

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50514081 (CHEMBL4471466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein by Michaelis-menten analysis | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50514086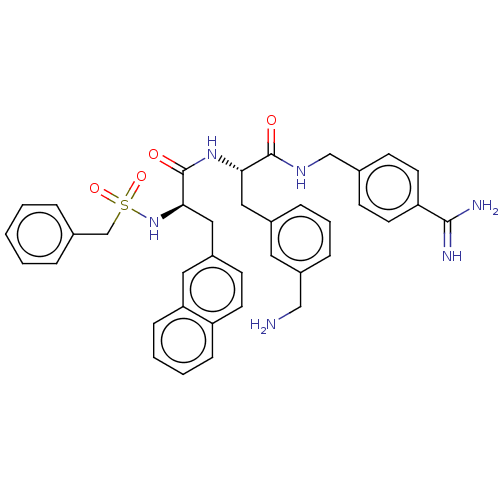 (CHEMBL4524734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using S2302 as substrate after 10 mins by UV/Vis photometry | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108110 (US8598206, Table 6, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using S2302 as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50514081 (CHEMBL4471466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of activated protein kinase C (unknown origin) | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514082 (CHEMBL4462811) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate after 10 mins by UV/Vis photometry | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50440040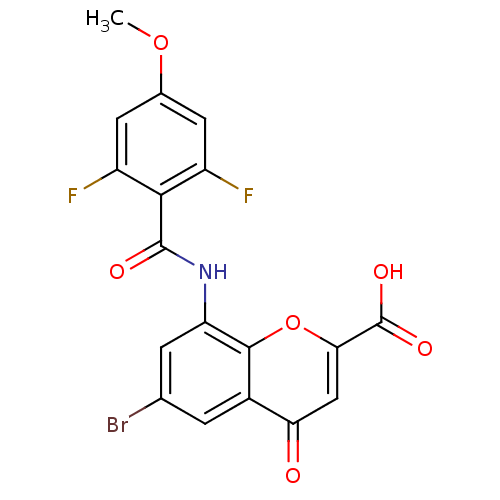 (CHEMBL2425821) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108098 (US8598206, Table 6, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50514082 (CHEMBL4462811) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using S2302 as substrate after 10 mins