

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113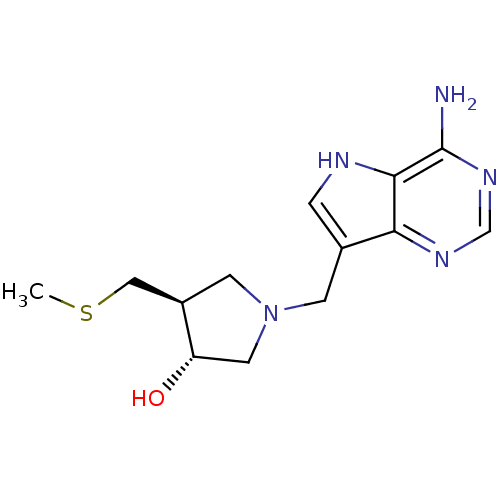 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate assessed as inhibition... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093582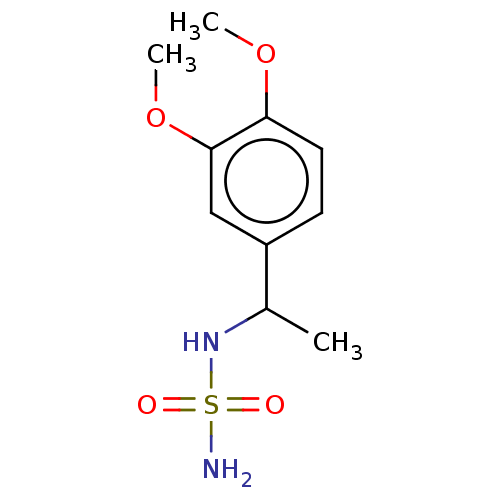 (CHEMBL3585782) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093588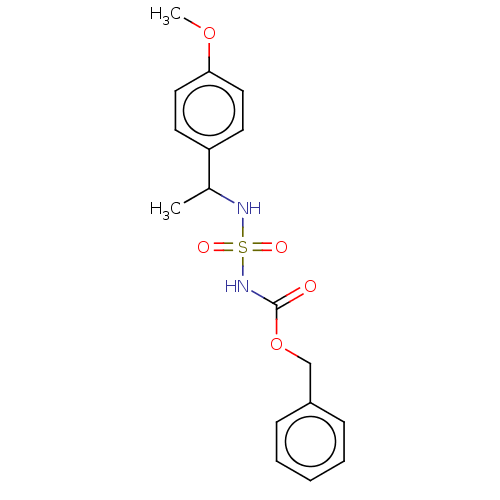 (CHEMBL3585776) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093587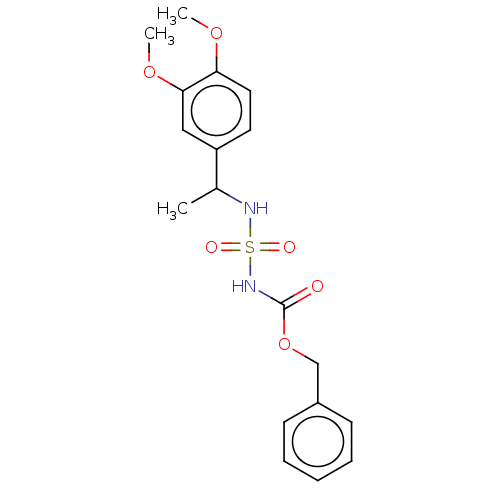 (CHEMBL3585777) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093589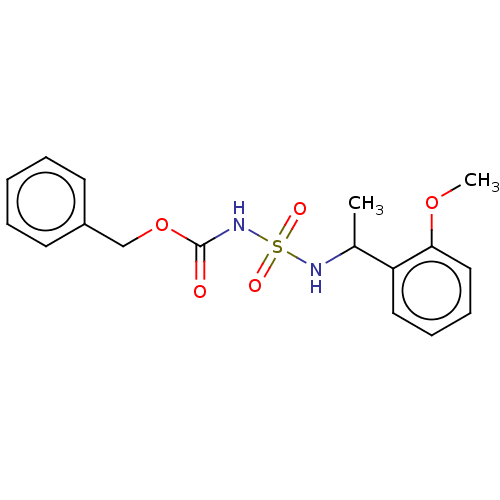 (CHEMBL3585775) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of esterase activity of carbonic anhydrase 2 in human erythrocytes using PNF as substrate by spectrophotometer analysis | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093583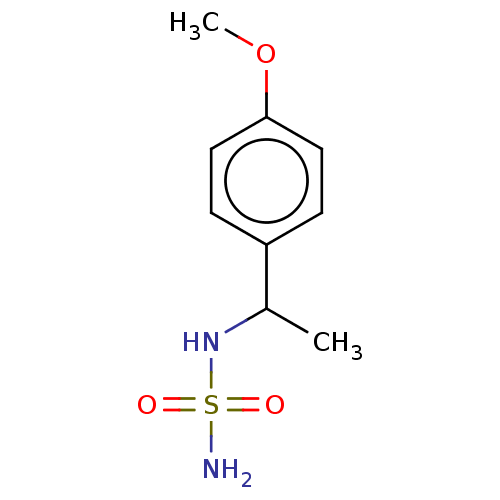 (CHEMBL3585781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093581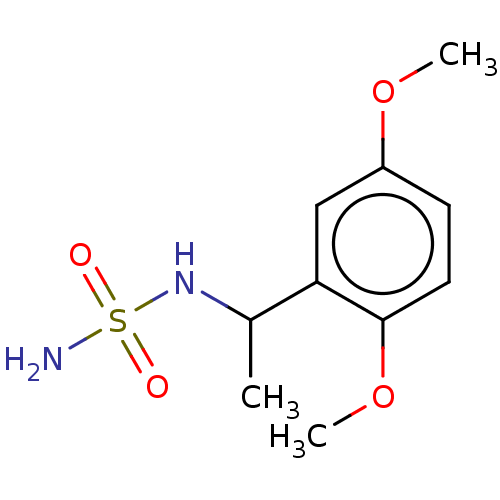 (CHEMBL3585783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388434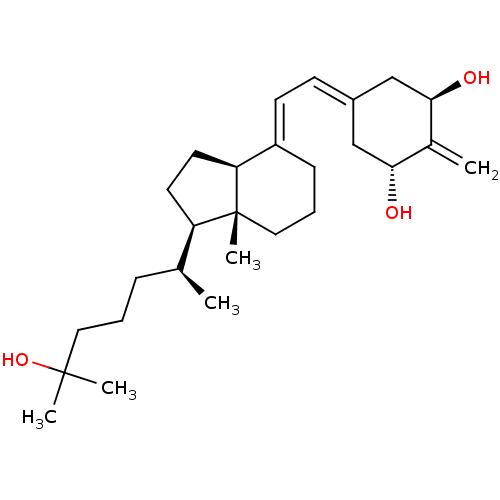 (CHEMBL605525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093597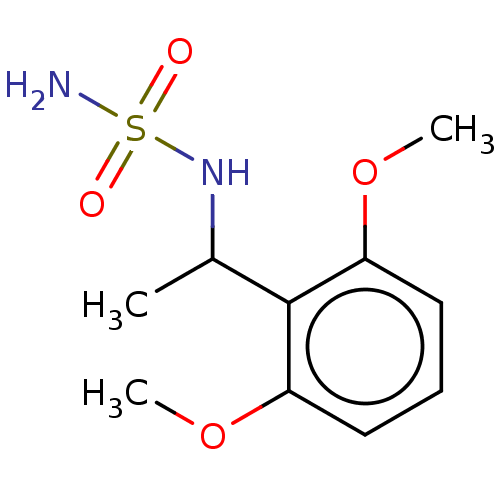 (CHEMBL3585784) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) assessed as AChI hydrolysis using AChI and DTNB as substrate by Ellman's method | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417515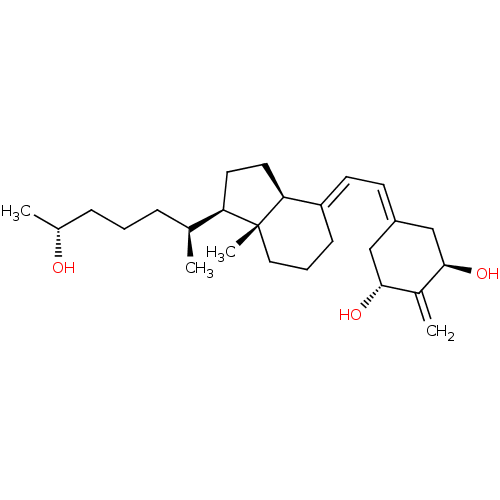 (CHEMBL1630755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50093585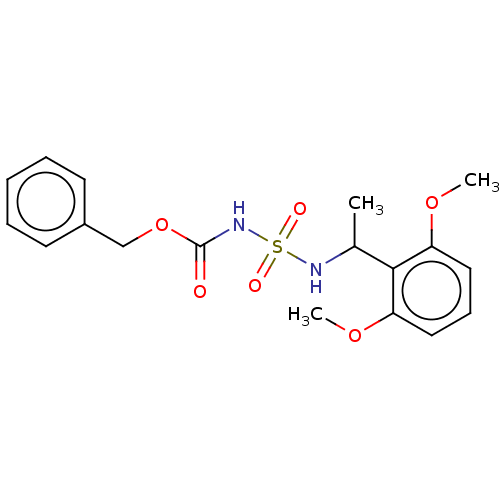 (CHEMBL3585779) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AgriIbrahim£e£en University Curated by ChEMBL | Assay Description Inhibition of esterase activity of carbonic anhydrase 1 in human erythrocytes using PNF as substrate by spectrophotometer analysis | Bioorg Med Chem 23: 3592-602 (2015) Article DOI: 10.1016/j.bmc.2015.04.019 BindingDB Entry DOI: 10.7270/Q2J104XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM272984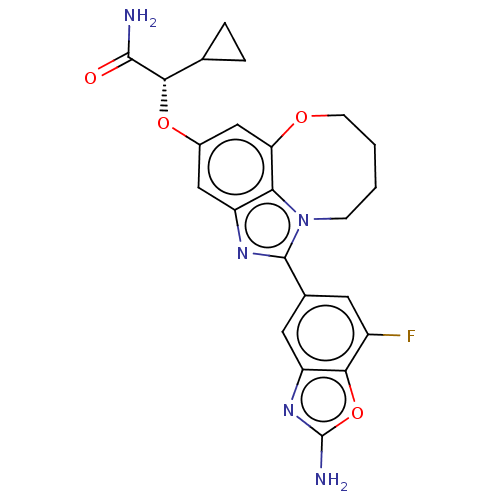 ((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description TBD | US Patent US10435414 (2019) BindingDB Entry DOI: 10.7270/Q2GX4DXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM272989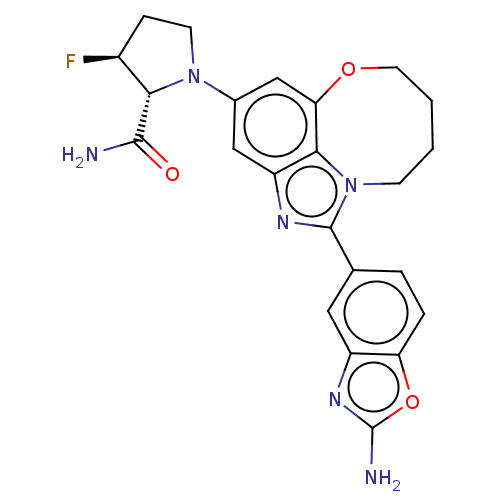 ((2R,3S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description TBD | US Patent US10435414 (2019) BindingDB Entry DOI: 10.7270/Q2GX4DXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM504044 (US11034692, Compound 313) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM504049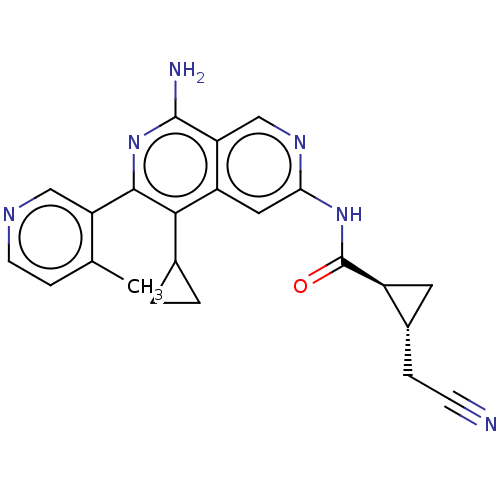 ((1R,2S)—N-(8-amino-5-cyclopropyl-6-(4-methylp...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM504051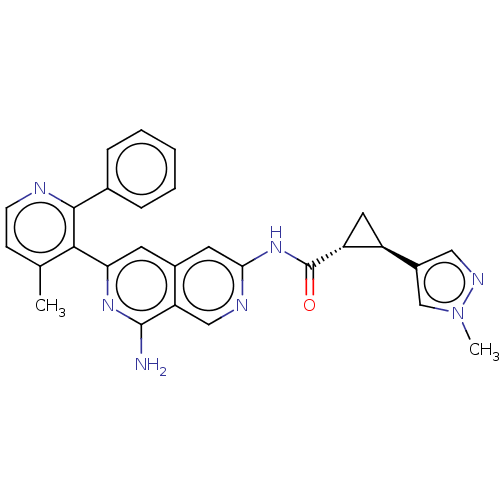 ((1S,2S)—N-(8-amino-6-(4-methyl-2-phenylpyridi...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM504062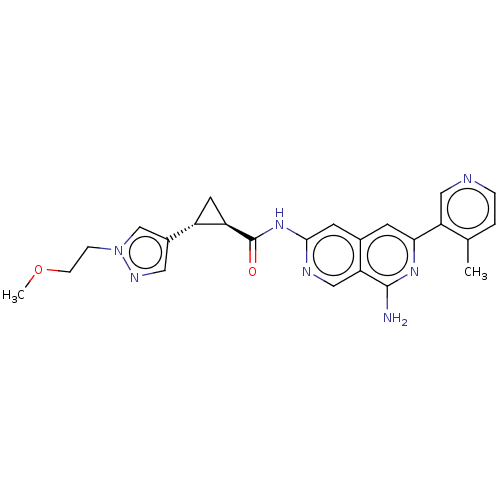 ((1R,2R)-N-(8-amino-6-(4-methylpyridin- 3-yl)-2,7-n...