 Found 1720 hits with Last Name = 'cooke' and Initial = 'a'
Found 1720 hits with Last Name = 'cooke' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060938
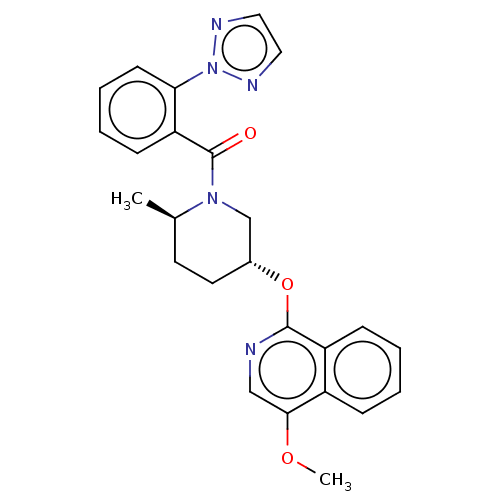
(CHEMBL3394847)Show SMILES COc1cnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c2ccccc12 |r| Show InChI InChI=1S/C25H25N5O3/c1-17-11-12-18(33-24-20-8-4-3-7-19(20)23(32-2)15-26-24)16-29(17)25(31)21-9-5-6-10-22(21)30-27-13-14-28-30/h3-10,13-15,17-18H,11-12,16H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692
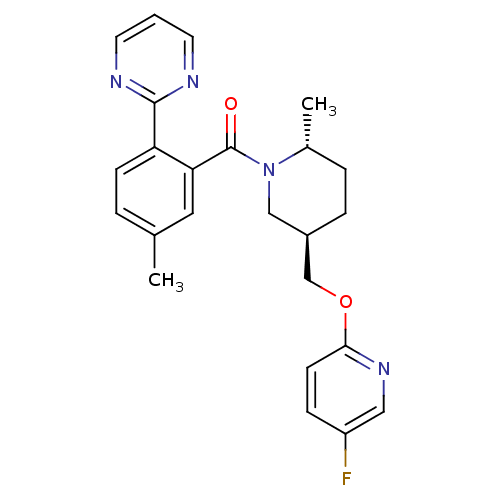
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120778
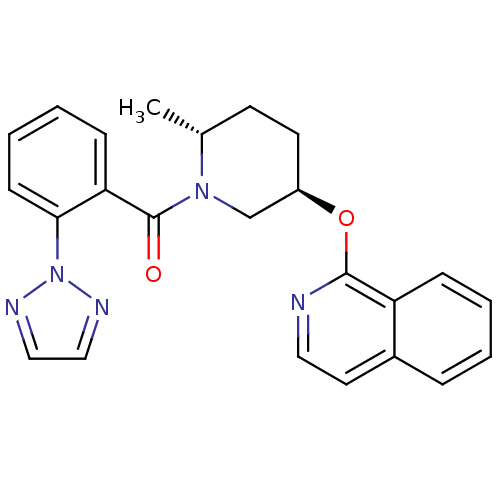
(US8710076, F-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-19(31-23-20-7-3-2-6-18(20)12-13-25-23)16-28(17)24(30)21-8-4-5-9-22(21)29-26-14-15-27-29/h2-9,12-15,17,19H,10-11,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060938

(CHEMBL3394847)Show SMILES COc1cnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c2ccccc12 |r| Show InChI InChI=1S/C25H25N5O3/c1-17-11-12-18(33-24-20-8-4-3-7-19(20)23(32-2)15-26-24)16-29(17)25(31)21-9-5-6-10-22(21)30-27-13-14-28-30/h3-10,13-15,17-18H,11-12,16H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120777
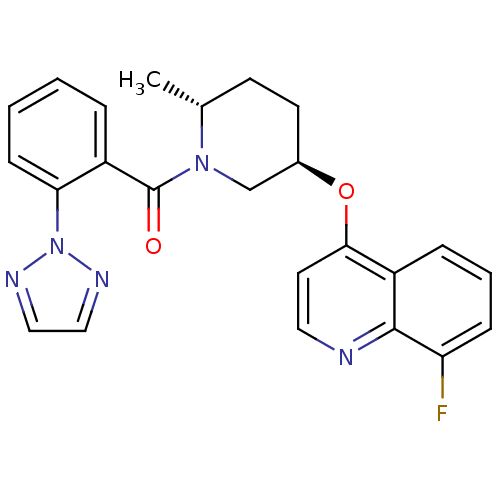
(US8710076, E-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C24H22FN5O2/c1-16-9-10-17(32-22-11-12-26-23-19(22)6-4-7-20(23)25)15-29(16)24(31)18-5-2-3-8-21(18)30-27-13-14-28-30/h2-8,11-14,16-17H,9-10,15H2,1H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060939

(CHEMBL3394845)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-c1ccccn1)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-12-13-19(33-25-14-16-30-26-22(25)9-6-10-23(26)28)17-31(18)27(32)21-8-3-2-7-20(21)24-11-4-5-15-29-24/h2-11,14-16,18-19H,12-13,17H2,1H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060945
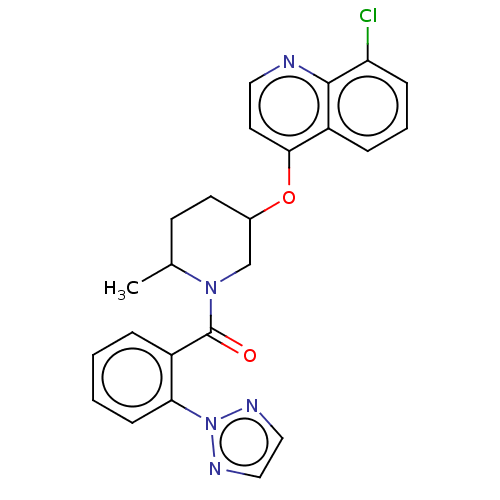
(CHEMBL3394840)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2c(Cl)cccc12 Show InChI InChI=1S/C24H22ClN5O2/c1-16-9-10-17(32-22-11-12-26-23-19(22)6-4-7-20(23)25)15-29(16)24(31)18-5-2-3-8-21(18)30-27-13-14-28-30/h2-8,11-14,16-17H,9-10,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060933