by UV/Vis photometry | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436006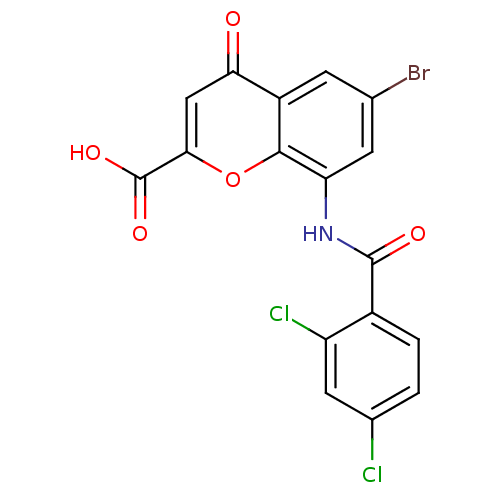 (CHEMBL2392171) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.938 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514086 (CHEMBL4524734) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate after 10 mins by UV/Vis photometry | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436000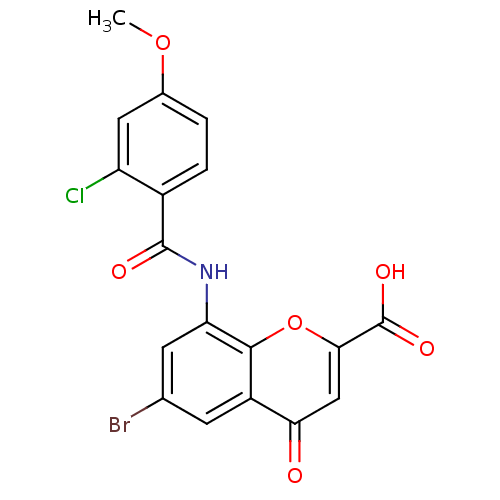 (CHEMBL2392179) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50440042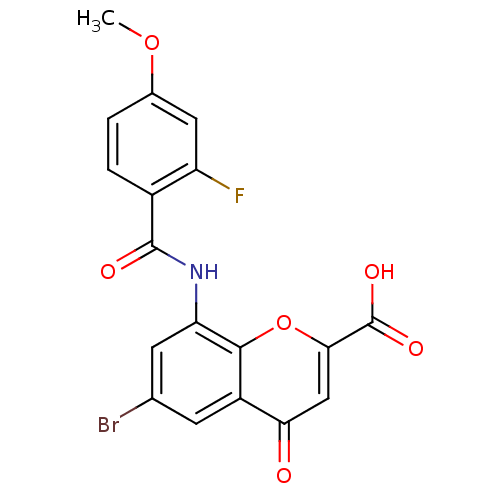 (CHEMBL2425819) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM108110 (US8598206, Table 6, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using S2302 as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50440041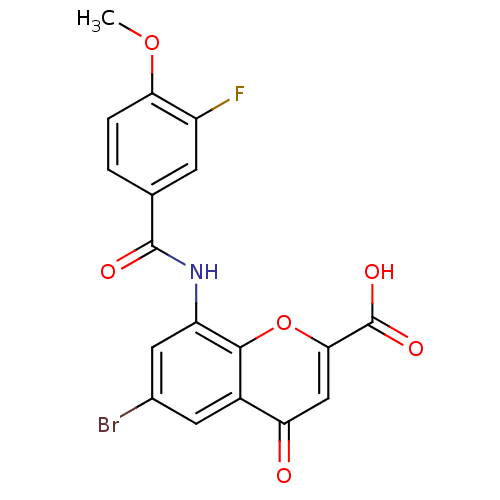 (CHEMBL2425820) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50440043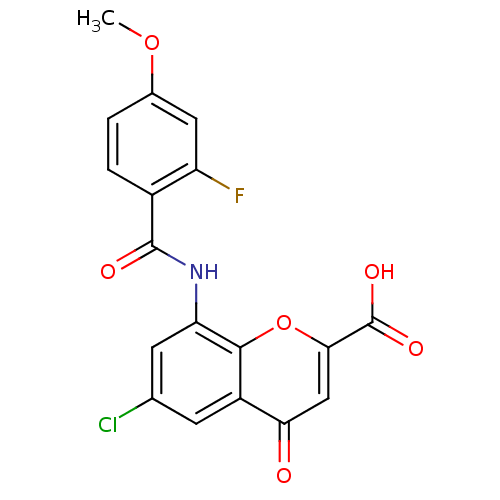 (CHEMBL2425824) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50510071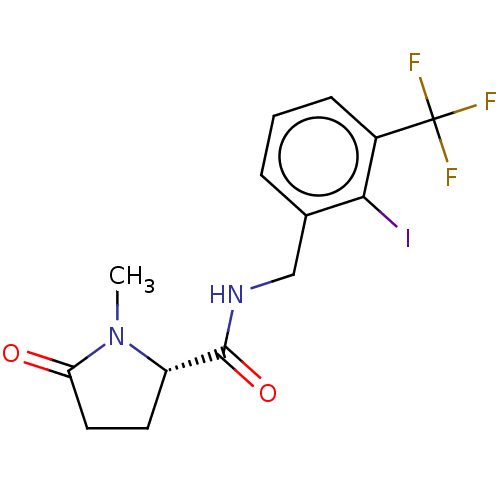 (CHEMBL4541082) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... | Bioorg Med Chem Lett 29: 1476-1480 (2019) Article DOI: 10.1016/j.bmcl.2019.04.018 BindingDB Entry