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM503940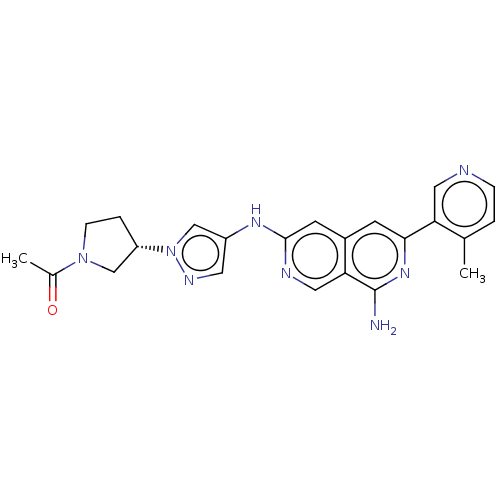 (1-(3-(4-(8-amino-6-(4-methylpyridin-3- yl)-2,7-nap...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM503988 (2-[[8-amino-6-(4-methylpyridin-3-yl)-2,7- naphthyr...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM503863 (US11034692, Compound 146 | trans-N-[8-amino-6-(4-m...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM503879 (1-[3-[4-[[8-amino-6-(4-methyl-3-pyridyl)- 2,7-naph...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM503914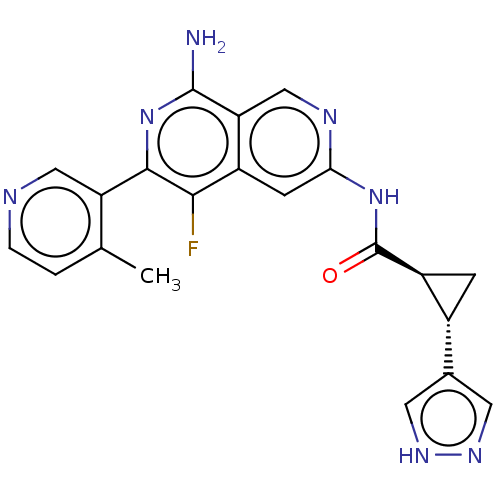 ((1S,2S)-N-(8-amino-5-fluoro-6-(4- methylpyridin-3-...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Lanth: For the binding assay, 4 ul 2×HPK1 and Eu-anti-GST antibody were added to each well of the assay plate using a Multidrop reagent dispenser. Th... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CV4MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 5 (Homo sapiens (Human)) | BDBM50597886 (CHEMBL5180177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01208 