 Found 574 hits with Last Name = 'sudom' and Initial = 'a'
Found 574 hits with Last Name = 'sudom' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499195

(CHEMBL3735504)Show SMILES CC(C)(C)C(=O)Nc1ccc2n([C@@H]3CC[C@H](CO)CC3)c(NC(=O)c3cccc(c3)C(F)(F)F)nc2c1 |r,wD:12.11,15.15,(-7.45,.88,;-6.39,1.5,;-7.46,2.11,;-6.4,2.74,;-5.05,.74,;-5.04,-.49,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.36,-7.18,;4.54,-7.52,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C27H31F3N4O3/c1-26(2,3)24(37)31-19-9-12-22-21(14-19)32-25(34(22)20-10-7-16(15-35)8-11-20)33-23(36)17-5-4-6-18(13-17)27(28,29)30/h4-6,9,12-14,16,20,35H,7-8,10-11,15H2,1-3H3,(H,31,37)(H,32,33,36)/t16-,20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499203
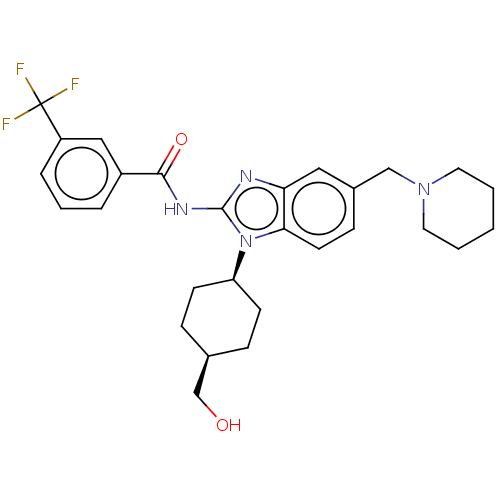
(CHEMBL3736036)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C28H33F3N4O2/c29-28(30,31)22-6-4-5-21(16-22)26(37)33-27-32-24-15-20(17-34-13-2-1-3-14-34)9-12-25(24)35(27)23-10-7-19(18-36)8-11-23/h4-6,9,12,15-16,19,23,36H,1-3,7-8,10-11,13-14,17-18H2,(H,32,33,37)/t19-,23+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499205
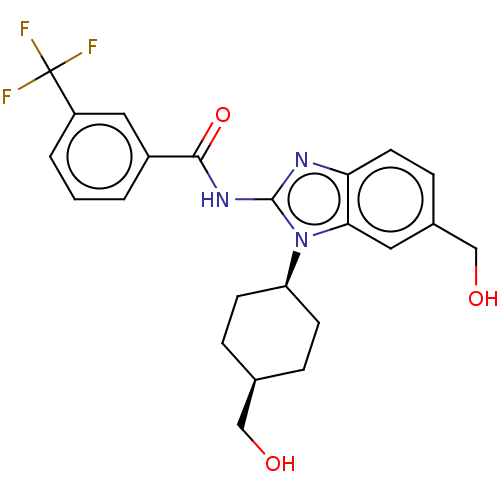
(CHEMBL3734814)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2ccc(CO)cc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.81,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;9,-2.3,;10.23,-2.29,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-2.38,-.77,;-3.72,-1.53,;-3.72,-2.76,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C23H24F3N3O3/c24-23(25,26)17-3-1-2-16(11-17)21(32)28-22-27-19-9-6-15(13-31)10-20(19)29(22)18-7-4-14(12-30)5-8-18/h1-3,6,9-11,14,18,30-31H,4-5,7-8,12-13H2,(H,27,28,32)/t14-,18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499208
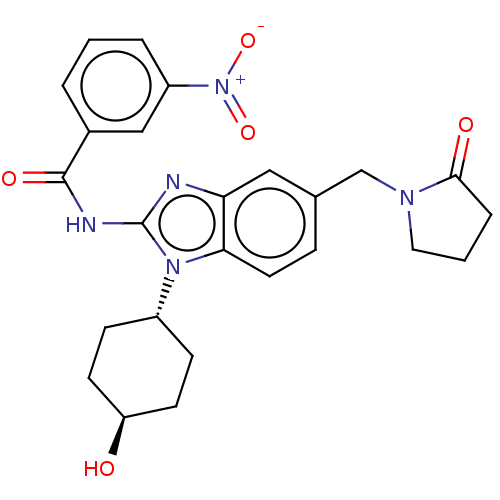
(CHEMBL3734872)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCC3=O)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-5.17,-.78,;-6.68,-1.11,;-7.46,.21,;-6.44,1.37,;-6.7,2.57,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H27N5O5/c31-20-9-7-18(8-10-20)29-22-11-6-16(15-28-12-2-5-23(28)32)13-21(22)26-25(29)27-24(33)17-3-1-4-19(14-17)30(34)35/h1,3-4,6,11,13-14,18,20,31H,2,5,7-10,12,15H2,(H,26,27,33)/t18-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29882
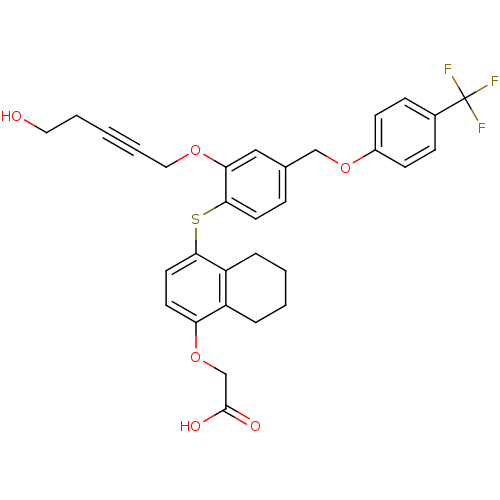
(alkynyl ether, 24)Show SMILES OCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O6S/c32-31(33,34)22-9-11-23(12-10-22)39-19-21-8-14-29(27(18-21)38-17-5-1-4-16-35)41-28-15-13-26(40-20-30(36)37)24-6-2-3-7-25(24)28/h8-15,18,35H,2-4,6-7,16-17,19-20H2,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable cofactor NADPH concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499197
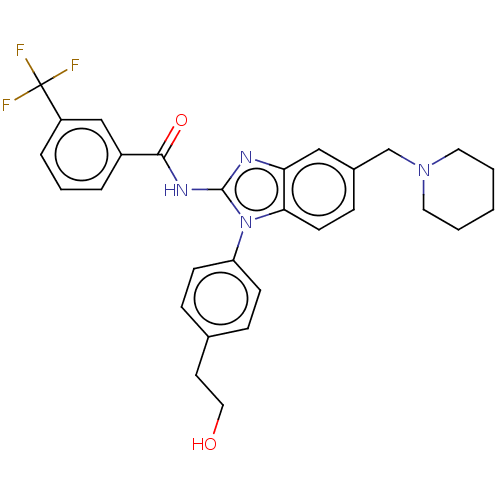
(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499206