(CHEMBL3394828)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1cc(C)ccc1-n1nccn1)Oc1cccc2ccccc12 |r| Show InChI InChI=1S/C26H26N4O2/c1-18-10-13-24(30-27-14-15-28-30)23(16-18)26(31)29-17-21(12-11-19(29)2)32-25-9-5-7-20-6-3-4-8-22(20)25/h3-10,13-16,19,21H,11-12,17H2,1-2H3/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060940
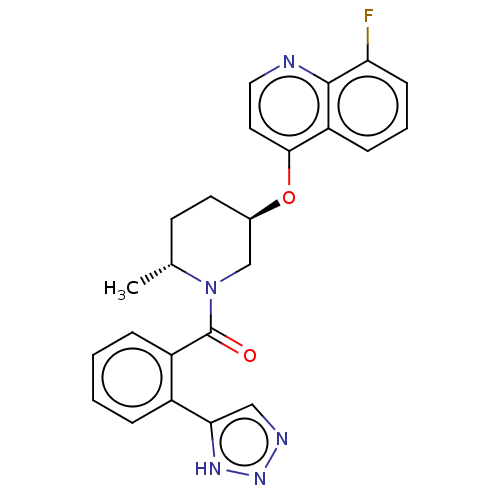
(CHEMBL3394844)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-c1cnn[nH]1)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-9-10-16(32-22-11-12-26-23-19(22)7-4-8-20(23)25)14-30(15)24(31)18-6-3-2-5-17(18)21-13-27-29-28-21/h2-8,11-13,15-16H,9-10,14H2,1H3,(H,27,28,29)/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060937
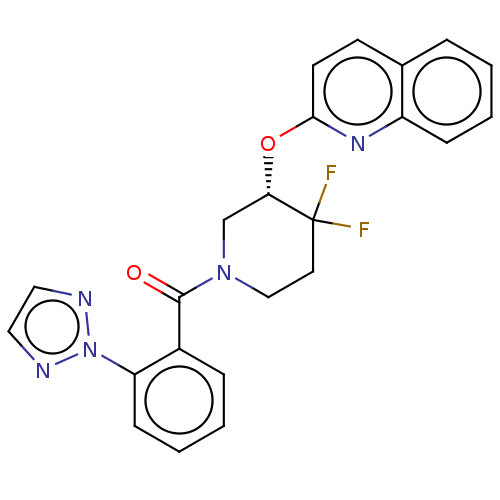
(CHEMBL3394848)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C23H19F2N5O2/c24-23(25)11-14-29(22(31)17-6-2-4-8-19(17)30-26-12-13-27-30)15-20(23)32-21-10-9-16-5-1-3-7-18(16)28-21/h1-10,12-13,20H,11,14-15H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060942
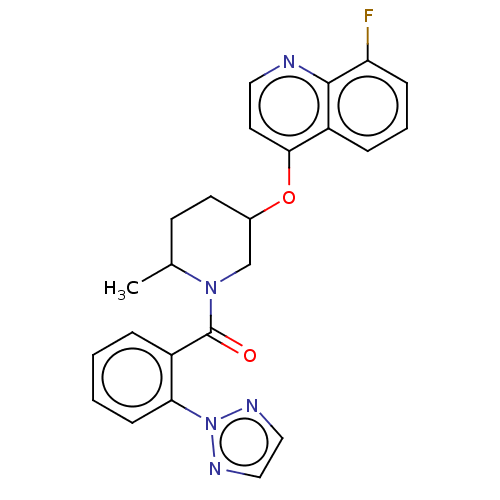
(CHEMBL3394841)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2c(F)cccc12 Show InChI InChI=1S/C24H22FN5O2/c1-16-9-10-17(32-22-11-12-26-23-19(22)6-4-7-20(23)25)15-29(16)24(31)18-5-2-3-8-21(18)30-27-13-14-28-30/h2-8,11-14,16-17H,9-10,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060946
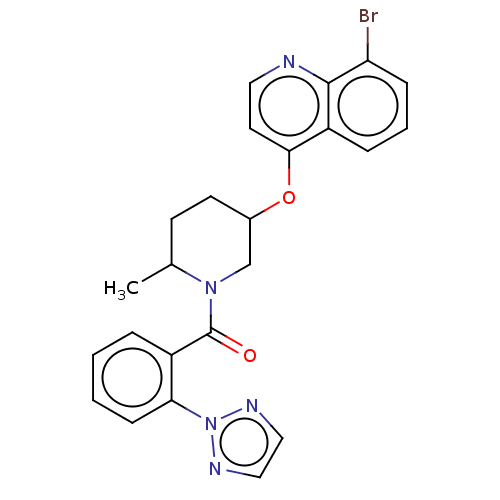
(CHEMBL3394839)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2c(Br)cccc12 Show InChI InChI=1S/C24H22BrN5O2/c1-16-9-10-17(32-22-11-12-26-23-19(22)6-4-7-20(23)25)15-29(16)24(31)18-5-2-3-8-21(18)30-27-13-14-28-30/h2-8,11-14,16-17H,9-10,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203736
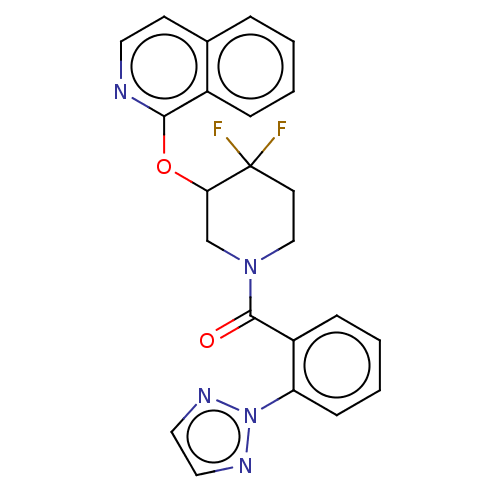
(CHEMBL3915136)Show SMILES FC1(F)CCN(CC1Oc1nccc2ccccc12)C(=O)c1ccccc1-n1nccn1 Show InChI InChI=1S/C23H19F2N5O2/c24-23(25)10-14-29(22(31)18-7-3-4-8-19(18)30-27-12-13-28-30)15-20(23)32-21-17-6-2-1-5-16(17)9-11-26-21/h1-9,11-13,20H,10,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060934