DOI: 10.7270/Q2C250RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123327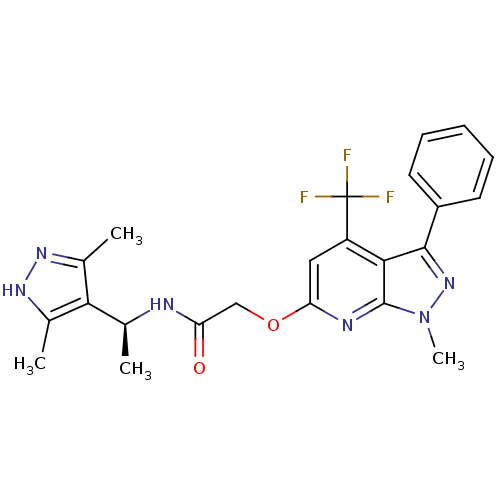 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123341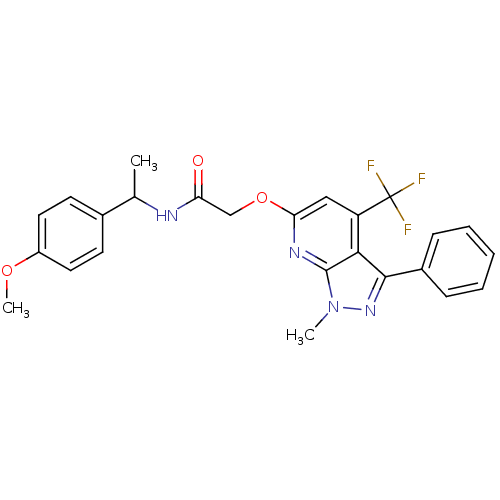 (US8742106, 1.45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123325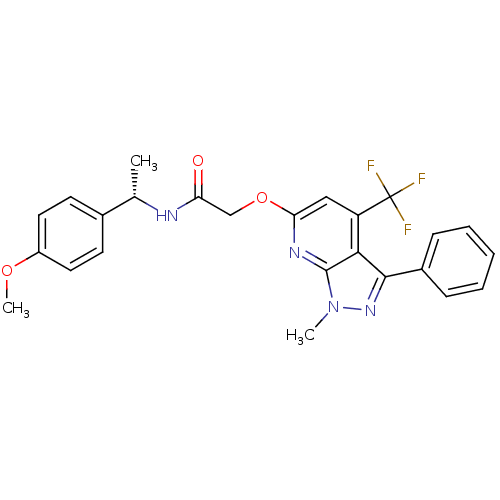 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM29388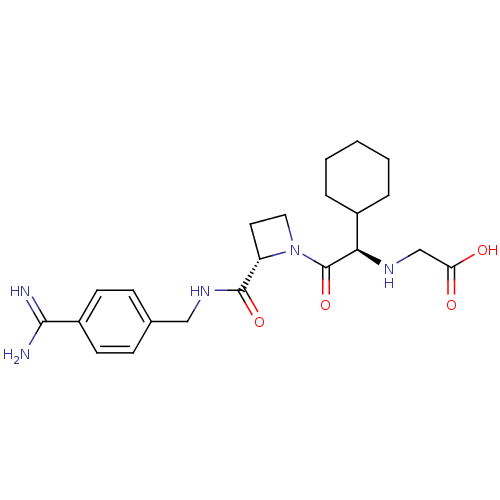 (Exanta | Melagatran | US11584714, Compound 999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of thrombin (unknown origin) | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436003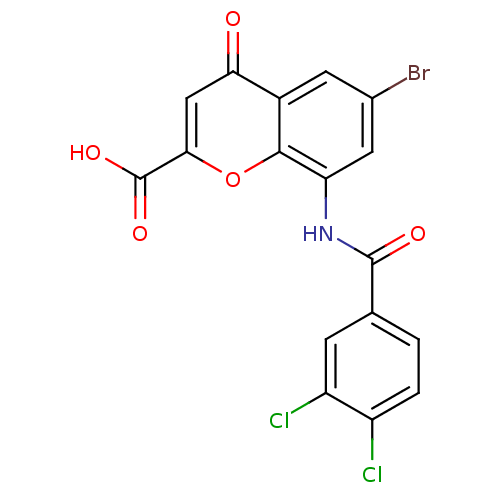 (CHEMBL2392172) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514081 (CHEMBL4471466) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin by Michaelis-menten analysis | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259915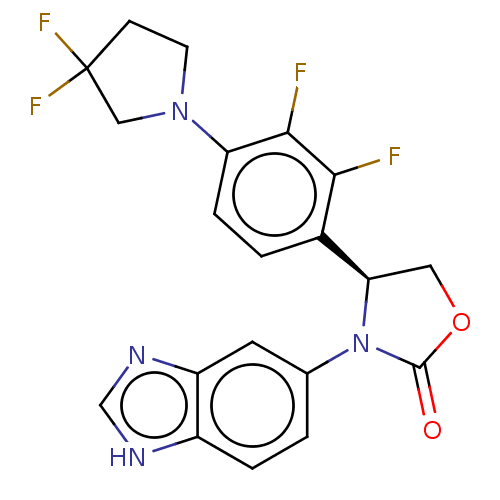 (US9512115, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.49 | -49.9 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50510072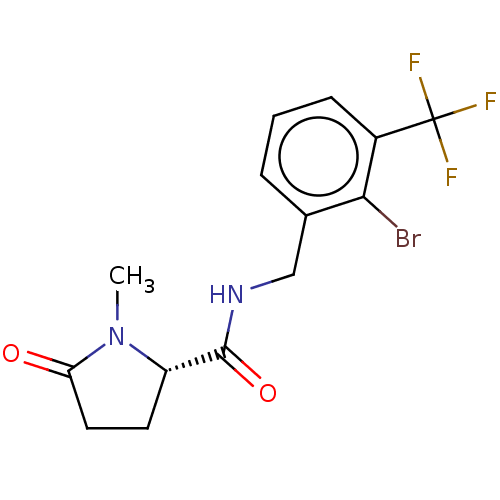 (CHEMBL4435339) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... | Bioorg Med Chem Lett 29: 1476-1480 (2019) Article DOI: 