

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110577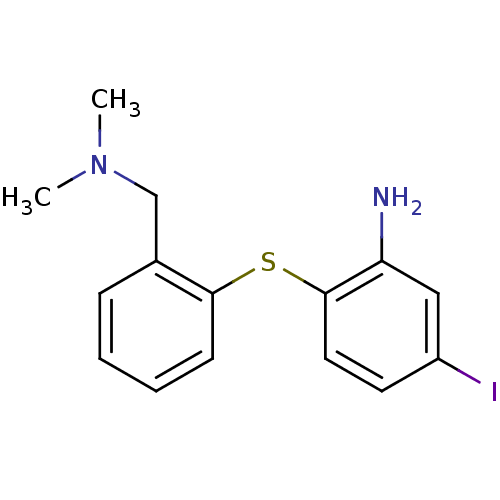 (2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110578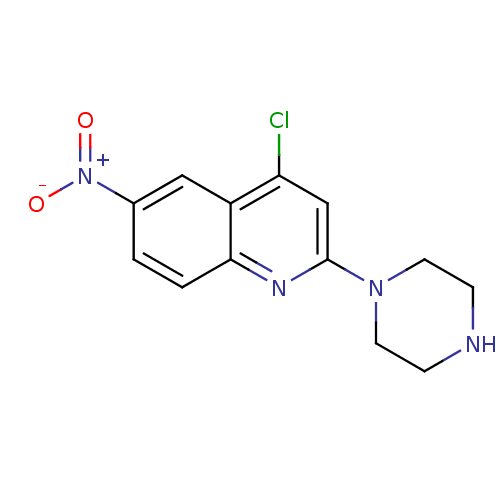 (4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328391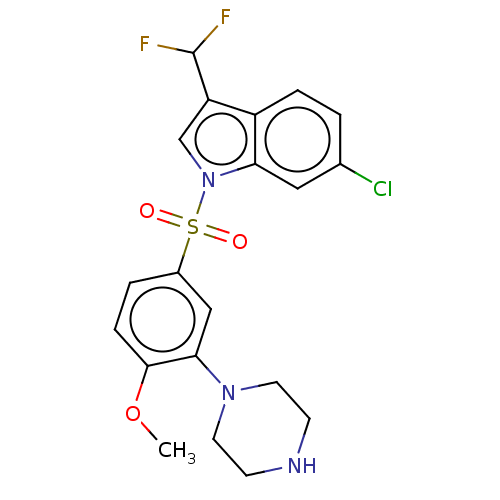 (6-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264121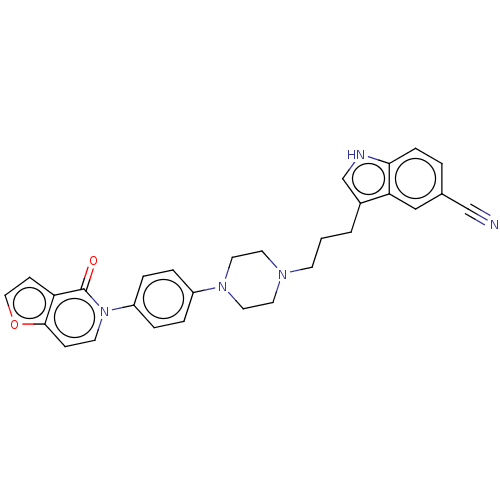 (3-(3-(4-(4-(4-oxofuro[3,2-c]pyridin-5 (4H)-yl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264120 (3-(4-(4-(4-(4-oxofuro[3,2-c]pyridin-5 (4H)-yl)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266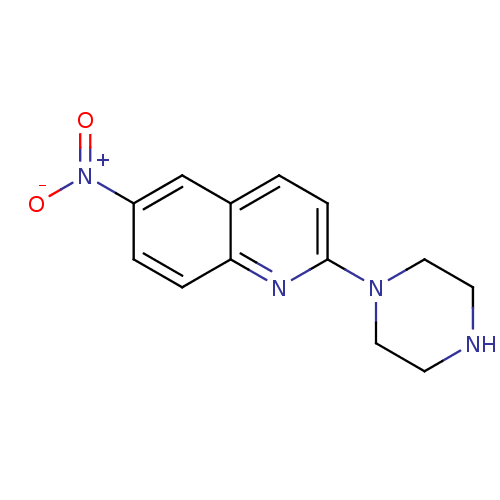 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264112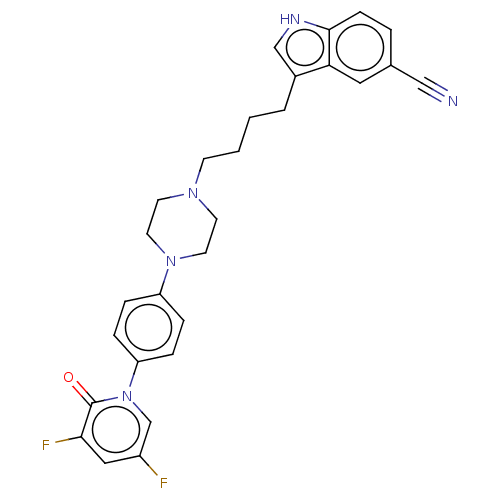 (3-(4-(4-(4-(3,5-difluoro-2-oxopyridin-1(2H)-yl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50592784 (CHEMBL5177311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50298610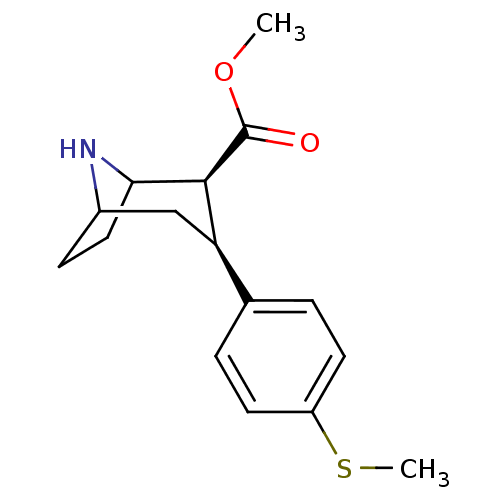 (3beta-(4-Methylthiophenyl)nortropane-2beta-carboxy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5-HTT | Bioorg Med Chem 17: 5126-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.052 