(CHEMBL3394833)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc2ncccc12 Show InChI InChI=1S/C24H23N5O2/c1-17-11-12-18(31-23-10-4-8-21-19(23)7-5-13-25-21)16-28(17)24(30)20-6-2-3-9-22(20)29-26-14-15-27-29/h2-10,13-15,17-18H,11-12,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060928
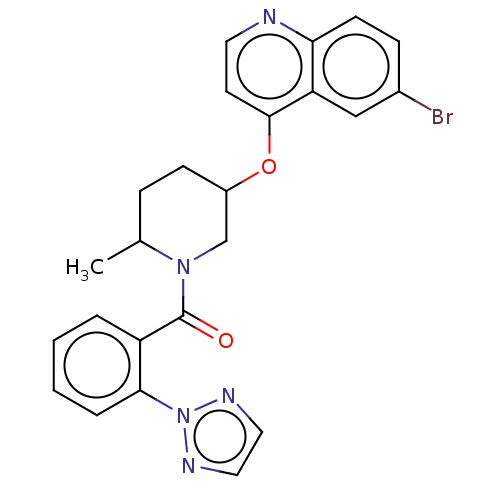
(CHEMBL3394838)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2ccc(Br)cc12 Show InChI InChI=1S/C24H22BrN5O2/c1-16-6-8-18(32-23-10-11-26-21-9-7-17(25)14-20(21)23)15-29(16)24(31)19-4-2-3-5-22(19)30-27-12-13-28-30/h2-5,7,9-14,16,18H,6,8,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060944
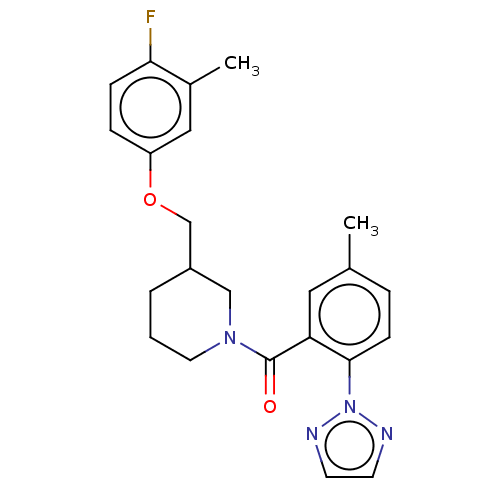
(CHEMBL3394825)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(COc2ccc(F)c(C)c2)C1)-n1nccn1 Show InChI InChI=1S/C23H25FN4O2/c1-16-5-8-22(28-25-9-10-26-28)20(12-16)23(29)27-11-3-4-18(14-27)15-30-19-6-7-21(24)17(2)13-19/h5-10,12-13,18H,3-4,11,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060935

(CHEMBL3394832)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2ccccc12 Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-18(31-23-12-13-25-21-8-4-2-6-19(21)23)16-28(17)24(30)20-7-3-5-9-22(20)29-26-14-15-27-29/h2-9,12-15,17-18H,10-11,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060948
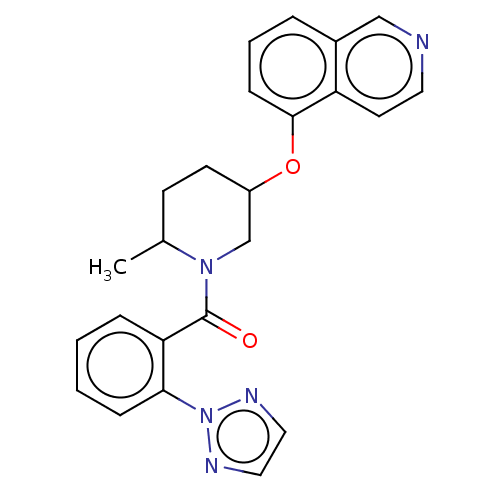
(CHEMBL3394830)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc2cnccc12 Show InChI InChI=1S/C24H23N5O2/c1-17-9-10-19(31-23-8-4-5-18-15-25-12-11-20(18)23)16-28(17)24(30)21-6-2-3-7-22(21)29-26-13-14-27-29/h2-8,11-15,17,19H,9-10,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060944

(CHEMBL3394825)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(COc2ccc(F)c(C)c2)C1)-n1nccn1 Show InChI InChI=1S/C23H25FN4O2/c1-16-5-8-22(28-25-9-10-26-28)20(12-16)23(29)27-11-3-4-18(14-27)15-30-19-6-7-21(24)17(2)13-19/h5-10,12-13,18H,3-4,11,14-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060949
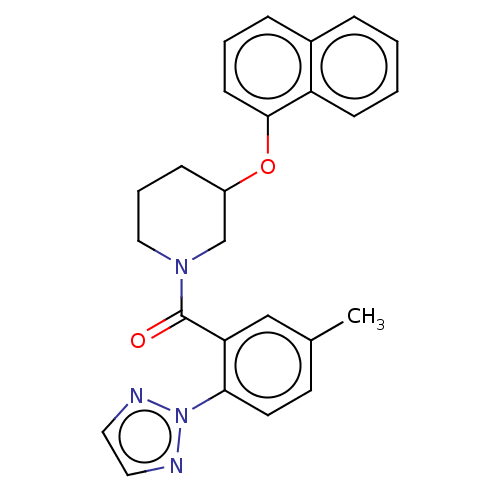
(CHEMBL3394827)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(C1)Oc1cccc2ccccc12)-n1nccn1 Show InChI InChI=1S/C25H24N4O2/c1-18-11-12-23(29-26-13-14-27-29)22(16-18)25(30)28-15-5-8-20(17-28)31-24-10-4-7-19-6-2-3-9-21(19)24/h2-4,6-7,9-14,16,20H,5,8,15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by FLIPR assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203756
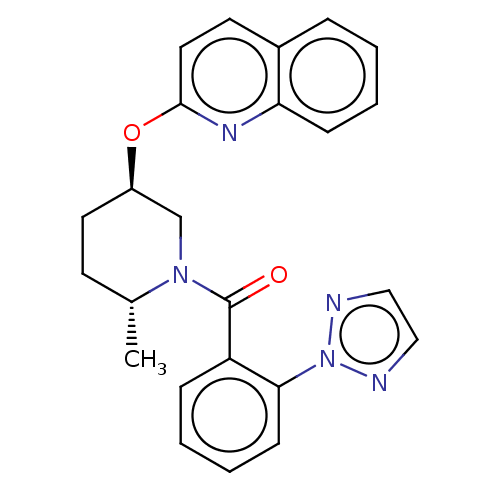
(CHEMBL3951917)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1ccc2ccccc2n1 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-12-19(31-23-13-11-18-6-2-4-8-21(18)27-23)16-28(17)24(30)20-7-3-5-9-22(20)29-25-14-15-26-29/h2-9,11,13-15,17,19H,10,12,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060936
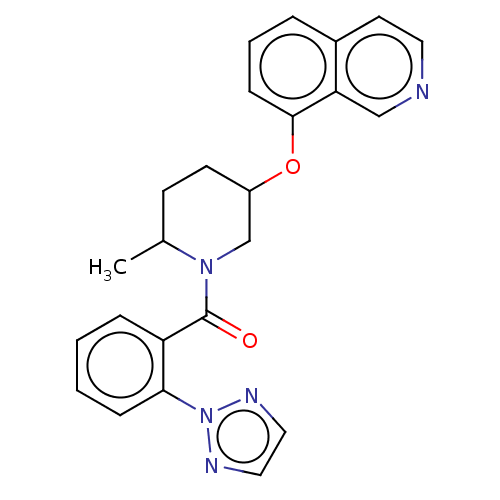
(CHEMBL3394831)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc2ccncc12 Show InChI InChI=1S/C24H23N5O2/c1-17-9-10-19(31-23-8-4-5-18-11-12-25-15-21(18)23)16-28(17)24(30)20-6-2-3-7-22(20)29-26-13-14-27-29/h2-8,11-15,17,19H,9-10,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060932
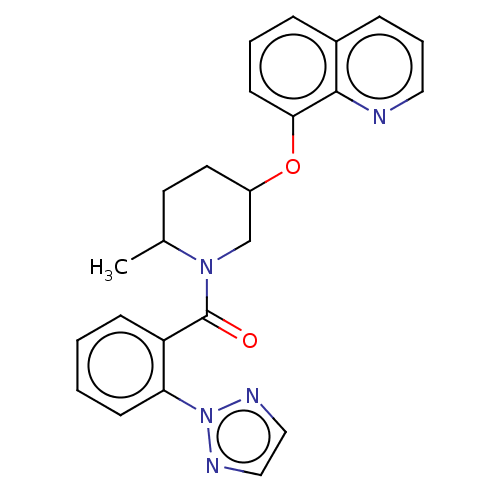
(CHEMBL3394834)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc2cccnc12 Show InChI InChI=1S/C24H23N5O2/c1-17-11-12-19(31-22-10-4-6-18-7-5-13-25-23(18)22)16-28(17)24(30)20-8-2-3-9-21(20)29-26-14-15-27-29/h2-10,13-15,17,19H,11-12,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203741
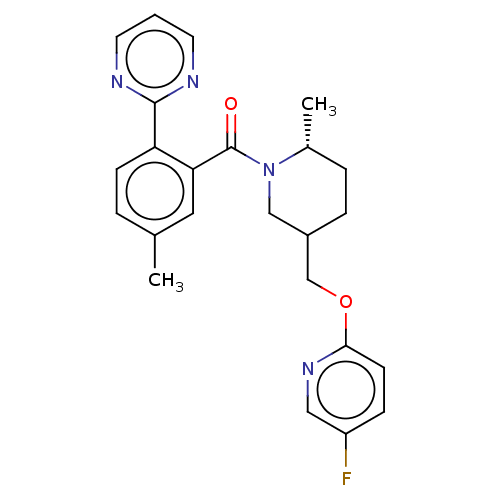
(CHEMBL3928138)Show SMILES C[C@@H]1CCC(COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM120778

(US8710076, F-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-19(31-23-20-7-3-2-6-18(20)12-13-25-23)16-28(17)24(30)21-8-4-5-9-22(21)29-26-14-15-27-29/h2-9,12-15,17,19H,10-11,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060933

