

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402859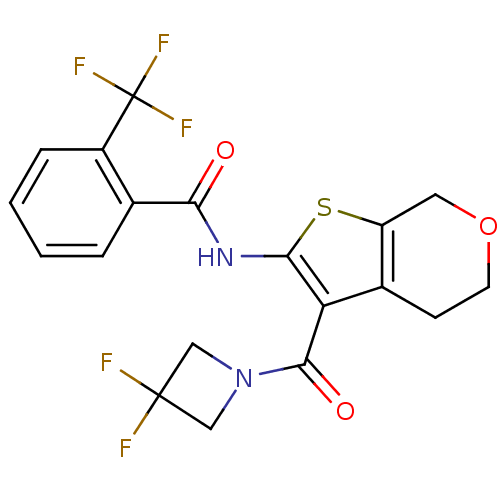 (CHEMBL2205591) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402858 (CHEMBL2205592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402864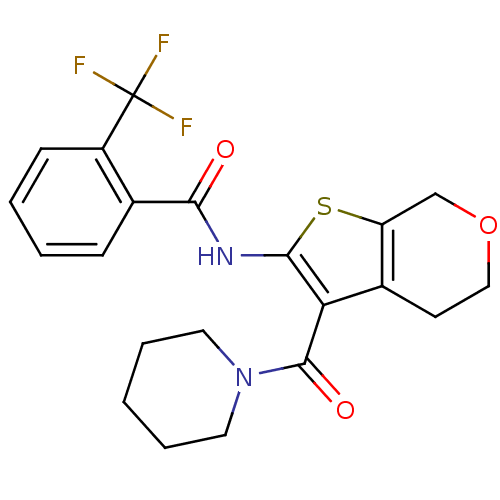 (CHEMBL2205615) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402863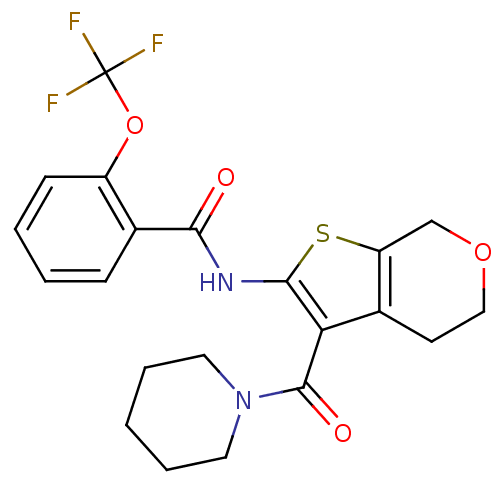 (CHEMBL2205616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402857 (CHEMBL2205593) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402859 (CHEMBL2205591) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402857 (CHEMBL2205593) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402860 (CHEMBL2205588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402861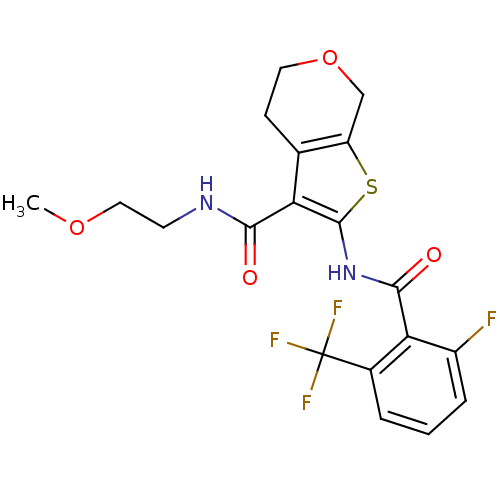 (CHEMBL2205584) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402862 (CHEMBL2205620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402864 (CHEMBL2205615) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402861 (CHEMBL2205584) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402863 (CHEMBL2205616) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402862 (CHEMBL2205620) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402860 (CHEMBL2205588) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402858 (CHEMBL2205592) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402859 (CHEMBL2205591) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402857 (CHEMBL2205593) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402864 (CHEMBL2205615) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402857 (CHEMBL2205593) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402864 (CHEMBL2205615) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402863 (CHEMBL2205616) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402863 (CHEMBL2205616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402862 (CHEMBL2205620) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402861 (CHEMBL2205584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402860 (CHEMBL2205588) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402858 (CHEMBL2205592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402862 (CHEMBL2205620) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402861 (CHEMBL2205584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402860 (CHEMBL2205588) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402859 (CHEMBL2205591) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402858 (CHEMBL2205592) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202199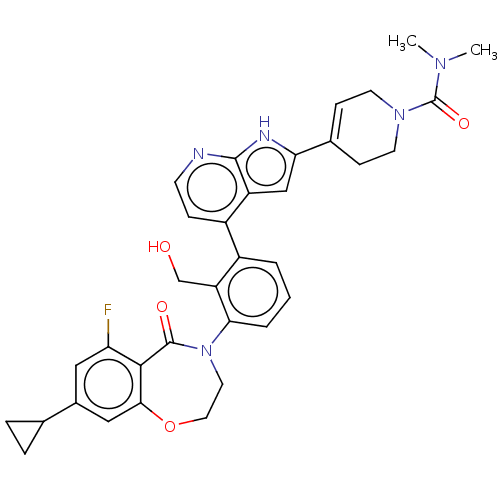 (US9233983, P-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202131 (US9233983, I-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202200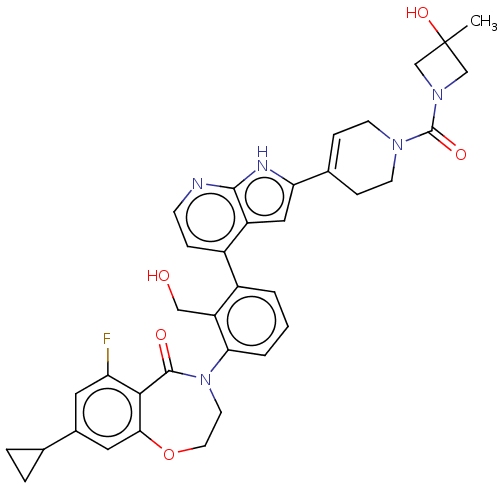 (US9233983, P-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50450836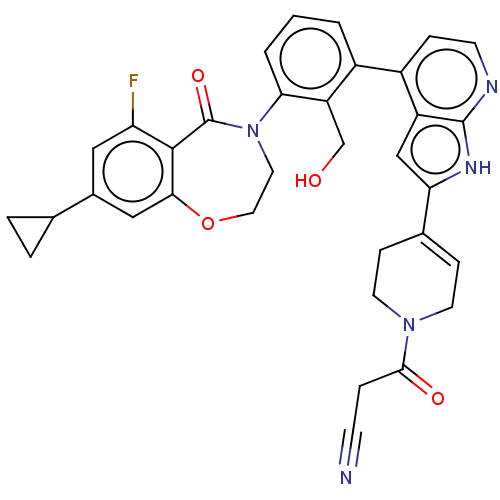 (CHEMBL4216939) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202208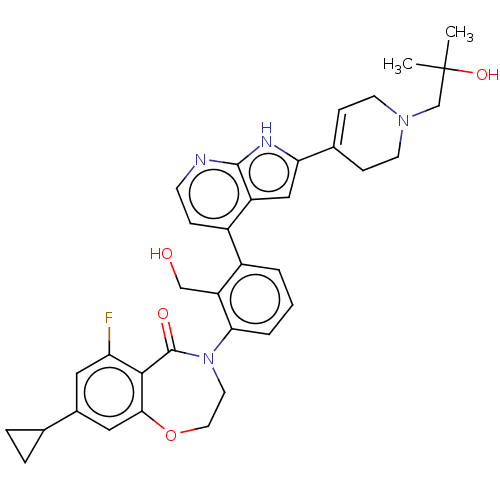 (US9233983, P-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202122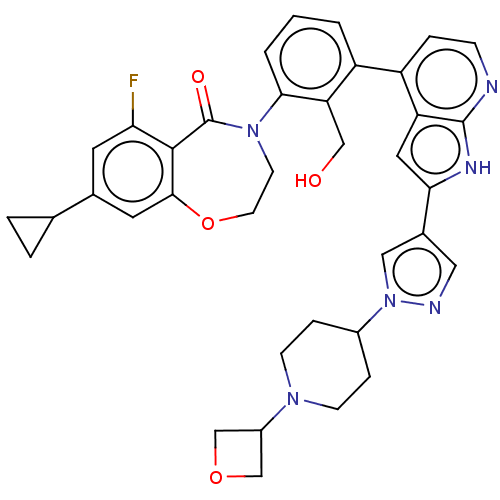 (US9233983, D-14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202141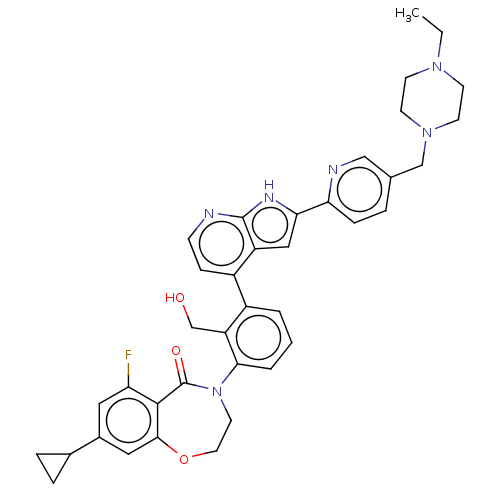 (US9233983, I-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202127 (US9233983, G-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202197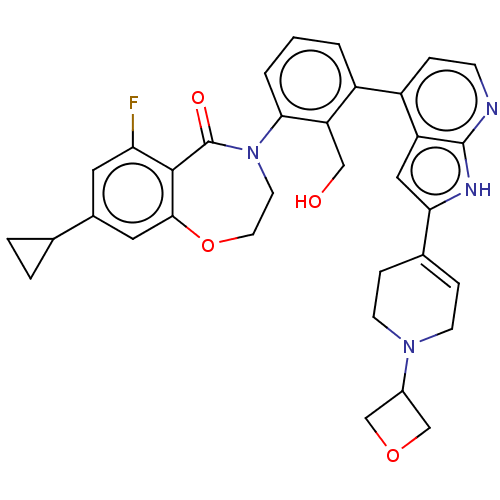 (US9233983, P-12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202128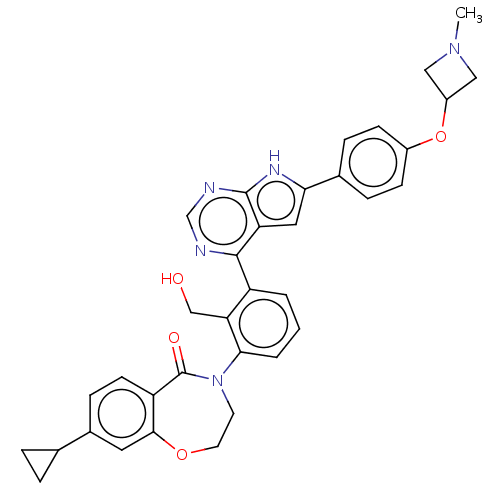 (US9233983, G-2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202130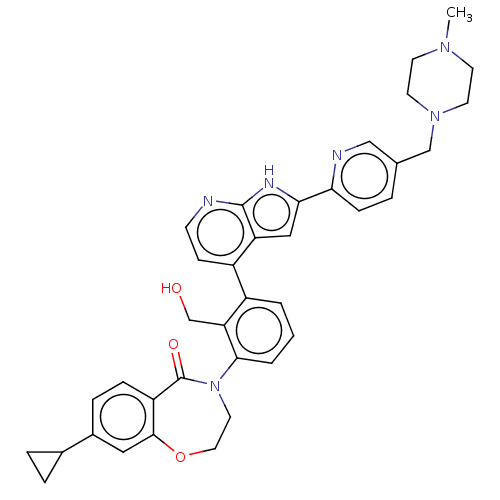 (US9233983, I-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202196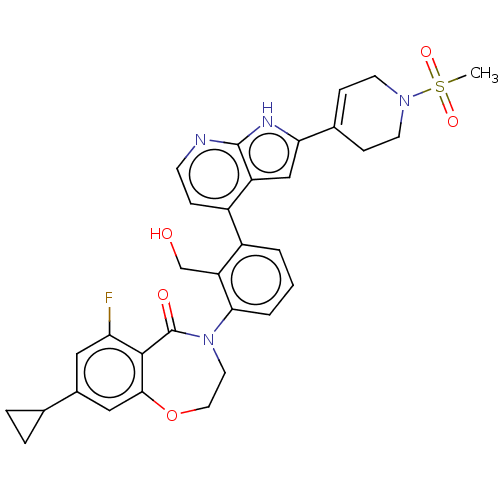 (US9233983, P-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Mus musculus) | BDBM202131 (US9233983, I-2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of C57BL/6J mouse BTK assessed as decrease in anti-mouse IgM antibody stimulated BCR mediated CD69 upregulation preincubated for 30 mins f... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Mus musculus) | BDBM202199 (US9233983, P-14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of C57BL/6J mouse BTK assessed as decrease in anti-mouse IgM antibody stimulated BCR mediated CD69 upregulation preincubated for 30 mins f... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202109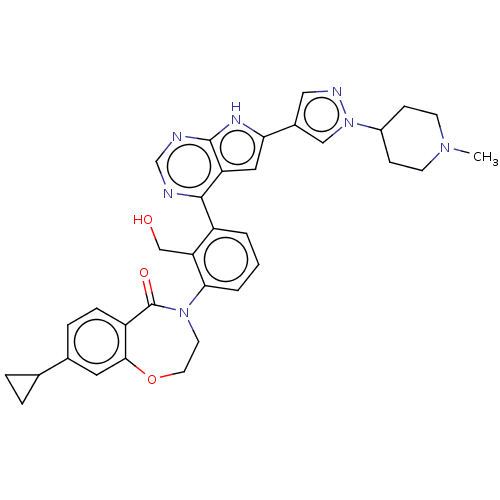 (US9233983, D-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202082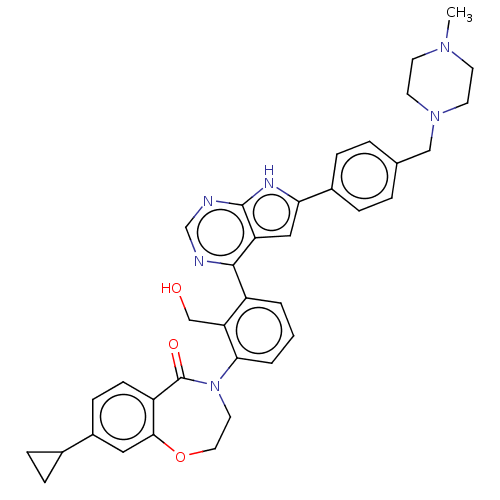 (US9233983, B-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202087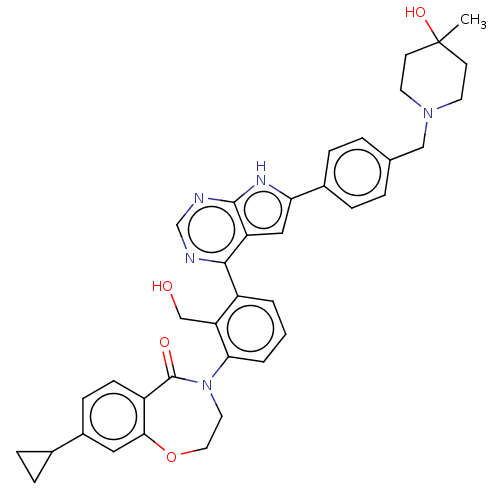 (US9233983, B-6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM202086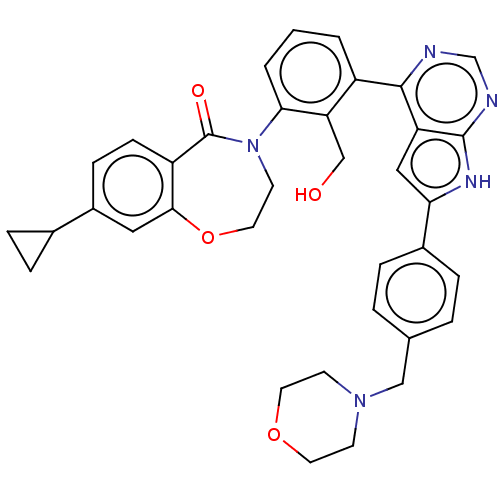 (US9233983, B-5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human full length BTK preincubated for 10 mins followed by Tk-biotin peptide substrate addition after 90 mins by TR-FRET as... | Bioorg Med Chem Lett 27: 1867-1873 (2017) Article DOI: 10.1016/j.bmcl.2017.02.026 BindingDB Entry DOI: 10.7270/Q2K35X74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |