

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM233016 (p-Xylidine-bis(dithiocarbamate) sodium salt (II)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | -37.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 27 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM233016 (p-Xylidine-bis(dithiocarbamate) sodium salt (II)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 640 | -35.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 27 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM233016 (p-Xylidine-bis(dithiocarbamate) sodium salt (II)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 950 | -35.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 37 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM233016 (p-Xylidine-bis(dithiocarbamate) sodium salt (II)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+3 | -35.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 37 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM50382706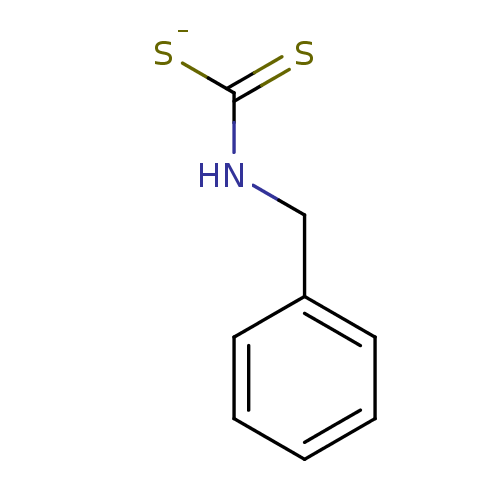 (Benzyldithiocarbamate sodium salt (I) | CHEMBL2023...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 6.8 | 37 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM50382706 (Benzyldithiocarbamate sodium salt (I) | CHEMBL2023...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | -31.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 37 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86064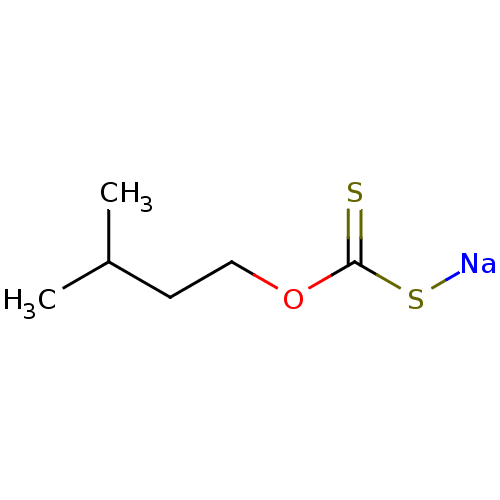 (Iso-pentyl xanthate, III) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10E+3 | -29.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86063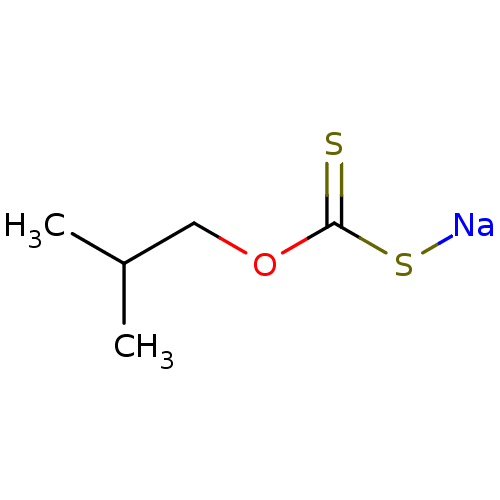 (Iso-butyl xanthate, II) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105934 (2-(6-Amino-purin-7-ylmethoxy)-ethanol | CHEMBL1260...