(CHEMBL3394828)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1cc(C)ccc1-n1nccn1)Oc1cccc2ccccc12 |r| Show InChI InChI=1S/C26H26N4O2/c1-18-10-13-24(30-27-14-15-28-30)23(16-18)26(31)29-17-21(12-11-19(29)2)32-25-9-5-7-20-6-3-4-8-22(20)25/h3-10,13-16,19,21H,11-12,17H2,1-2H3/t19-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203768
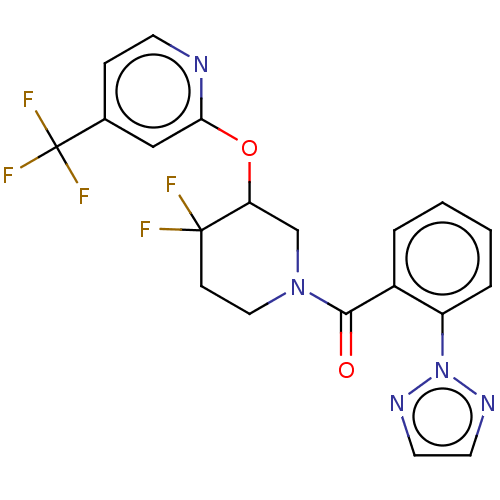
(CHEMBL3959896)Show SMILES FC(F)(F)c1ccnc(OC2CN(CCC2(F)F)C(=O)c2ccccc2-n2nccn2)c1 Show InChI InChI=1S/C20H16F5N5O2/c21-19(22)6-10-29(12-16(19)32-17-11-13(5-7-26-17)20(23,24)25)18(31)14-3-1-2-4-15(14)30-27-8-9-28-30/h1-5,7-9,11,16H,6,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203761
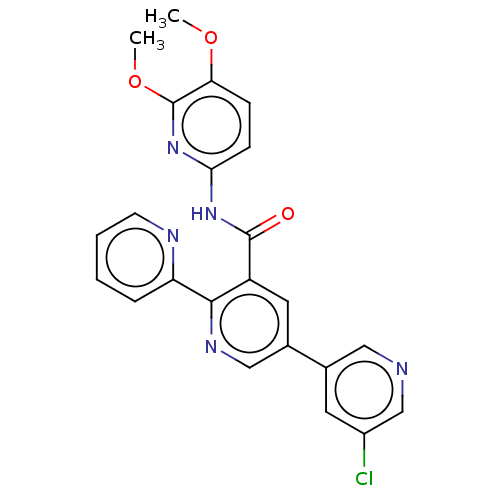
(CHEMBL3891586)Show SMILES COc1ccc(NC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C23H18ClN5O3/c1-31-19-6-7-20(29-23(19)32-2)28-22(30)17-10-15(14-9-16(24)13-25-11-14)12-27-21(17)18-5-3-4-8-26-18/h3-13H,1-2H3,(H,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060949

(CHEMBL3394827)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(C1)Oc1cccc2ccccc12)-n1nccn1 Show InChI InChI=1S/C25H24N4O2/c1-18-11-12-23(29-26-13-14-27-29)22(16-18)25(30)28-15-5-8-20(17-28)31-24-10-4-7-19-6-2-3-9-21(19)24/h2-4,6-7,9-14,16,20H,5,8,15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50384416

(CHEMBL2111553 | CHEMBL291536 | SB-334867)Show InChI InChI=1S/C17H13N5O2/c1-10-20-12-5-4-11(9-15(12)24-10)21-17(23)22-14-6-8-18-13-3-2-7-19-16(13)14/h2-9H,1H3,(H2,18,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of N6,10-rhodamine green-tagged orexin-A from human OX1 receptor expressed in CHO cells measured after 30 mins by syto62 staining based ... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203725
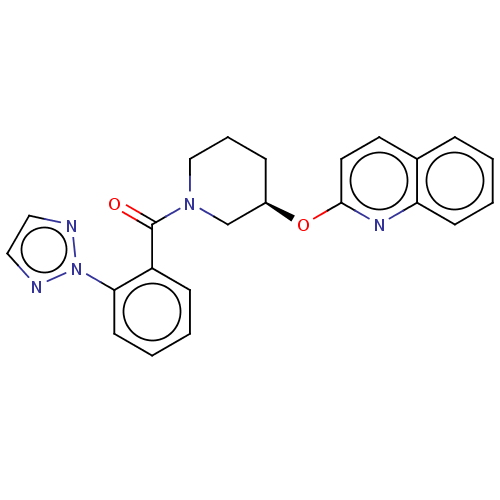
(CHEMBL3972735)Show SMILES O=C(N1CCC[C@H](C1)Oc1ccc2ccccc2n1)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C23H21N5O2/c29-23(19-8-2-4-10-21(19)28-24-13-14-25-28)27-15-5-7-18(16-27)30-22-12-11-17-6-1-3-9-20(17)26-22/h1-4,6,8-14,18H,5,7,15-16H2/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060931

(CHEMBL3394835)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(C)nc2ccccc12 Show InChI InChI=1S/C25H25N5O2/c1-17-15-24(20-7-3-5-9-22(20)28-17)32-19-12-11-18(2)29(16-19)25(31)21-8-4-6-10-23(21)30-26-13-14-27-30/h3-10,13-15,18-19H,11-12,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060943
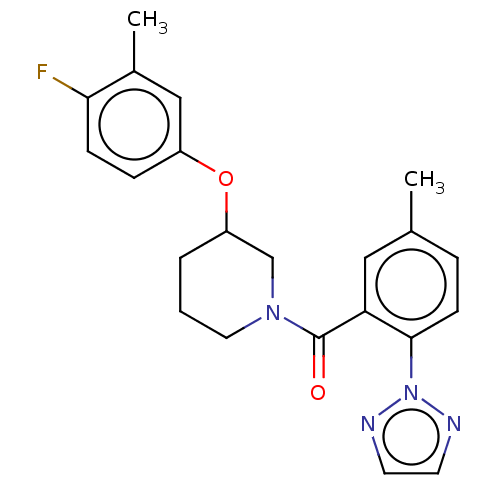
(CHEMBL3394826)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(C1)Oc1ccc(F)c(C)c1)-n1nccn1 Show InChI InChI=1S/C22H23FN4O2/c1-15-5-8-21(27-24-9-10-25-27)19(12-15)22(28)26-11-3-4-18(14-26)29-17-6-7-20(23)16(2)13-17/h5-10,12-13,18H,3-4,11,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060941

