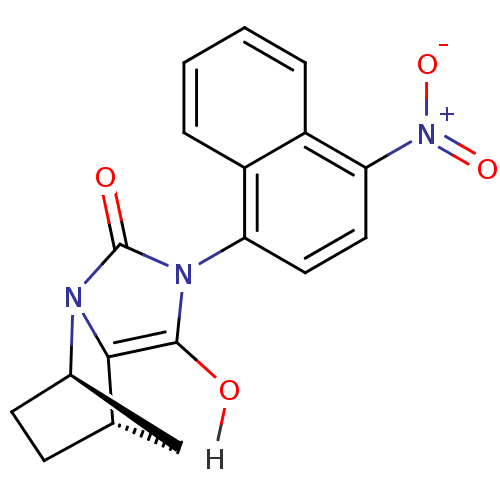 ((1R,6R,7S)-4-(4-nitronaphthalen-1-yl)-2,4-diazatri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | 385 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50161646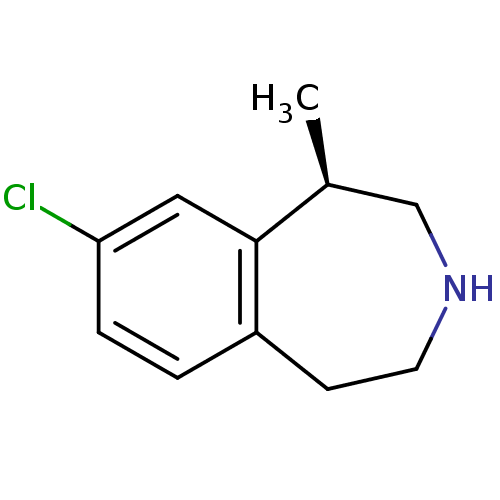 ((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at 5-HT2A receptor (unknown origin) | Bioorg Med Chem Lett 23: 330-5 (2012) Article DOI: 10.1016/j.bmcl.2012.10.091 BindingDB Entry DOI: 10.7270/Q2V12656 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50161646 ((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Agonist activity at 5-HT2B receptor (unknown origin) | Bioorg Med Chem Lett 23: 330-5 (2012) Article DOI: 10.1016/j.bmcl.2012.10.091 BindingDB Entry DOI: 10.7270/Q2V12656 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11530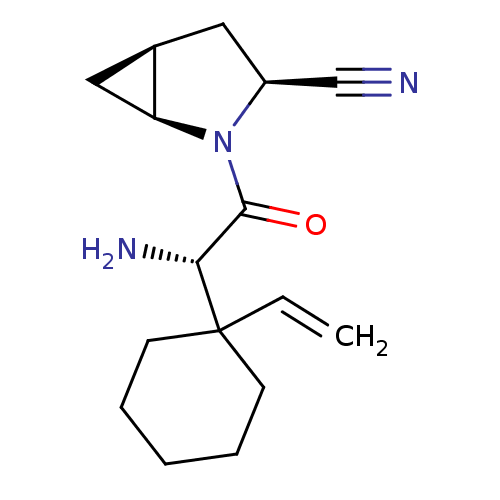 ((1S,3S,5S)-2-[(2S)-2-amino-2-(1-ethenylcyclohexyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120796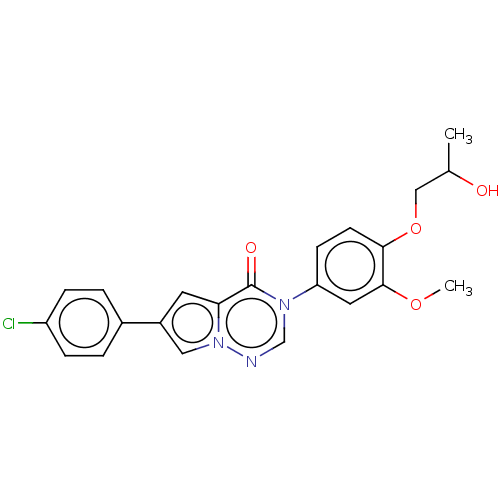 (CHEMBL3618363) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120628 (CHEMBL3618334) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120676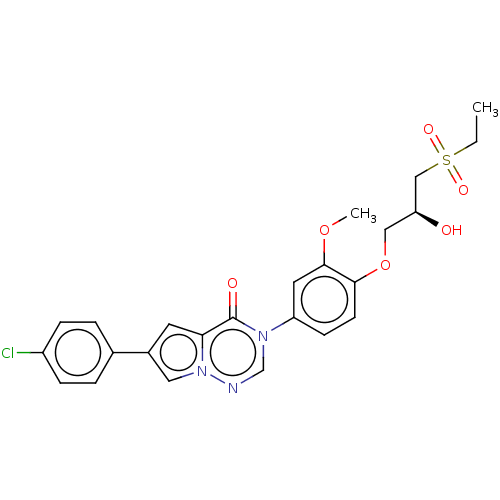 (CHEMBL3618340) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18169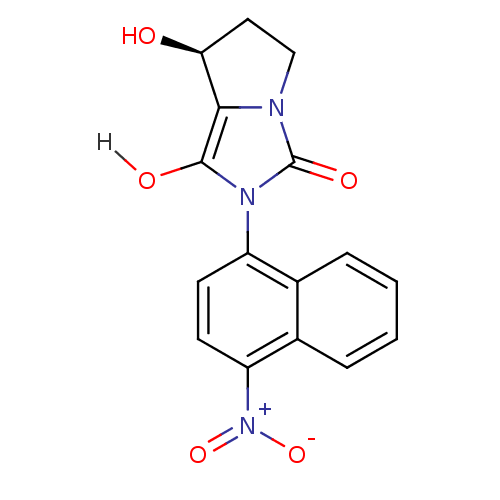 ((7S,7aR)-7-hydroxy-2-(4-nitronaphthalen-1-yl)-hexa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | 281 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18165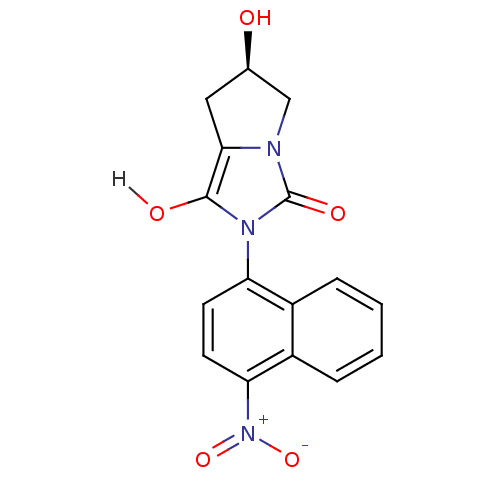 ((6R)-6-hydroxy-2-(4-nitronaphthalen-1-yl)-hexahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | 320 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18164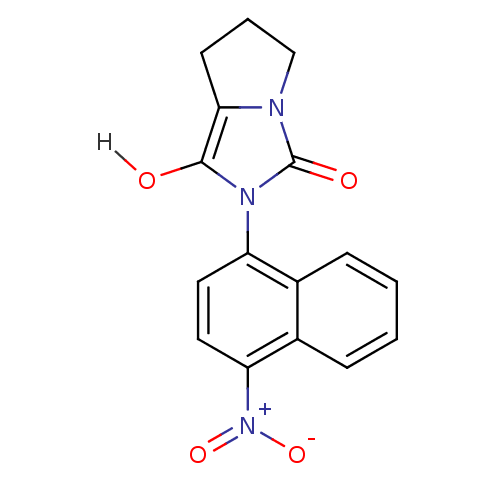 (2-(4-nitronaphthalen-1-yl)-hexahydro-1H-pyrrolo[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | -49.6 | n/a | n/a | 270 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120629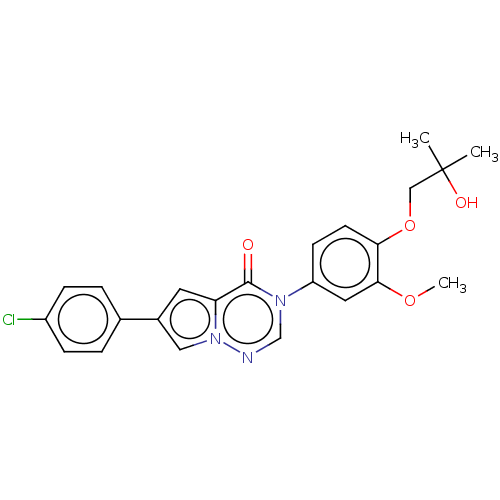 (CHEMBL3618336) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11544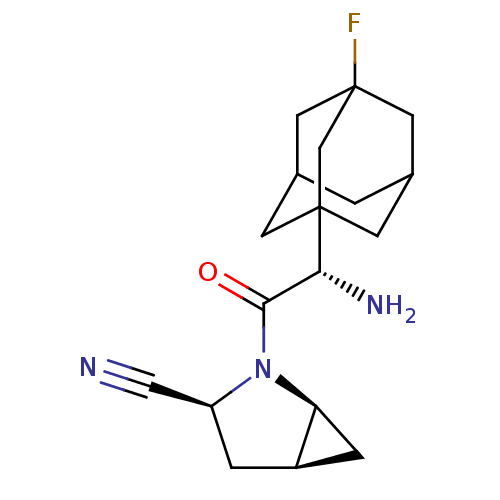 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3-fluoroadamantan-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50492229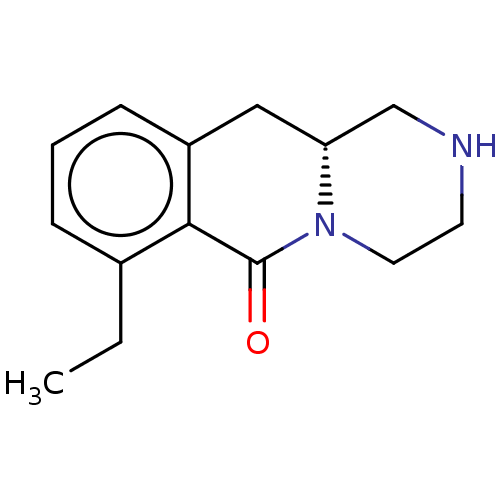 (CHEMBL2397890) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]LSD from human recombinant 5-HT2B receptor expressed in HEK293E cells after 45 mins by Top counting analysis | Bioorg Med Chem Lett 23: 3914-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.061 