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM50382706 (Benzyldithiocarbamate sodium salt (I) | CHEMBL2023...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | 6.8 | 27 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86062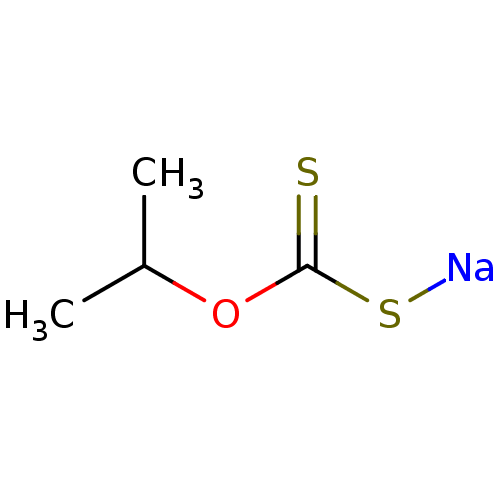 (Iso-propyl xanthate, I) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 9.80E+3 | -28.1 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86062 (Iso-propyl xanthate, I) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 1.29E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 1 (Agaricus bisporus (White button mushroom)) | BDBM50382706 (Benzyldithiocarbamate sodium salt (I) | CHEMBL2023...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 6.8 | 27 |
University of Tehran | Assay Description The assay was performed as previously described with slight modifications [Chen et al., J. Agric. Food Chem., 50:4108-12]. The reaction medium was 1 ... | J Enzyme Inhib Med Chem 25: 272-81 (2010) Article DOI: 10.1080/14756360903179351 BindingDB Entry DOI: 10.7270/Q2000101 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50029651 (CHEMBL143926 | [2-(6-Amino-purin-9-ylmethoxy)-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory potency was determined by competitive inhibition of Adenosine deaminase | J Med Chem 38: 4648-59 (1995) BindingDB Entry DOI: 10.7270/Q2V40T7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86063 (Iso-butyl xanthate, II) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.18E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM86064 (Iso-pentyl xanthate, III) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.22E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 20 |
