

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | ALK tyrosine kinase receptor | ||
| Ligand | BDBM50157641 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEBML_1568746 | ||
| IC50 | 18.0±n/a nM | ||
| Citation |  Zhang, P; Dong, J; Zhong, B; Zhang, D; Yuan, H; Jin, C; Xu, X; Li, H; Zhou, Y; Liang, Z; Ji, M; Xu, T; Song, G; Zhang, L; Chen, G; Meng, X; Sun, D; Shih, J; Zhang, R; Hou, G; Wang, C; Jin, Y; Yang, Q Design and synthesis of novel 3-sulfonylpyrazol-4-amino pyrimidines as potent anaplastic lymphoma kinase (ALK) inhibitors. Bioorg Med Chem Lett26:1910-8 (2016) [PubMed] Article Zhang, P; Dong, J; Zhong, B; Zhang, D; Yuan, H; Jin, C; Xu, X; Li, H; Zhou, Y; Liang, Z; Ji, M; Xu, T; Song, G; Zhang, L; Chen, G; Meng, X; Sun, D; Shih, J; Zhang, R; Hou, G; Wang, C; Jin, Y; Yang, Q Design and synthesis of novel 3-sulfonylpyrazol-4-amino pyrimidines as potent anaplastic lymphoma kinase (ALK) inhibitors. Bioorg Med Chem Lett26:1910-8 (2016) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| ALK tyrosine kinase receptor | |||
| Name: | ALK tyrosine kinase receptor | ||
| Synonyms: | ALK | ALK tyrosine kinase receptor (ALK) | ALK_HUMAN | Anaplastic lymphoma kinase | CD_antigen: CD246 | ||
| Type: | Protein | ||
| Mol. Mass.: | 176453.10 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q9UM73 | ||
| Residue: | 1620 | ||
| Sequence: |
| ||
| BDBM50157641 | |||
| n/a | |||
| Name | BDBM50157641 | ||
| Synonyms: | CHEMBL3787658 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C33H45ClN8O4S | ||
| Mol. Mass. | 685.28 | ||
| SMILES | CC(C)S(=O)(=O)c1nn(C)cc1Nc1nc(Nc2cc(C)c(cc2OC2CC2)[C@H]2CC[C@@H](CC2)N2CCN(CC2)C(C)=O)ncc1Cl |r,wU:28.30,wD:31.37,(-4.74,-11.34,;-3.82,-10.52,;-4.07,-9.32,;-2.36,-11.01,;-2.11,-12.22,;-3.28,-11.83,;-1.21,-9.99,;.3,-10.31,;1.07,-8.98,;2.29,-8.85,;.04,-7.83,;-1.35,-8.47,;-2.68,-7.7,;-2.68,-6.15,;-1.34,-5.39,;-1.34,-3.85,;-0,-3.08,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.4,1.39,;,1.54,;1.33,.77,;1.33,-.77,;2.67,-1.54,;4,-.77,;5.46,-.81,;4.69,.52,;,3.08,;-1.33,3.85,;-1.34,5.39,;-0,6.16,;1.33,5.39,;1.33,3.85,;-.01,7.7,;-1.34,8.47,;-1.34,10.01,;-.01,10.78,;1.33,10.01,;1.33,8.47,;-0,12.32,;-1.07,12.94,;1.06,12.94,;-2.67,-3.07,;-4.01,-3.84,;-4.01,-5.38,;-5.08,-5.99,)| | ||
| Structure | 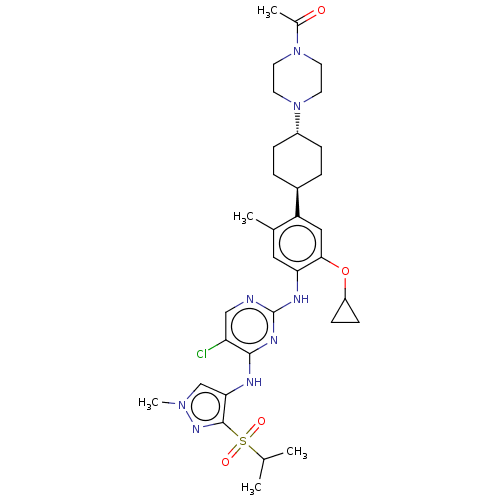
| ||