BindingDB Entry DOI: 10.7270/Q2T156KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Sus scrofa (pig)) | BDBM50287059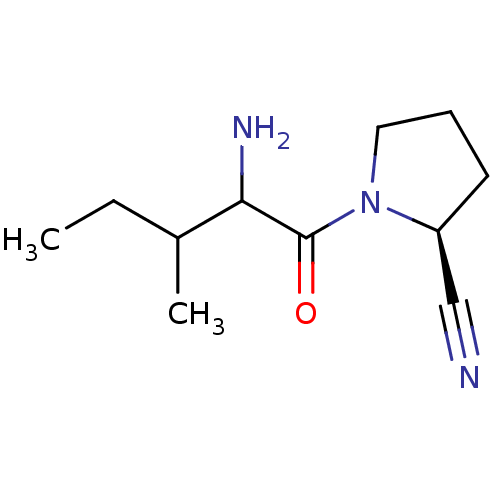 ((S)-1-(2-Amino-3-methyl-pentanoyl)-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of porcine Dipeptidylpeptidase IV. | J Med Chem 47: 2587-98 (2004) Article DOI: 10.1021/jm049924d BindingDB Entry DOI: 10.7270/Q2FT8MSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212876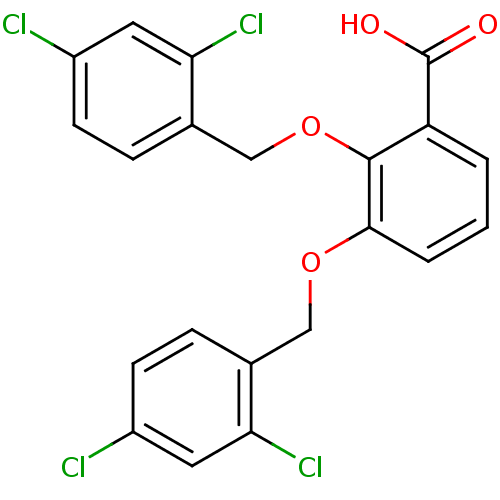 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50212876 (2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from eFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11543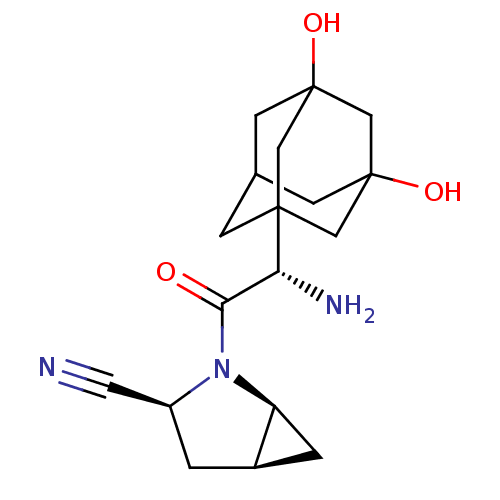 ((1S,3S,5S)-2-[(2S)-2-amino-2-(3,5-dihydroxyadamant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute | Assay Description Inhibition of human DPP-IV activity was measured under steady-state conditions by following the absorbance increase at 405 nm upon the substrate clea... | J Med Chem 48: 5025-37 (2005) Article DOI: 10.1021/jm050261p BindingDB Entry DOI: 10.7270/Q2FN14DM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18168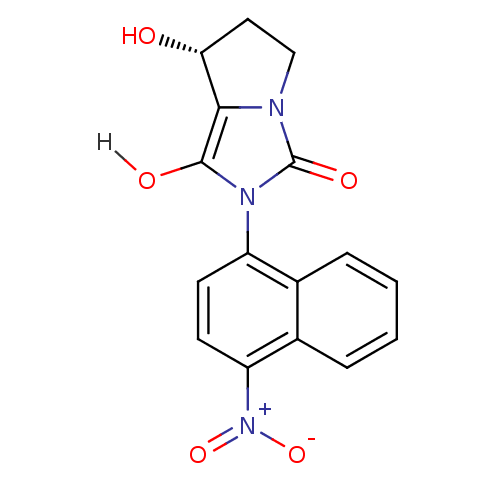 ((7R,7aS)-7-hydroxy-2-(4-nitronaphthalen-1-yl)-hexa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | -49.0 | n/a | n/a | 1.5 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120629 (CHEMBL3618336) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120670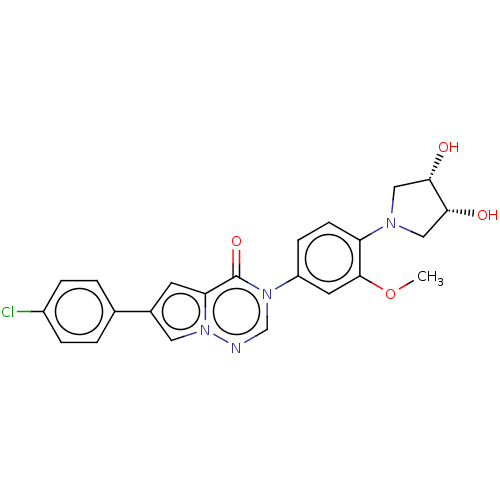 (CHEMBL3618338) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120676 (CHEMBL3618340) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50192462 ((S)-2-(2,3-bis(2-chlorobenzyloxy)phenyl)-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to ap2 | J Med Chem 49: 5013-7 (2006) Article DOI: 10.1021/jm060360i BindingDB Entry DOI: 10.7270/Q2TQ6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120634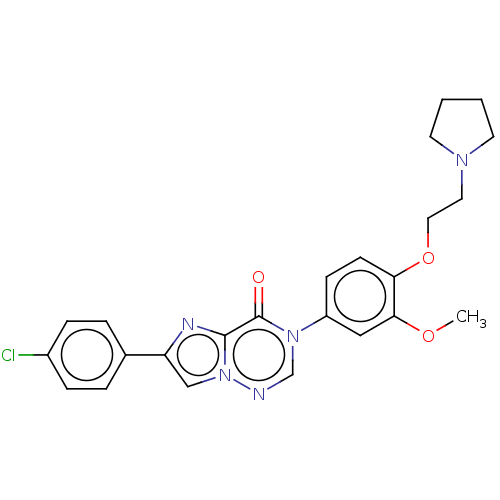 (CHEMBL3618326) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212885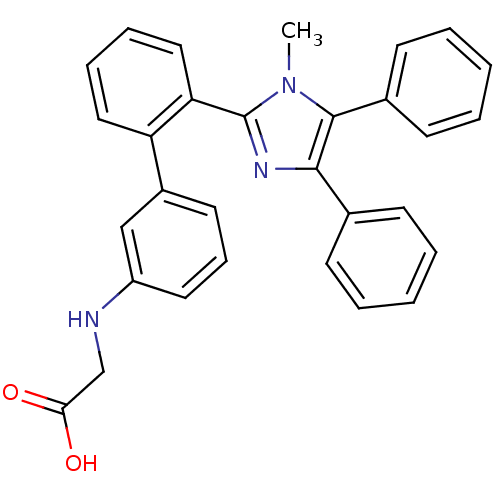 (CHEMBL245282 | [2'-(1-methyl-4,5-diphenyl-1H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120633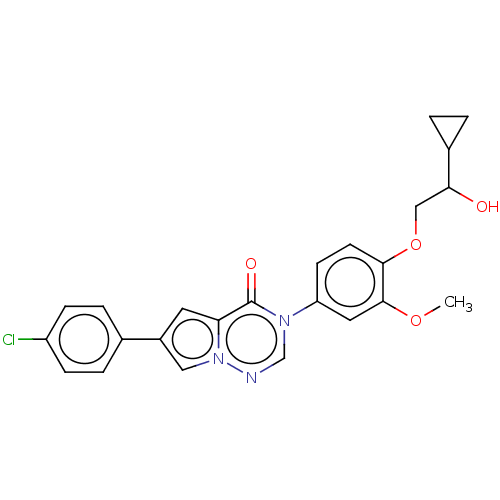 (CHEMBL3618327) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50309343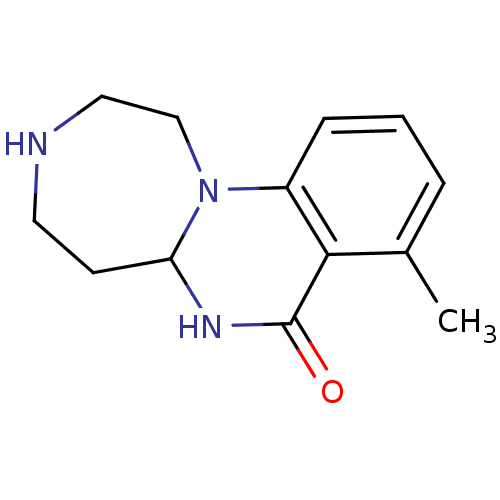 (8-methyl-2,3,4,5,5a,6-hexahydro-[1,4]diazepino[1,7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293E cells | Bioorg Med Chem Lett 20: 1128-33 (2010) Article DOI: 10.1016/j.bmcl.2009.12.014 BindingDB Entry DOI: 10.7270/Q2K074DK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50225073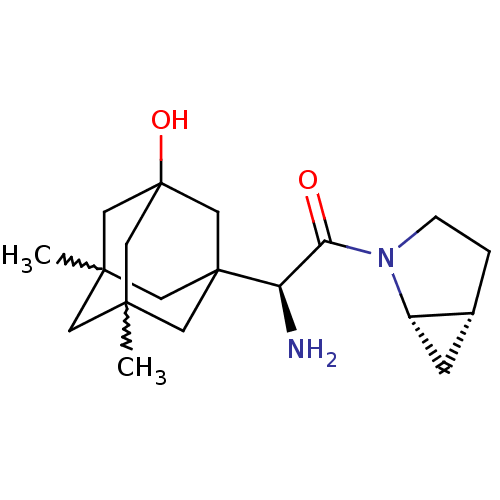 ((S)-2-amino-1-(1S,5R)-2-aza-bicyclo[3.1.0]hex-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human DPP4 | Bioorg Med Chem Lett 17: 6476-80 (2007) Article DOI: 10.1016/j.bmcl.2007.09.090 BindingDB Entry DOI: 10.7270/Q2B56JGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM103905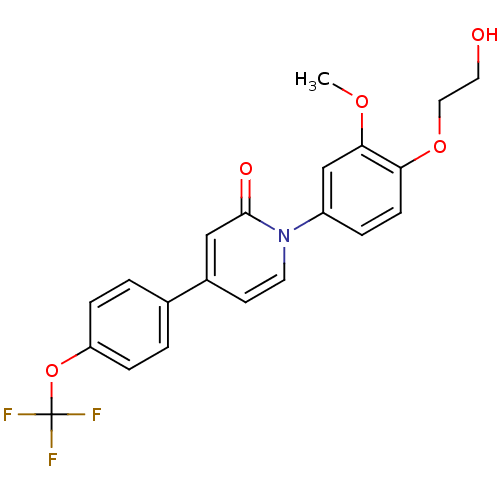 (JNK3 inhibitor 2 | US8563583, C-1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds were characterized in an in vitro binding assay to determine their Ki or ability to antagonized binding of a peptide agonist to the human m... | US Patent US8563583 (2013) BindingDB Entry DOI: 10.7270/Q22F7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50192463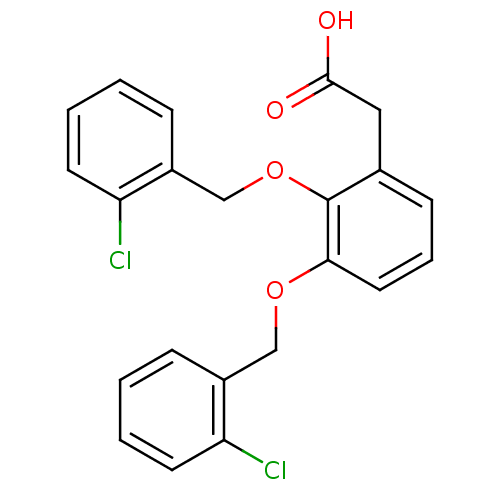 (2-(2,3-bis(2-chlorobenzyloxy)phenyl)acetic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity to human kFABP | J Med Chem 49: 5013-7 (2006) Article DOI: 10.1021/jm060360i BindingDB Entry DOI: 10.7270/Q2TQ6157 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120670 (CHEMBL3618338) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18171 (4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.20 | -48.0 | n/a | n/a | 2.30 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... | J Med Chem 49: 7596-9 (2006) Article DOI: 10.1021/jm061101w BindingDB Entry DOI: 10.7270/Q2862DQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212884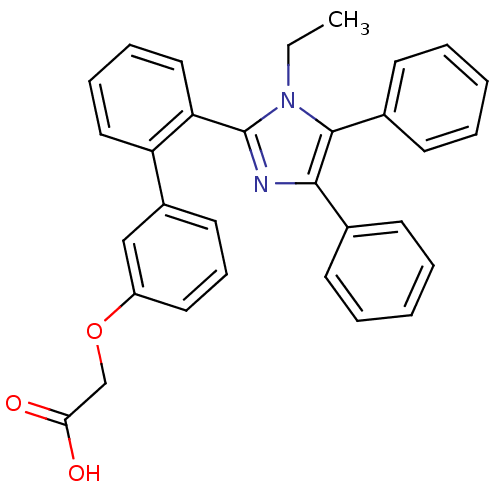 (CHEMBL245284 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-2,3-bis[(2,4-dichlorobenzyl)oxy]benzoic acid from aFABP | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120675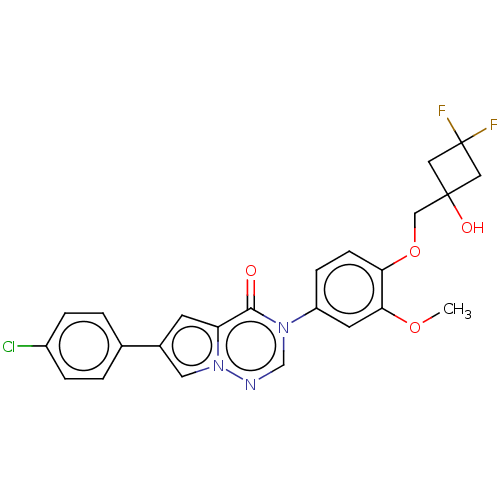 (CHEMBL3618339) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212877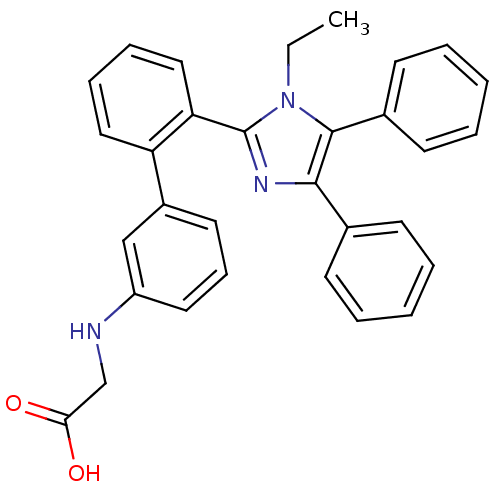 (CHEMBL245653 | [2'-(1-ethyl-4,5-diphenyl-1H-imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50212886 (CHEMBL247529 | [2'-(3-ethyl-4,5-diphenyl-furan-2-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of 1,8-ANS from aFABP by fluorescence based-assay | Bioorg Med Chem Lett 17: 3511-5 (2007) Article DOI: 10.1016/j.bmcl.2006.12.044 BindingDB Entry DOI: 10.7270/Q24B3119 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120633 (CHEMBL3618327) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3452 total ) | Next | Last >> |