BindingDB Entry DOI: 10.7270/Q2611186 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301953 (3-(3-(4-(4,6-Dimethoxypyrimidin-2-yl)piperazin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264097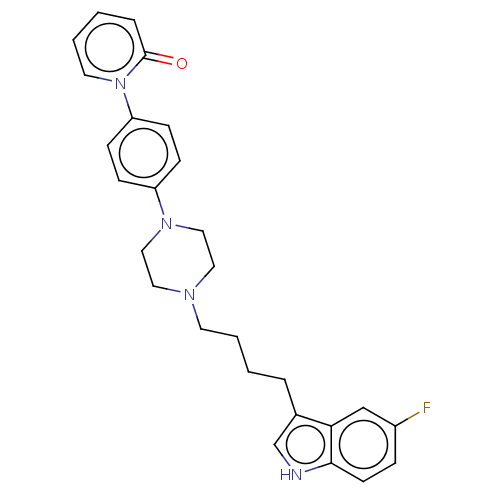 (1-(4-(4-(4-(5-fluoro-1H-indol-3-yl)butyl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301980 (3-(3-(4-(4-(Trifluoromethyl)pyrimidin-2-yl)piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328379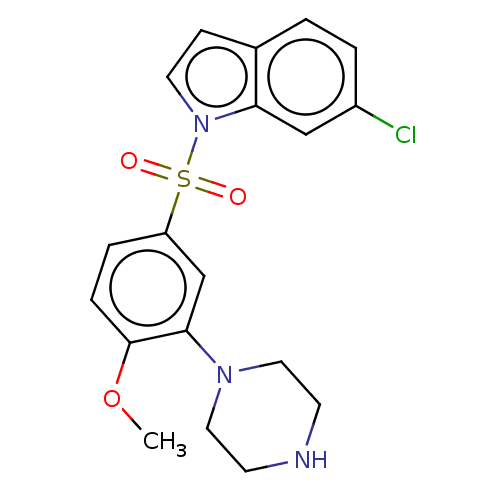 (6-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264096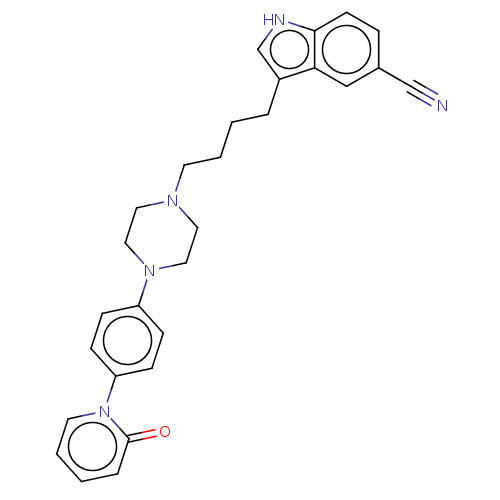 (3-(4-(4-(4-(2-oxopyridin-1(2H)-yl)phenyl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50063266 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328389 (3-(difluoromethyl)-6-fluoro-1-((4-methoxy-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50130285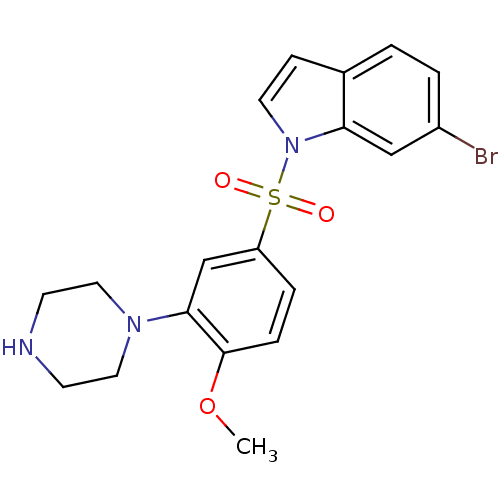 (6-Bromo-1-(4-methoxy-3-piperazin-1-yl-benzenesulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50592785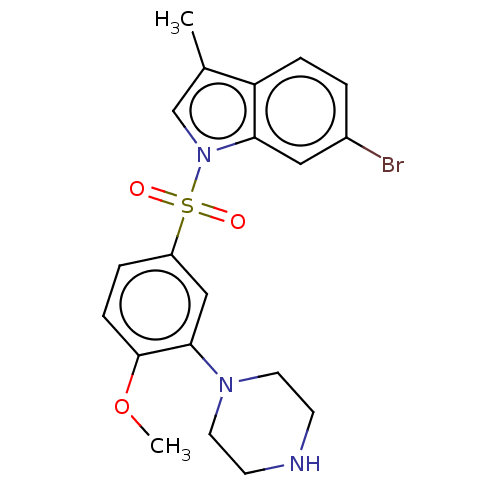 (CHEMBL5206617) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301982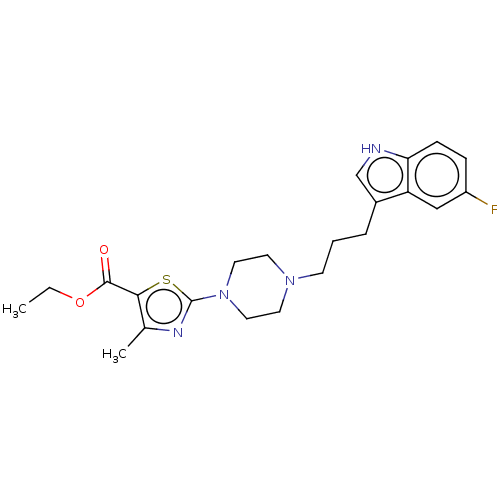 (Ethyl 2-(4-(3-(5-fluoro-1H-indol-3-yl)propyl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301963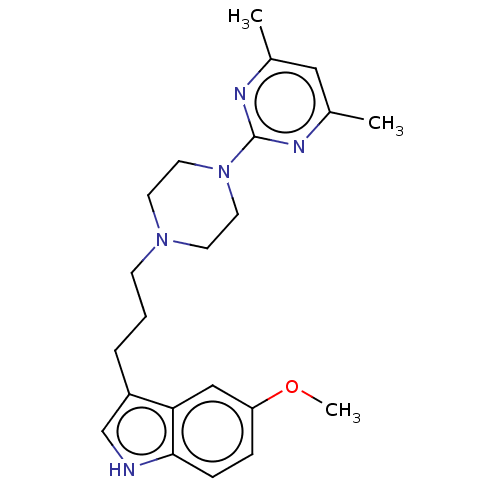 (3-(3-(4-(4,6-Dimethylpyrimidin-2-yl)piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301984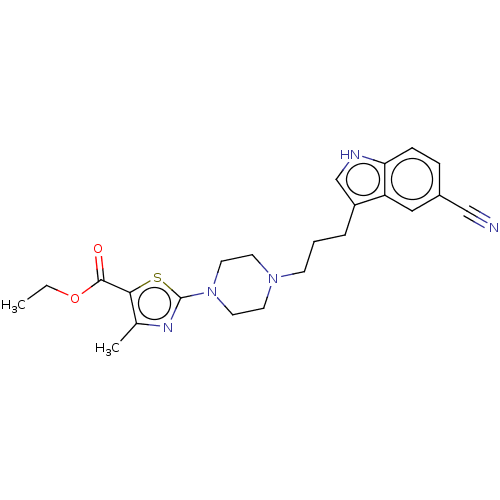 (Ethyl 2-(4-(3-(5-cyano-1H-indol-3-yl)propyl)pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264113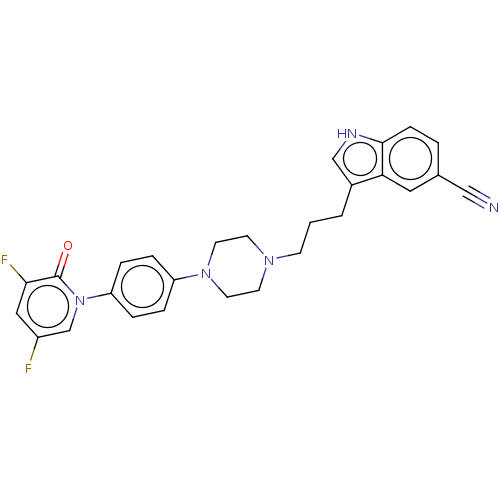 (3-(3-(4-(4-(3,5-difluoro-2-oxopyridin-1(2H)-yl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110584 (3-(3-Fluoro-propyl)-6-nitro-2-piperazin-1-yl-quino...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328393 (3-(difluoromethyl)-6-bromo-1-((4-methoxy-3-(pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50110574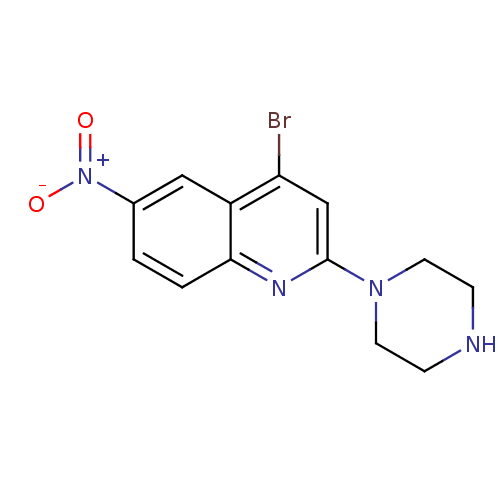 (4-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394084 (US9974785, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116950 BindingDB Entry DOI: 10.7270/Q2CC14NC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328408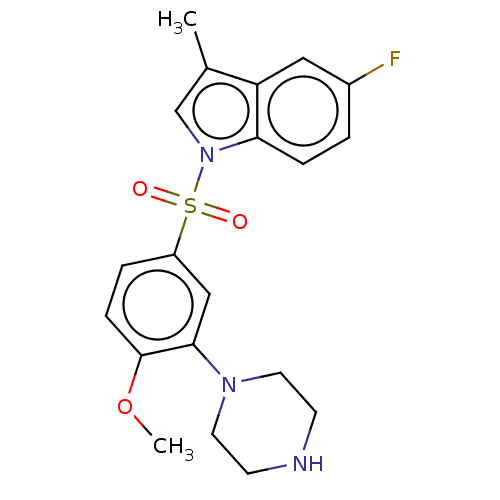 (5-fluoro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394084 (US9974785, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc. | Assay Description 32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... | J Med Chem 52: 3047-62 (2009) BindingDB Entry DOI: 10.7270/Q27W6FH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208769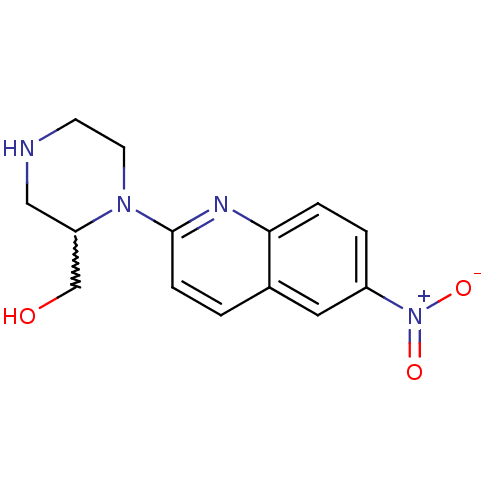 (2-[2-(hydroxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208770 (2-[2-(ethoxymethyl)piperazin-1-yl]-6-nitroquinolin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328383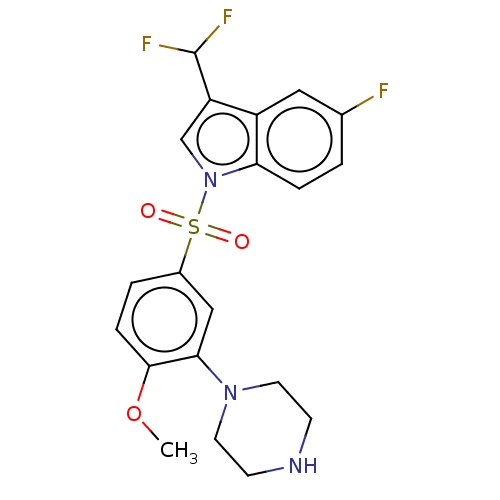 (3-(difluoromethyl)-5-fluoro-1-((4-methoxy-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394083 (US9974785, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc. | Assay Description 32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... | J Med Chem 52: 3047-62 (2009) BindingDB Entry DOI: 10.7270/Q27W6FH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394083 (US9974785, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116950 BindingDB Entry DOI: 10.7270/Q2CC14NC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264104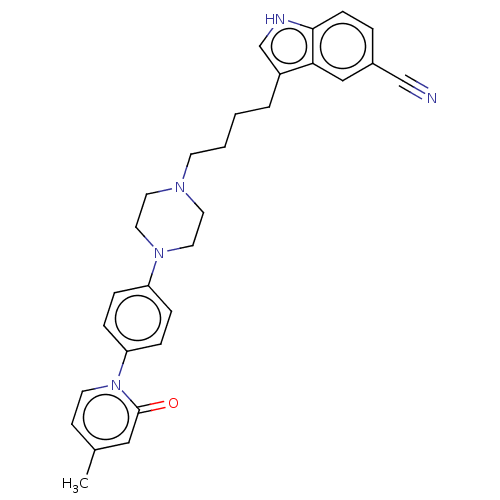 (3-(4-(4-(4-(4-methyl-2-oxopyridin-1(2H)-yl)phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264118 (3-(4-(4-(4-(5-oxo-1,6-naphthyridin-6(5H)-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394090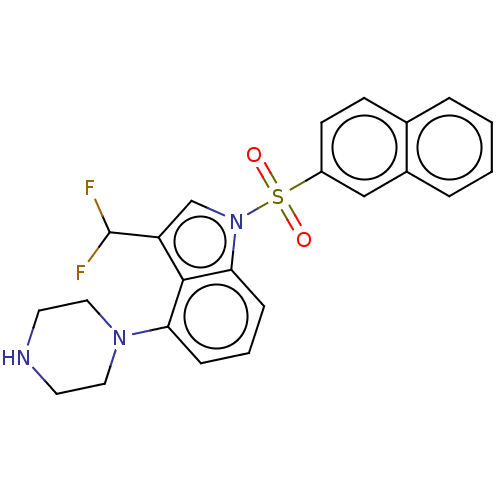 (US9974785, Example 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc. | Assay Description 32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... | J Med Chem 52: 3047-62 (2009) BindingDB Entry DOI: 10.7270/Q27W6FH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50590516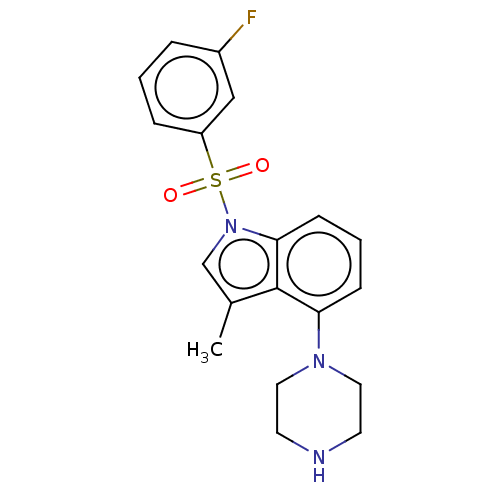 (CHEMBL5196479) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116950 BindingDB Entry DOI: 10.7270/Q2CC14NC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394086 (US9974785, Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116950 BindingDB Entry DOI: 10.7270/Q2CC14NC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394086 (US9974785, Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc. | Assay Description 32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... | J Med Chem 52: 3047-62 (2009) BindingDB Entry DOI: 10.7270/Q27W6FH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortex | Bioorg Med Chem Lett 10: 1559-62 (2000) BindingDB Entry DOI: 10.7270/Q20P10J2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) | Bioorg Med Chem Lett 12: 811-5 (2002) BindingDB Entry DOI: 10.7270/Q2S46SHN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328387 (5-bromo-3-(difluoromethyl)-1-((4-methoxy-3-(pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50298606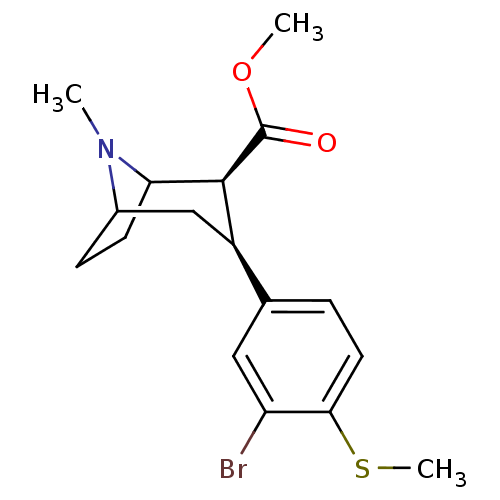 (3beta-(3-Bromo-4-methylthiophenyl)tropane-2beta-ca...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]paroxetine from 5-HTT | Bioorg Med Chem 17: 5126-32 (2009) Article DOI: 10.1016/j.bmc.2009.05.052 BindingDB Entry DOI: 10.7270/Q2611186 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264109 (3-(3-(4-(4-(4-methoxy-2-oxopyridin-1(2H)-yl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328385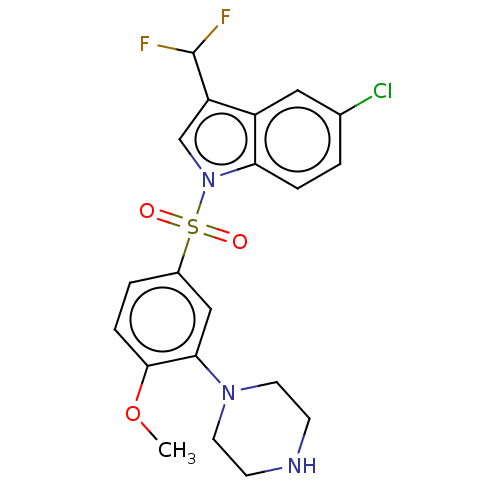 (5-chloro-3-(difluoromethyl)-1-((4-methoxy-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM301967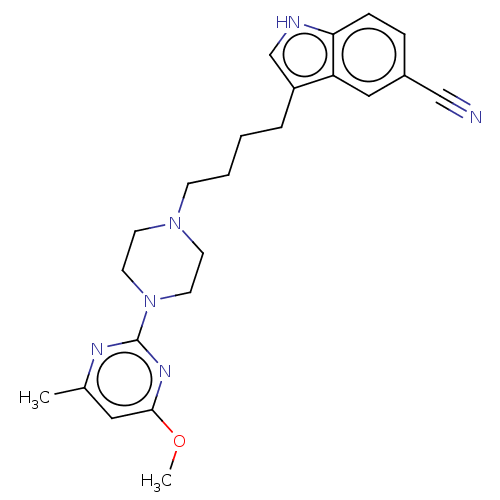 (3-(4-(4-(4-Methoxy-6-methylpyrimidin-2-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-OH-DPAT (Perkin-Elmer) in the absence o... | US Patent US9598401 (2017) BindingDB Entry DOI: 10.7270/Q2GH9M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM328409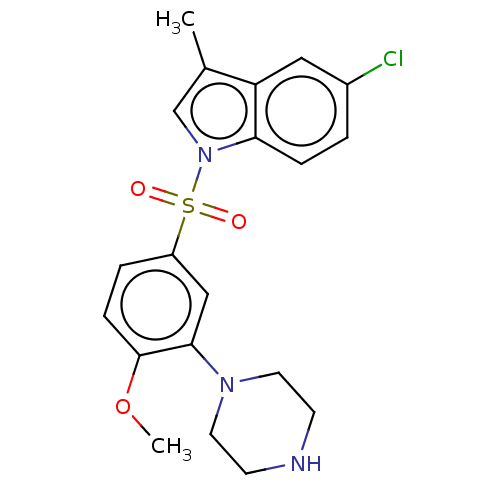 (5-chloro-1-((4-methoxy-3-(piperazin-1-yl)phenyl)su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116917 BindingDB Entry DOI: 10.7270/Q21C21V3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM394087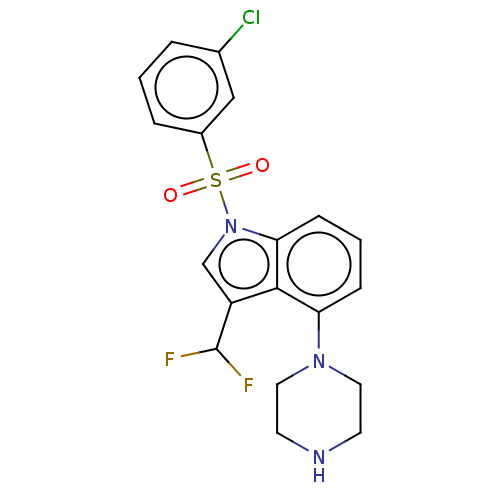 (US9974785, Example 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys Inc. | Assay Description 32 μg membrane proteins of CHO cell expressing human 5-HT6 receptor, 2 nM of radioactive marker [3H]LSD, a compound of the present invention hav... | J Med Chem 52: 3047-62 (2009) BindingDB Entry DOI: 10.7270/Q27W6FH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50208771 (2-[(2-methoxymethyl)piperazin-1-yl]-6-nitroquinoli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from Sprague-Dawley rat SERT | Bioorg Med Chem 15: 3499-504 (2007) Article DOI: 10.1016/j.bmc.2007.03.001 BindingDB Entry DOI: 10.7270/Q2SN08N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2368 total ) | Next | Last >> |