10.1016/j.bmcl.2019.04.018 BindingDB Entry DOI: 10.7270/Q2C250RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259915 (US9512115, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.53 | -49.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259914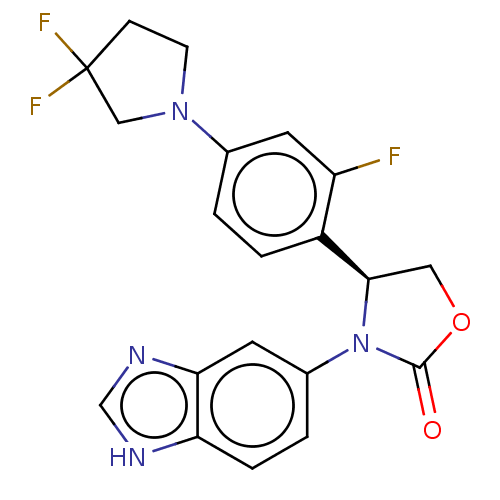 (US9512115, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.69 | -49.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259898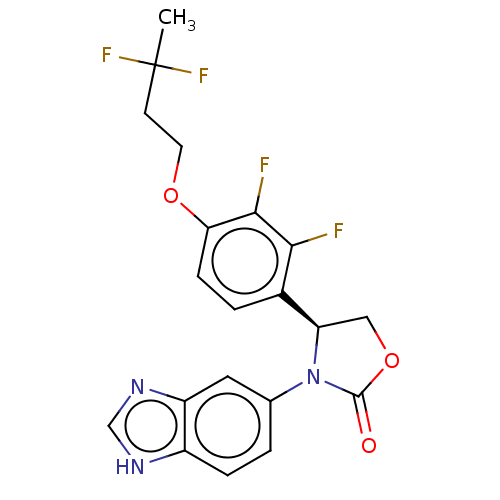 (US9512115, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.90 | -49.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259914 (US9512115, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.92 | -49.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123338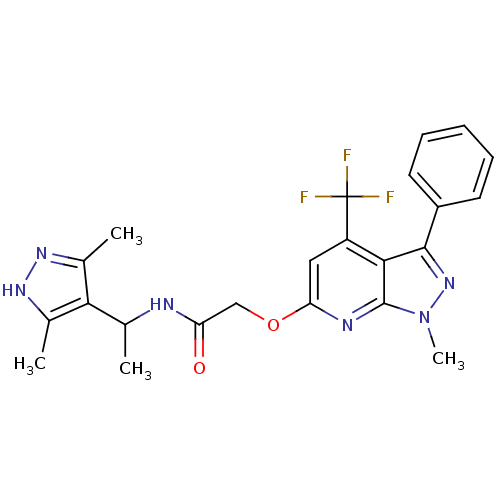 (US8742106, 1.38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM108101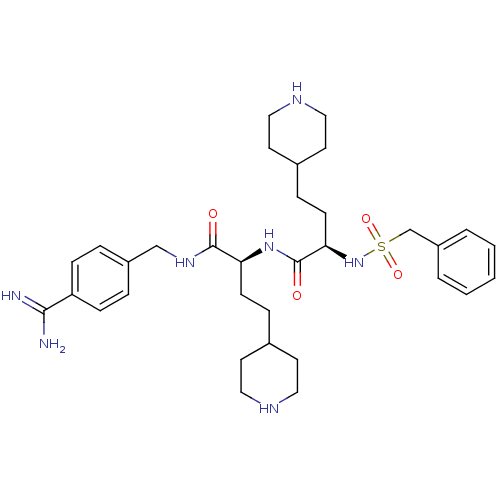 (US8598206, Table 6, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin using tosyl-Gly-Pro-Lys-pNA as substrate | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123327 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 1 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50416603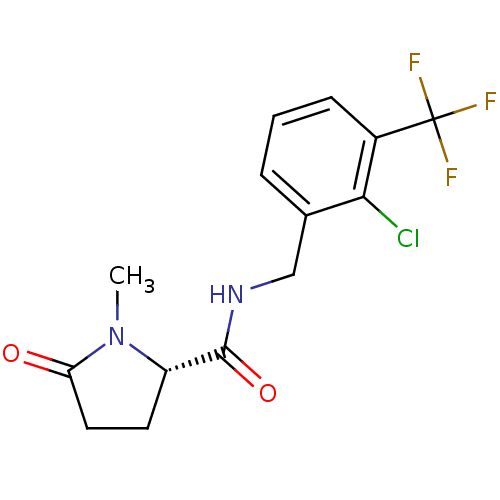 (CHEMBL1222883) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Competitive displacement of [11C]GSK1482160 from human recombinant P2X7 receptor expressed in HEK293 cell membranes incubated for 30 mins by scintill... | Bioorg Med Chem Lett 29: 1476-1480 (2019) Article DOI: 10.1016/j.bmcl.2019.04.018 BindingDB Entry DOI: 10.7270/Q2C250RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50380629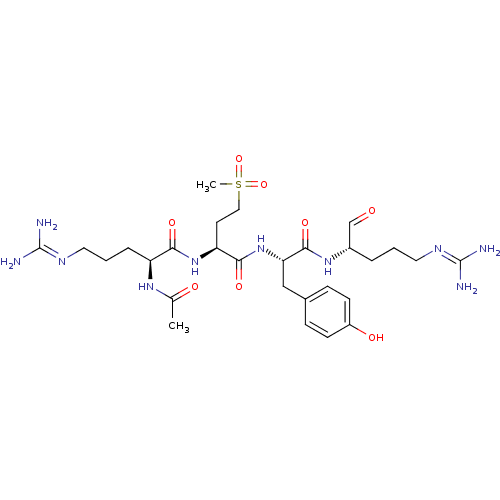 (CHEMBL2016877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in proteolysis activity in presence of fibrinogen after 90 mins by SDS-PAGE analysis | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50514066 (CHEMBL4589217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin assessed as reduction in proteolysis activity in presence of fibrinogen after 90 mins by SDS-PAGE analysis | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259907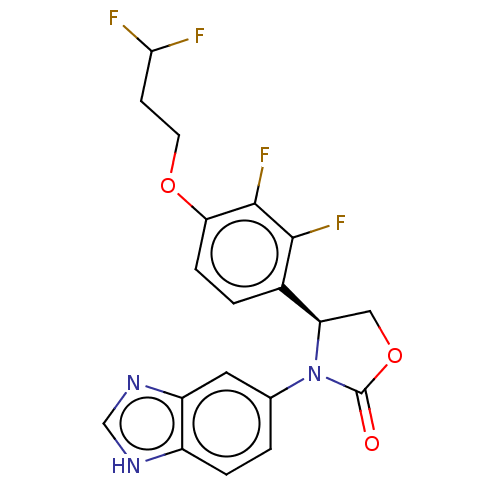 (US9512115, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.34 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259898 (US9512115, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.58 | -49.