University of Tehran | Assay Description Kinetic assay of catecholase and cresolase activities was carried out through depletion of MeBACat and MePAPh for 1 or 2 min with enzyme concentratio... | J Enzyme Inhib Med Chem 22: 239-46 (2007) Article DOI: 10.1080/14756360601114536 BindingDB Entry DOI: 10.7270/Q2XS5SZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50369958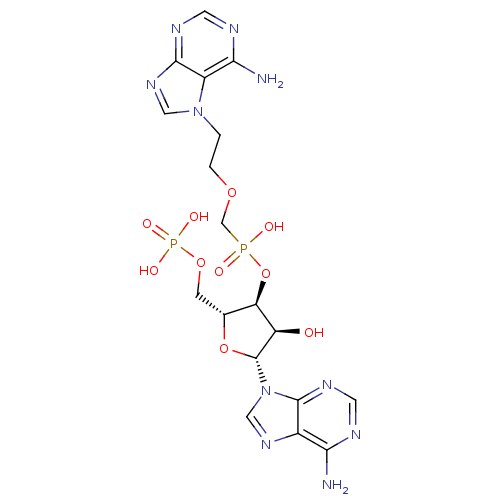 (CHEMBL1790862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50029650 (2-(6-Amino-purin-9-ylmethoxy)-ethanol | CHEMBL3775...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50029650 (2-(6-Amino-purin-9-ylmethoxy)-ethanol | CHEMBL3775...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory potency was determined by competitive inhibition of Adenosine deaminase | J Med Chem 38: 4648-59 (1995) BindingDB Entry DOI: 10.7270/Q2V40T7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50099681 (5-[2-(6-Amino-purin-9-yl)-ethylidene]-3,4-dimethox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Rate of deamination in the presence of calf mucosal adenosine deaminase (ADA) | J Med Chem 44: 1749-57 (2001) BindingDB Entry DOI: 10.7270/Q21V5D7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50369958 (CHEMBL1790862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50001103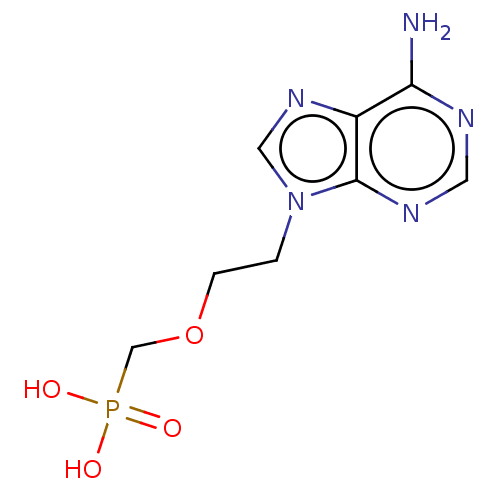 ((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50369957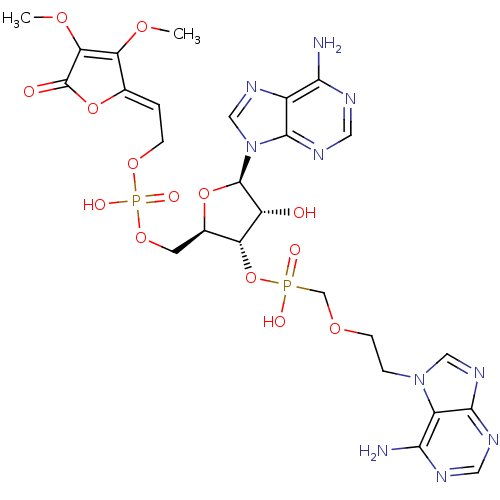 (CHEMBL1790864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105935 (CHEMBL121723 | [2-(6-Amino-purin-9-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50099682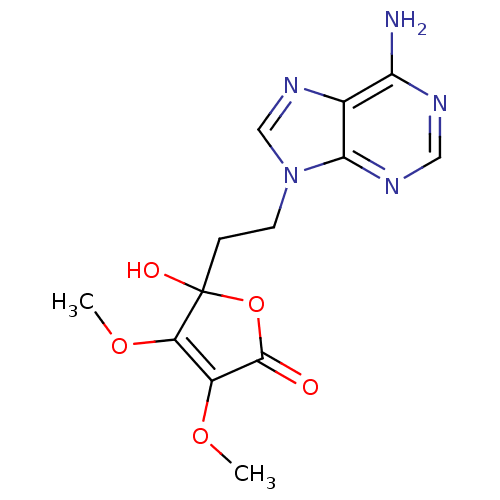 (5-(2-(6-amino-9H-purin-9-yl)ethyl)-5-hydroxy-3,4-d...