BindingDB Entry DOI: 10.7270/Q25X2DZR | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM272959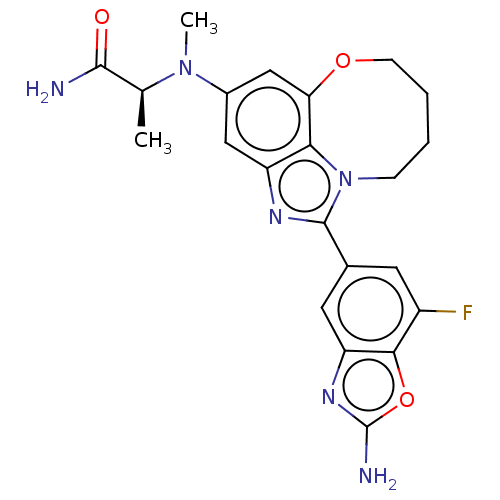 ((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description TBD | US Patent US10435414 (2019) BindingDB Entry DOI: 10.7270/Q2GX4DXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM414902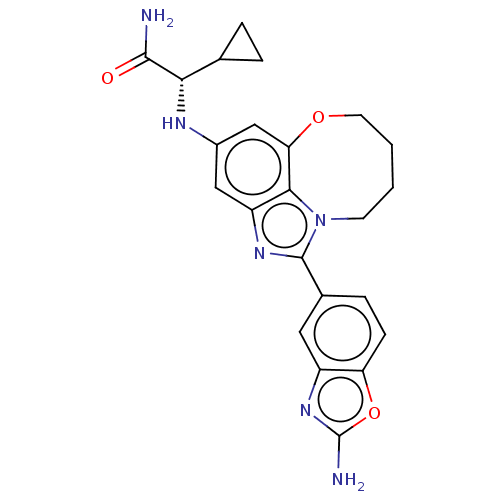 ((S)-2-((1-(2- aminobenzo [d]oxazol-5- yl)-7,8,9,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description TBD | US Patent US10435414 (2019) BindingDB Entry DOI: 10.7270/Q2GX4DXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135183 (CHEMBL3745798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100535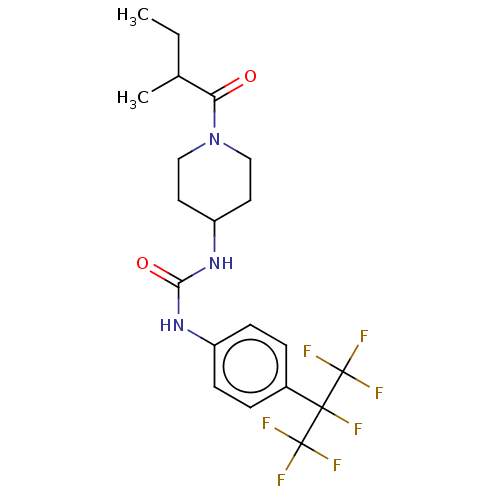 (CHEMBL3327073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50100528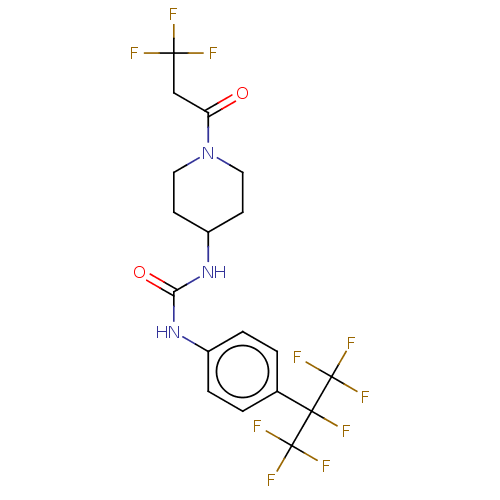 (CHEMBL3327081) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis Curated by ChEMBL | Assay Description Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay | J Med Chem 57: 