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123325 (US8742106, 1.4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [125l]orexin A from human orexin 2 receptor expressed in CHO cells after 1 hr | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123338 (US8742106, 1.38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123357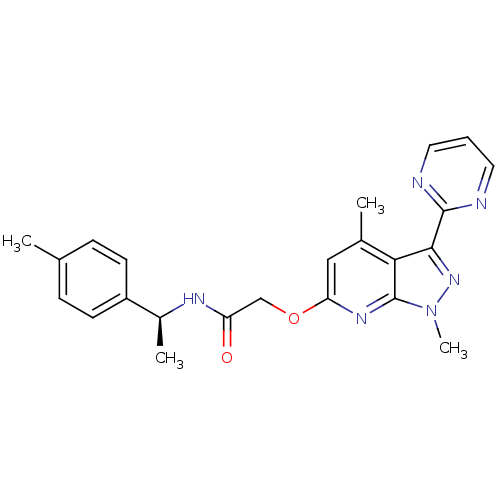 (US8742106, 4.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436008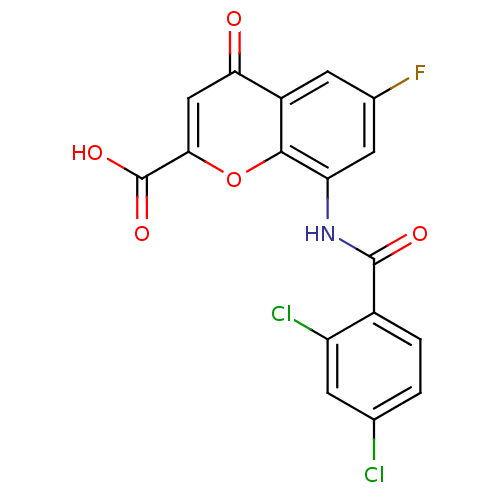 (CHEMBL2392160) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259916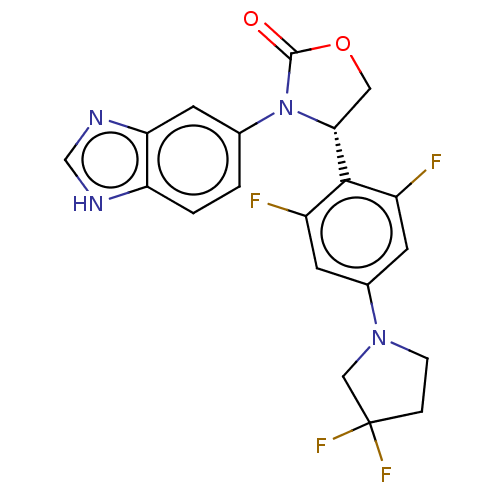 (US9512115, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.53 | -48.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436012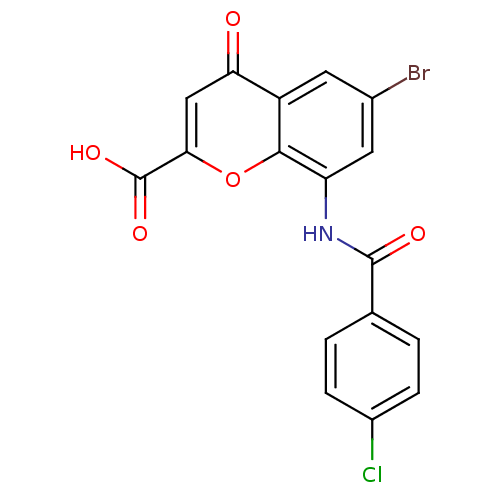 (CHEMBL2392170) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM123327 (US8742106, 1.6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 1 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM123357 (US8742106, 4.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor after 1 hr by Ca2+ sensitive Fluo4-AM fluorescent dye-based FLIPR assay | Bioorg Med Chem Lett 25: 5555-60 (2015) Article DOI: 10.1016/j.bmcl.2015.10.055 BindingDB Entry DOI: 10.7270/Q2WH2RVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259900 (US9512115, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.02 | -48.