(CHEMBL3394843)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-c1cccnc1)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-11-12-20(33-25-13-15-30-26-23(25)9-4-10-24(26)28)17-31(18)27(32)22-8-3-2-7-21(22)19-6-5-14-29-16-19/h2-10,13-16,18,20H,11-12,17H2,1H3/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060943

(CHEMBL3394826)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCC(C1)Oc1ccc(F)c(C)c1)-n1nccn1 Show InChI InChI=1S/C22H23FN4O2/c1-15-5-8-21(27-24-9-10-25-27)19(12-15)22(28)26-11-3-4-18(14-26)29-17-6-7-20(23)16(2)13-17/h5-10,12-13,18H,3-4,11,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203950
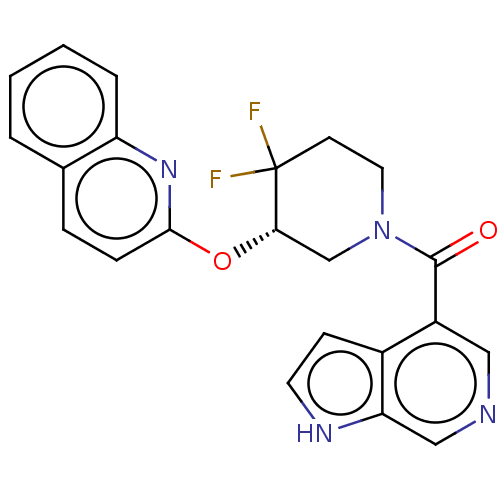
(CHEMBL3900983)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1cncc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18F2N4O2/c23-22(24)8-10-28(21(29)16-11-25-12-18-15(16)7-9-26-18)13-19(22)30-20-6-5-14-3-1-2-4-17(14)27-20/h1-7,9,11-12,19,26H,8,10,13H2/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203751
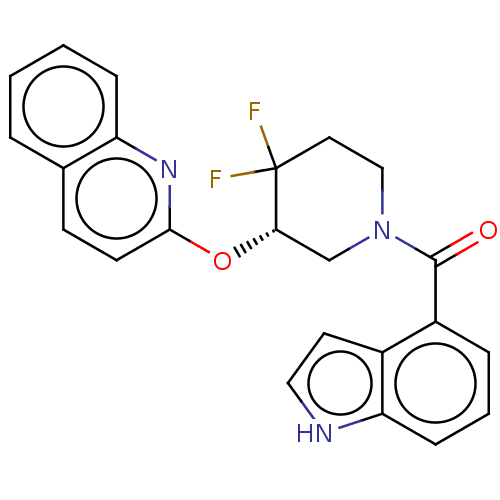
(CHEMBL3971815)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1cccc2[nH]ccc12 |r| Show InChI InChI=1S/C23H19F2N3O2/c24-23(25)11-13-28(22(29)17-5-3-7-19-16(17)10-12-26-19)14-20(23)30-21-9-8-15-4-1-2-6-18(15)27-21/h1-10,12,20,26H,11,13-14H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060930
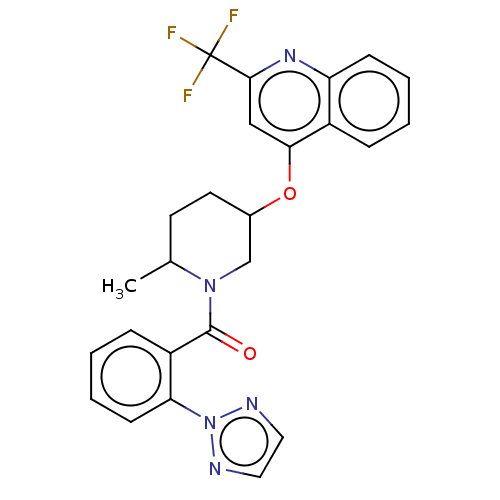
(CHEMBL3394836)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(nc2ccccc12)C(F)(F)F Show InChI InChI=1S/C25H22F3N5O2/c1-16-10-11-17(35-22-14-23(25(26,27)28)31-20-8-4-2-6-18(20)22)15-32(16)24(34)19-7-3-5-9-21(19)33-29-12-13-30-33/h2-9,12-14,16-17H,10-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060929
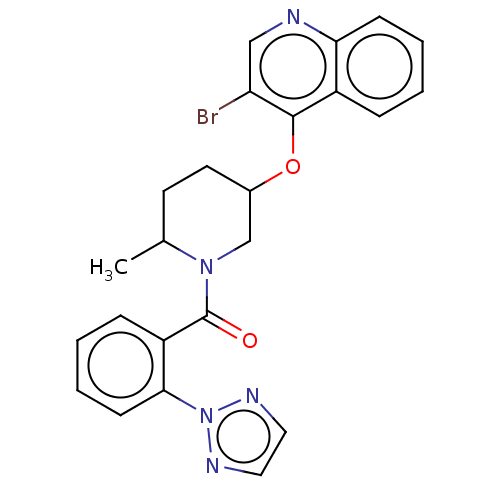
(CHEMBL3394837)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1c(Br)cnc2ccccc12 Show InChI InChI=1S/C24H22BrN5O2/c1-16-10-11-17(32-23-18-6-2-4-8-21(18)26-14-20(23)25)15-29(16)24(31)19-7-3-5-9-22(19)30-27-12-13-28-30/h2-9,12-14,16-17H,10-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203949
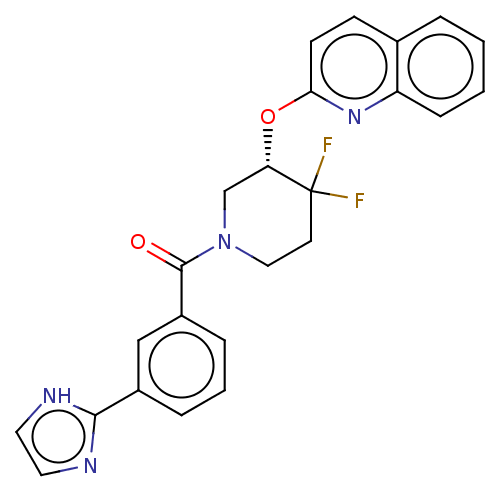