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Rate of deamination in the presence of calf mucosal adenosine deaminase (ADA) | J Med Chem 44: 1749-57 (2001) BindingDB Entry DOI: 10.7270/Q21V5D7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105931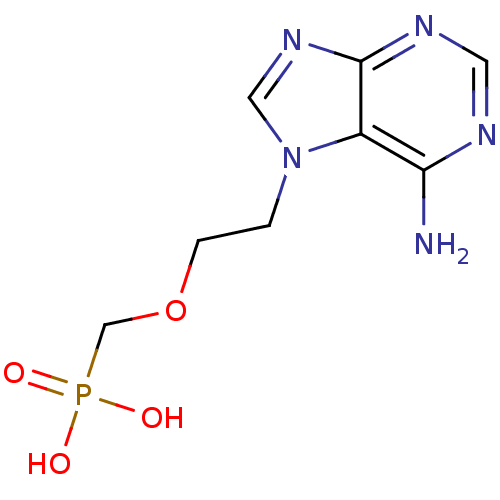 (CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50001103 ((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50105931 (CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250844 (CHEMBL4070191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250842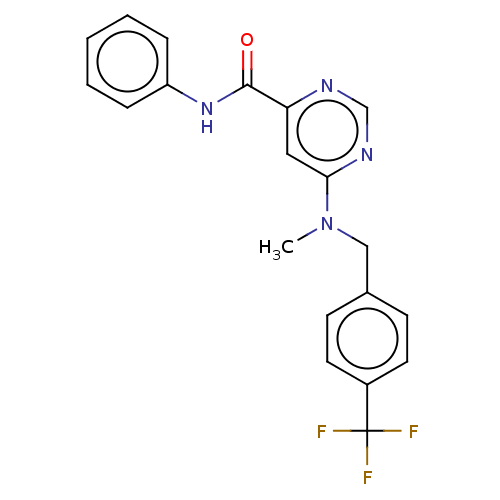 (CHEMBL4088721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250847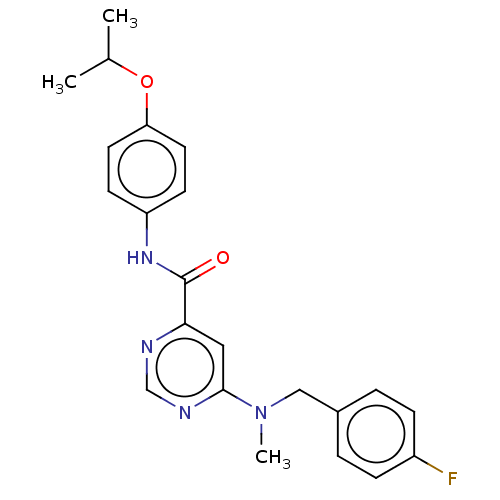 (CHEMBL4088719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250845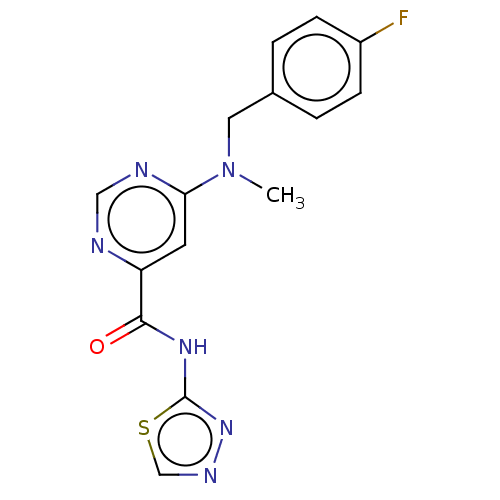 (CHEMBL4101551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250843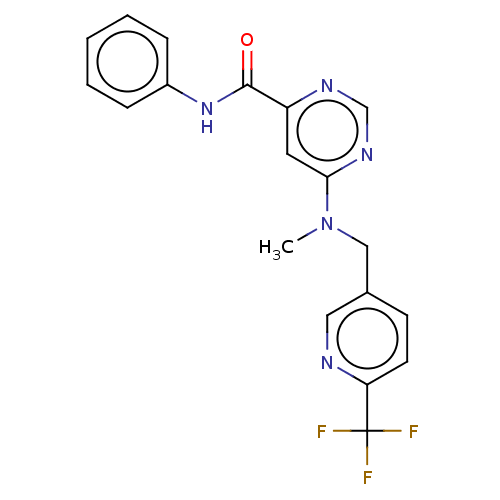 (CHEMBL4075071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50250846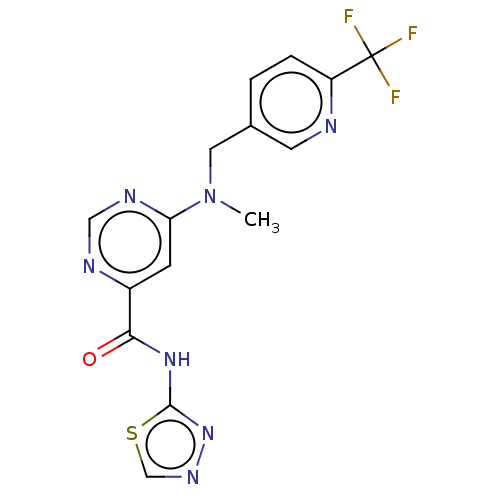 (CHEMBL4083549) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town Curated by ChEMBL | Assay Description Inhibition of full length human ERG expressed in CHO cells at -70 mV holding potential by patch clamp assay | J Med Chem 60: 10118-10134 (2017) Article DOI: 10.1021/acs.jmedchem.7b01347 BindingDB Entry DOI: 10.7270/Q2RR21P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50581159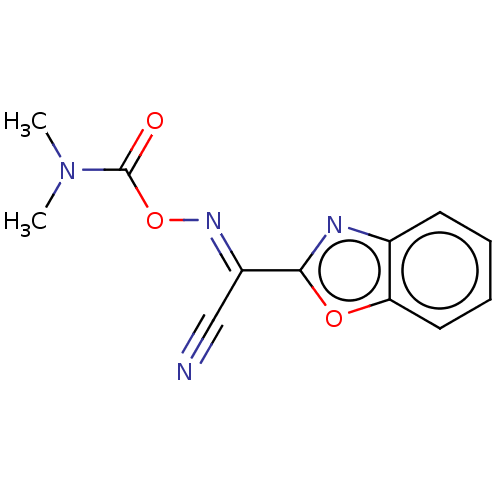 (CHEMBL5082703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by Ionworks electrophysiology assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00707 BindingDB Entry DOI: 10.7270/Q2P2730D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||