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259900 (US9512115, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.03 | -48.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259919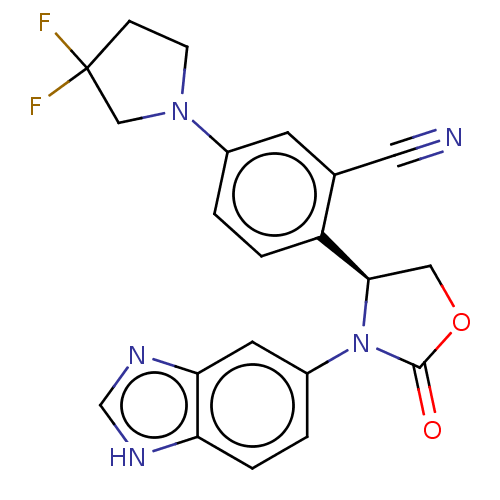 (US9512115, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.15 | -48.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 35 (Homo sapiens (Human)) | BDBM50436001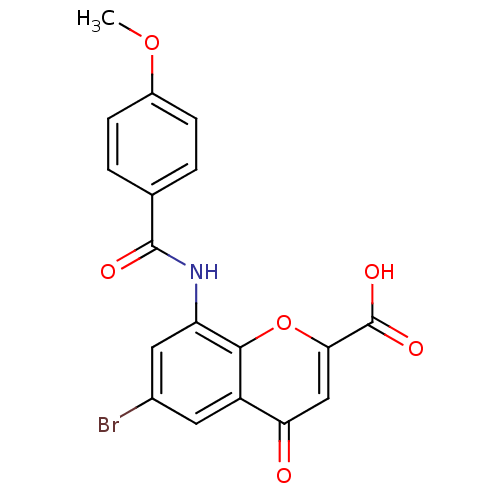 (CHEMBL2392174 | CHEMBL2425818) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-13253 from human recombinant GPR35 exprssed in CHO cells by liquid scintillation counting analysis | J Med Chem 56: 7084-99 (2013) Checked by Author Article DOI: 10.1021/jm4009373 BindingDB Entry DOI: 10.7270/Q2G16280 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM259916 (US9512115, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.29 | -48.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description All measurements were performed with a BioAssay Reader HTS-7000Plus for microplates (Perkin Elmer) at 30° C. QC activity was evaluated fluorometr... | US Patent US9512115 (2016) BindingDB Entry DOI: 10.7270/Q2XK8DG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2129 total ) | Next | Last >> |