(CHEMBL3930252)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1cccc(c1)-c1ncc[nH]1 |r| Show InChI InChI=1S/C24H20F2N4O2/c25-24(26)10-13-30(23(31)18-6-3-5-17(14-18)22-27-11-12-28-22)15-20(24)32-21-9-8-16-4-1-2-7-19(16)29-21/h1-9,11-12,14,20H,10,13,15H2,(H,27,28)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203743
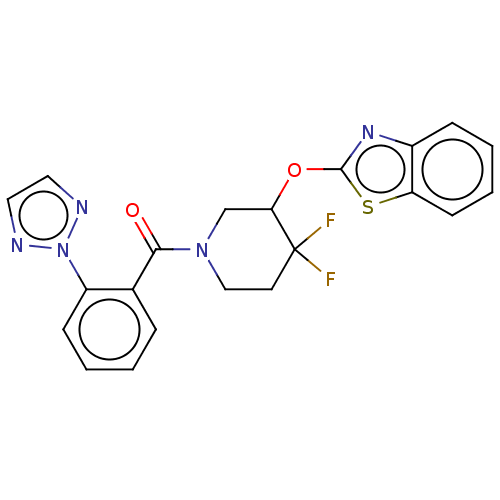
(CHEMBL3923070)Show SMILES FC1(F)CCN(CC1Oc1nc2ccccc2s1)C(=O)c1ccccc1-n1nccn1 Show InChI InChI=1S/C21H17F2N5O2S/c22-21(23)9-12-27(13-18(21)30-20-26-15-6-2-4-8-17(15)31-20)19(29)14-5-1-3-7-16(14)28-24-10-11-25-28/h1-8,10-11,18H,9,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203758
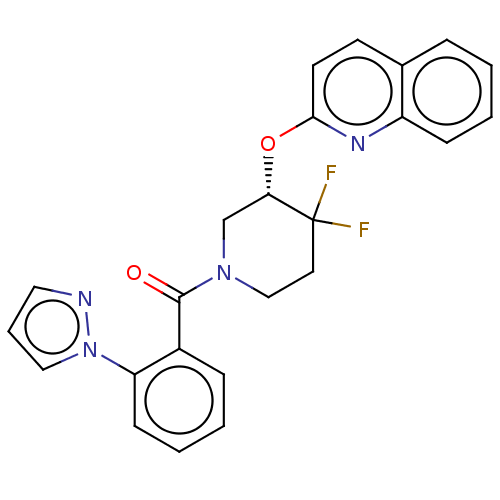
(CHEMBL3892723)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1ccccc1-n1cccn1 |r| Show InChI InChI=1S/C24H20F2N4O2/c25-24(26)12-15-29(23(31)18-7-2-4-9-20(18)30-14-5-13-27-30)16-21(24)32-22-11-10-17-6-1-3-8-19(17)28-22/h1-11,13-14,21H,12,15-16H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203760
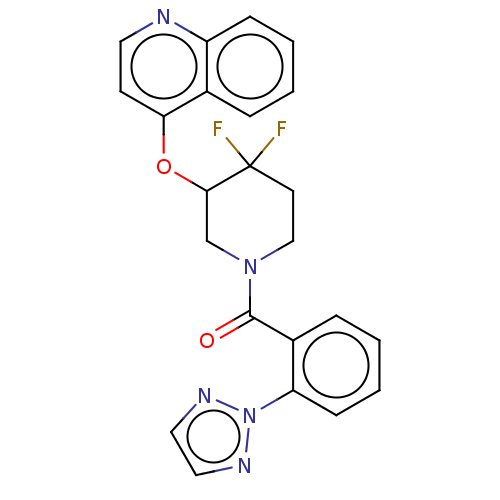
(CHEMBL3924149)Show SMILES FC1(F)CCN(CC1Oc1ccnc2ccccc12)C(=O)c1ccccc1-n1nccn1 Show InChI InChI=1S/C23H19F2N5O2/c24-23(25)10-14-29(22(31)17-6-2-4-8-19(17)30-27-12-13-28-30)15-21(23)32-20-9-11-26-18-7-3-1-5-16(18)20/h1-9,11-13,21H,10,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203748
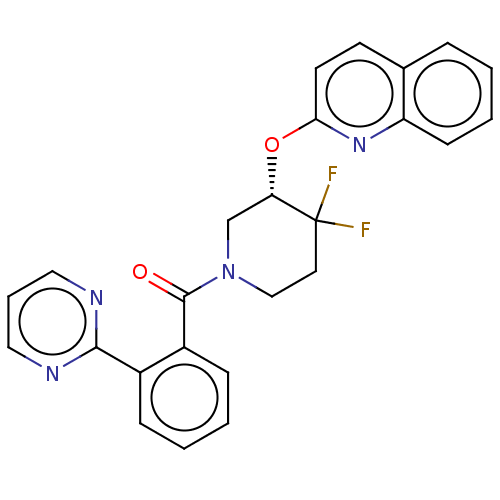
(CHEMBL3919876)Show SMILES FC1(F)CCN(C[C@@H]1Oc1ccc2ccccc2n1)C(=O)c1ccccc1-c1ncccn1 |r| Show InChI InChI=1S/C25H20F2N4O2/c26-25(27)12-15-31(16-21(25)33-22-11-10-17-6-1-4-9-20(17)30-22)24(32)19-8-3-2-7-18(19)23-28-13-5-14-29-23/h1-11,13-14,21H,12,15-16H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060947
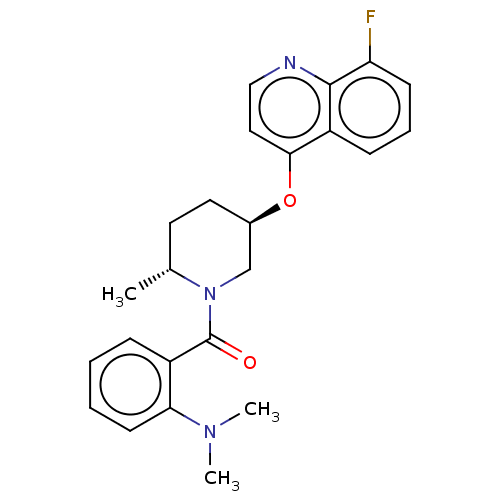
(CHEMBL3394846)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1N(C)C)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C24H26FN3O2/c1-16-11-12-17(30-22-13-14-26-23-19(22)8-6-9-20(23)25)15-28(16)24(29)18-7-4-5-10-21(18)27(2)3/h4-10,13-14,16-17H,11-12,15H2,1-3H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by FLIPR assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50203737