7016-30 (2014) Article DOI: 10.1021/jm500694p BindingDB Entry DOI: 10.7270/Q2FJ2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50304585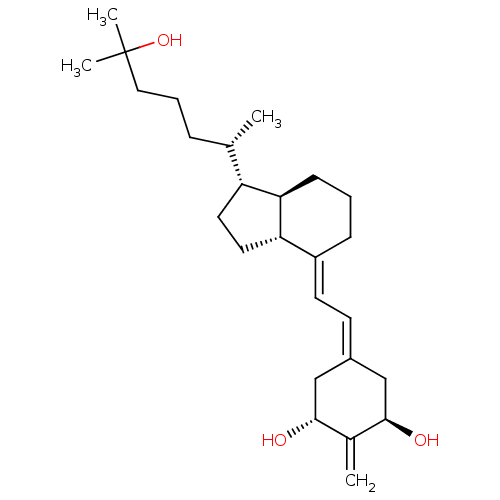 ((20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorv...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417519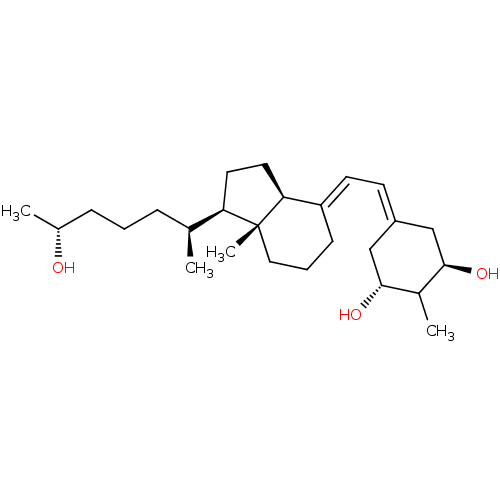 (CHEMBL1630759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388439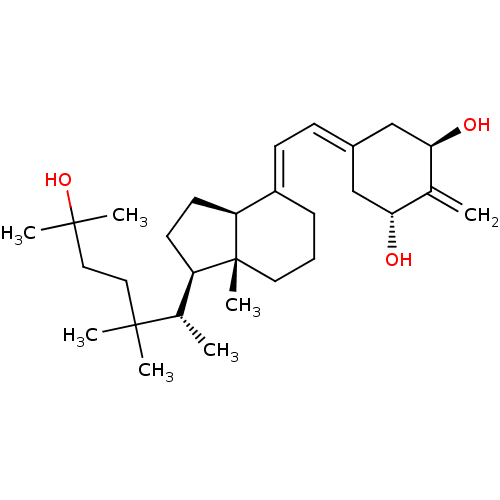 (CHEMBL2059272) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272941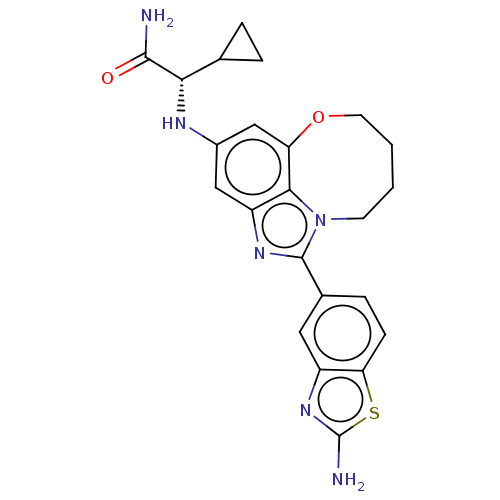 ((S)-2-((1-(2- Aminobenzo[d]thiazol- 5-yl)-7,8,9,10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272944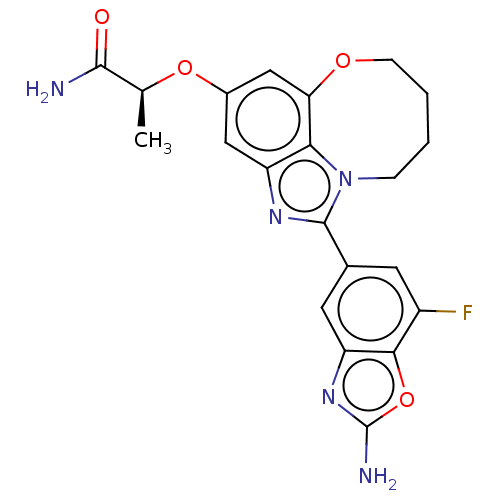 ((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272945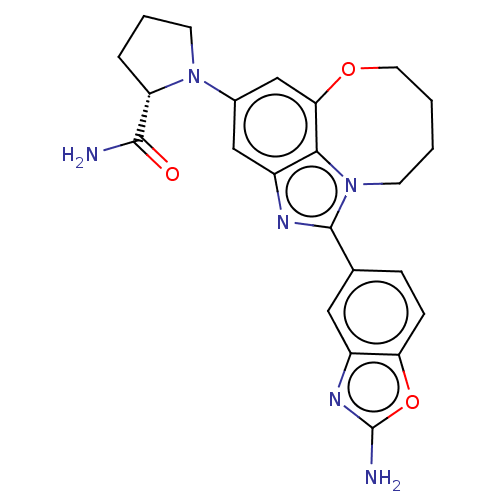 ((S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272949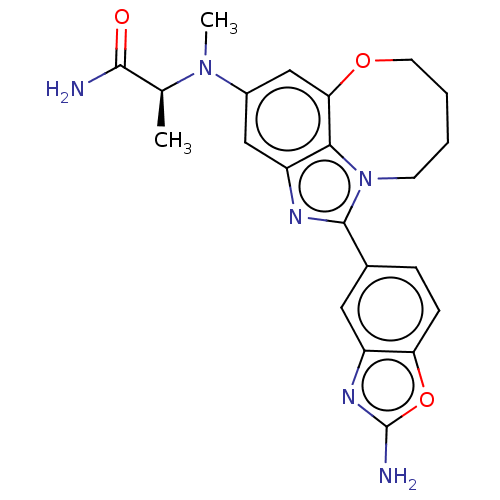 ((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272899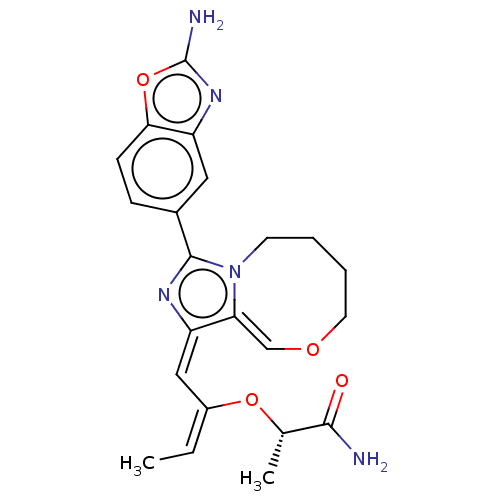 ((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272903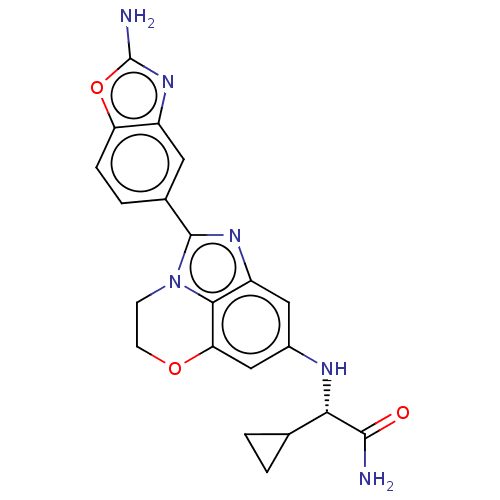 ((S)-2-((2-(2- aminobenzo[d]oxazol- 5-yl)-3,4-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272909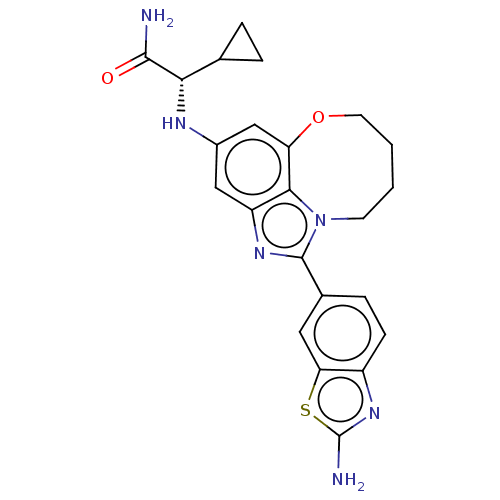 ((S)-2-((1-(2- aminobenzo[d]thiazol- 6-yl)-7,8,9,10...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272830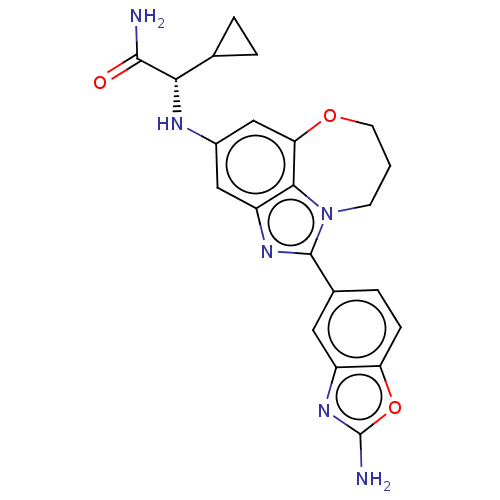 ((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-8,9-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272991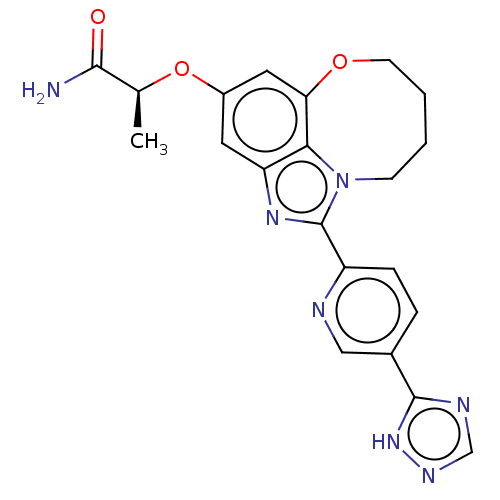 ((S)-2-((1-(5-(1H-1,2,4- triazol-5-yl)pyridin-2- yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM273000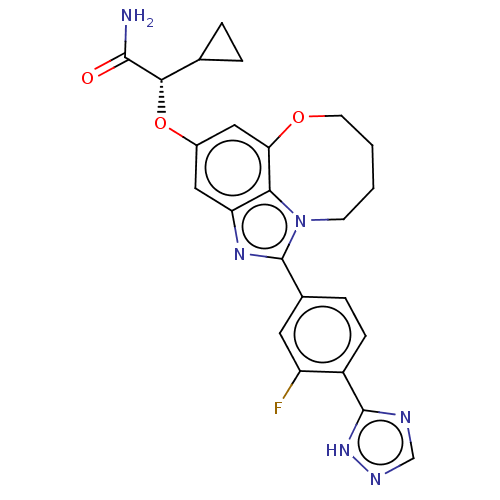 ((S)-2-cyclopropyl-2- ((1-(3-fluoro-4-(1H- 1,2,4-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM273002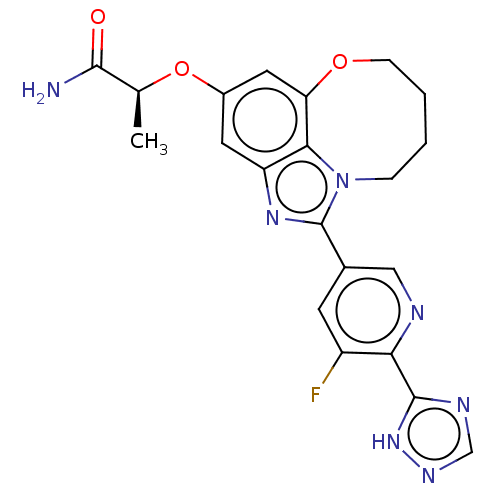 ((S)-2-((1-(5-fluoro-6- (1H-1,2,4-triazol-5- yl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM273010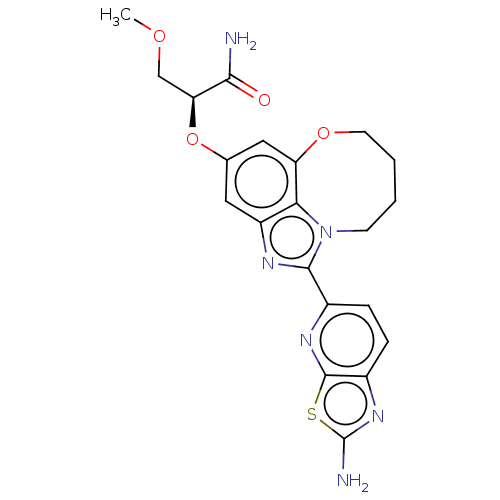 ((S)-2-((1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272956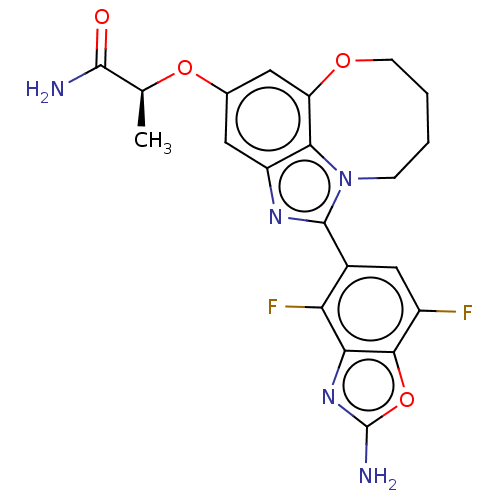 ((S)-2-((1-(2-amino-4,7- difluorobenzo[d]oxazol- 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272959 ((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272960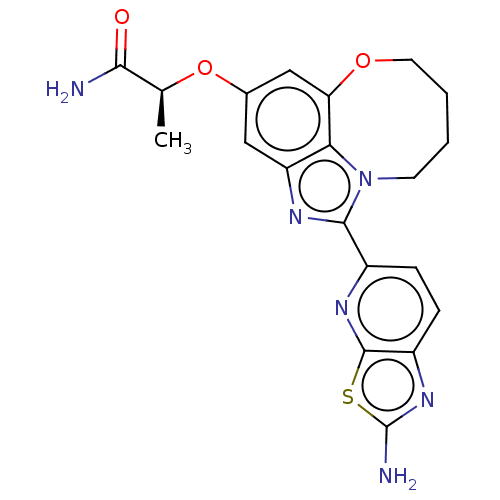 ((S)-2-((1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272964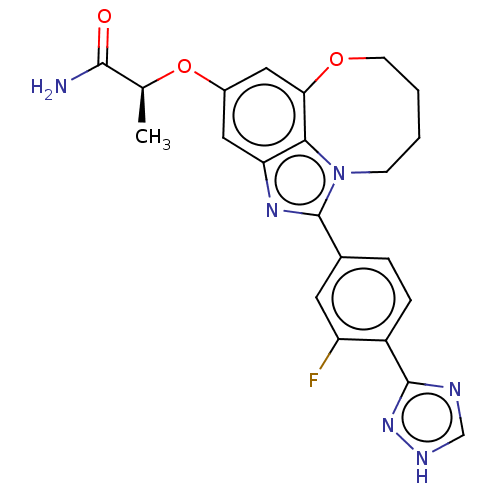 ((S)-2-((1-(3-fluoro-4- (1H-1,2,4-triazol-3- yl)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272966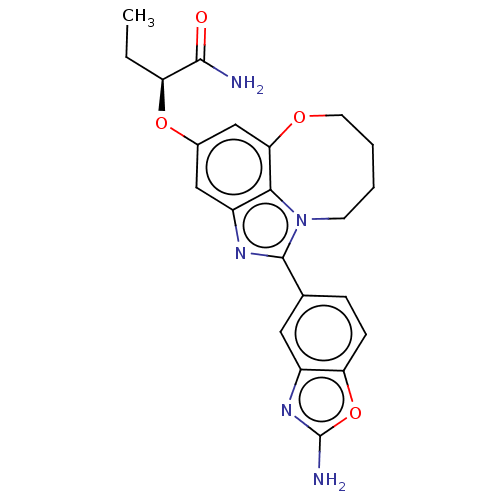 ((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272967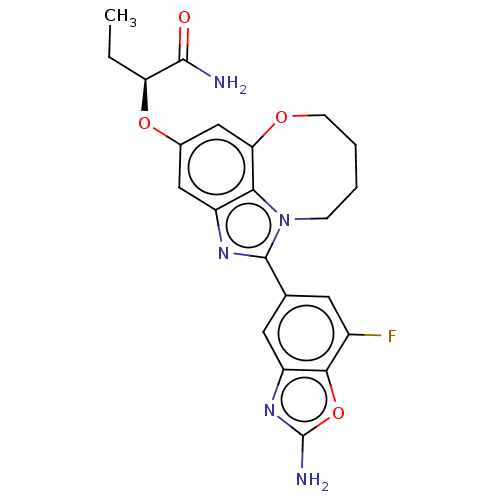 ((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM272975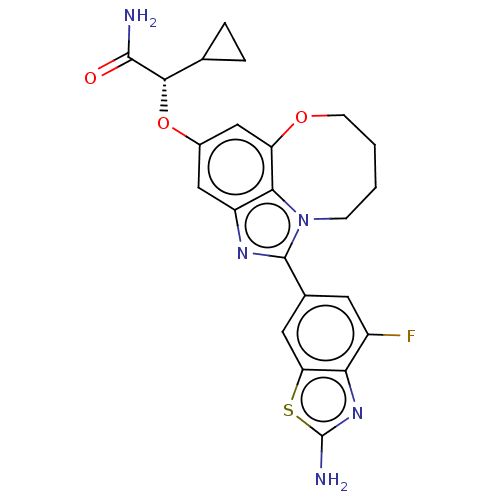 ((S)-2-((1-(2-amino-4- fluorobenzo[d]thiazol- 6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... | US Patent US10065970 (2018) BindingDB Entry DOI: 10.7270/Q27P91FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 31894 total ) | Next | Last >> |