(CHEMBL3929466)Show SMILES FC1(F)CCN(CC1Oc1cc2ccccc2cn1)C(=O)c1ccccc1-n1nccn1 Show InChI InChI=1S/C23H19F2N5O2/c24-23(25)9-12-29(22(31)18-7-3-4-8-19(18)30-27-10-11-28-30)15-20(23)32-21-13-16-5-1-2-6-17(16)14-26-21/h1-8,10-11,13-14,20H,9,12,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50060948

(CHEMBL3394830)Show SMILES CC1CCC(CN1C(=O)c1ccccc1-n1nccn1)Oc1cccc2cnccc12 Show InChI InChI=1S/C24H23N5O2/c1-17-9-10-19(31-23-8-4-5-18-15-25-12-11-20(18)23)16-28(17)24(30)21-6-2-3-7-22(21)29-26-13-14-27-29/h2-8,11-15,17,19H,9-10,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
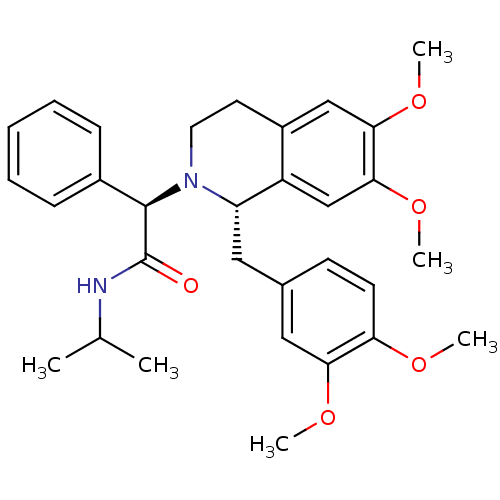
(Homo sapiens (Human)) | BDBM50438822
(CHEMBL2413367)Show SMILES COc1ccc(C[C@@H]2N(CCc3cc(OC)c(OC)cc23)[C@@H](C(=O)NC(C)C)c2ccccc2)cc1OC |r| Show InChI InChI=1S/C31H38N2O5/c1-20(2)32-31(34)30(22-10-8-7-9-11-22)33-15-14-23-18-28(37-5)29(38-6)19-24(23)25(33)16-21-12-13-26(35-3)27(17-21)36-4/h7-13,17-20,25,30H,14-16H2,1-6H3,(H,32,34)/t25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
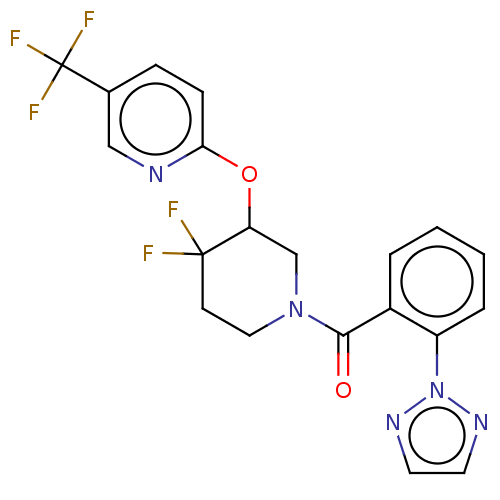
(Homo sapiens (Human)) | BDBM50203724
(CHEMBL3941376)Show SMILES FC(F)(F)c1ccc(OC2CN(CCC2(F)F)C(=O)c2ccccc2-n2nccn2)nc1 Show InChI InChI=1S/C20H16F5N5O2/c21-19(22)7-10-29(12-16(19)32-17-6-5-13(11-26-17)20(23,24)25)18(31)14-3-1-2-4-15(14)30-27-8-9-28-30/h1-6,8-9,11,16H,7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (2S)-N-(2-pyrrol-1-ylphenyl)-1-[2-[1-(tritritiomethyl)benzimidazol-2-yl]sulfanylacetyl]pyrrolidine-2-carboxamide from human OX2 recep... |
Bioorg Med Chem Lett 26: 5809-5814 (2016)
Article DOI: 10.1016/j.bmcl.2016.10.019
BindingDB Entry DOI: 10.7270/Q2MS3VRG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data