(CHEMBL3735949)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29881

(alkynyl ether, 23)Show SMILES OCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H27F3O6S/c31-30(32,33)21-8-10-22(11-9-21)38-18-20-7-13-28(26(17-20)37-16-4-3-15-34)40-27-14-12-25(39-19-29(35)36)23-5-1-2-6-24(23)27/h7-14,17,34H,1-2,5-6,15-16,18-19H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248569

((1-(((R)-3-methyl-4-(4-((S)-1,1,1-trifluoro-2-hydr...)Show SMILES C[C@@H]1CN(CC2(CC2)C(N)=O)CCN1S(=O)(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F |r| Show InChI InChI=1S/C19H26F3N3O4S/c1-13-11-24(12-18(7-8-18)16(23)26)9-10-25(13)30(28,29)15-5-3-14(4-6-15)17(2,27)19(20,21)22/h3-6,13,27H,7-12H2,1-2H3,(H2,23,26)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 using variable substrate cortisol concentration by Lineweaver burk plot |
Bioorg Med Chem 16: 8922-31 (2008)
Article DOI: 10.1016/j.bmc.2008.08.065
BindingDB Entry DOI: 10.7270/Q22F7N8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499198
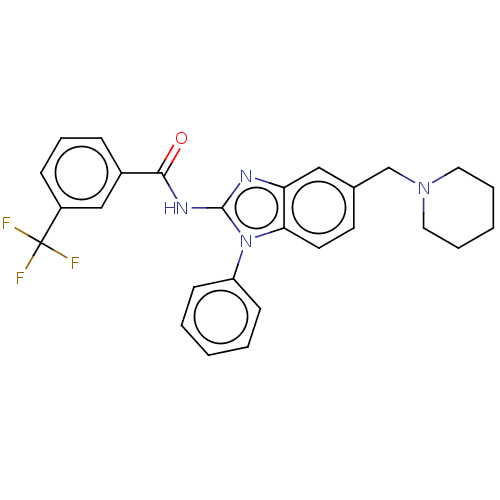
(CHEMBL3736278)Show SMILES FC(F)(F)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C27H25F3N4O/c28-27(29,30)21-9-7-8-20(17-21)25(35)32-26-31-23-16-19(18-33-14-5-2-6-15-33)12-13-24(23)34(26)22-10-3-1-4-11-22/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499202

(CHEMBL3735523)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCOCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C25H29N5O5/c31-21-7-5-19(6-8-21)29-23-9-4-17(16-28-10-12-35-13-11-28)14-22(23)26-25(29)27-24(32)18-2-1-3-20(15-18)30(33)34/h1-4,9,14-15,19,21,31H,5-8,10-13,16H2,(H,26,27,32)/t19-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29868
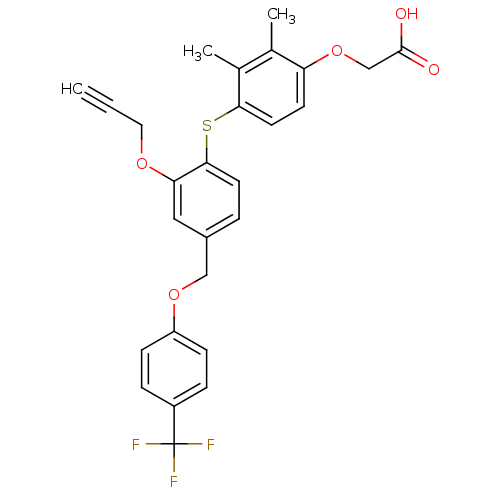
(alkynyl ether, 10)Show SMILES Cc1c(C)c(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCC#C)ccc1OCC(O)=O Show InChI InChI=1S/C27H23F3O5S/c1-4-13-33-23-14-19(15-34-21-8-6-20(7-9-21)27(28,29)30)5-11-25(23)36-24-12-10-22(17(2)18(24)3)35-16-26(31)32/h1,5-12,14H,13,15-16H2,2-3H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29885
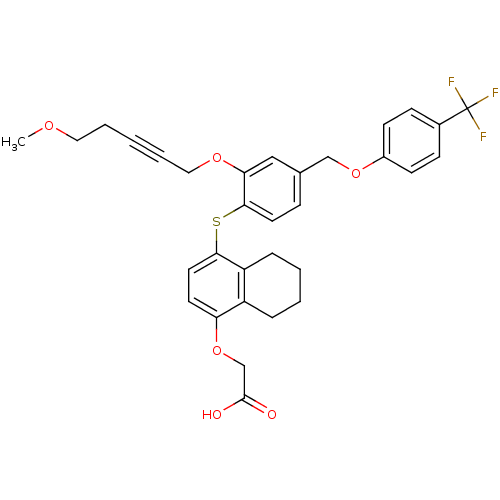
(alkynyl ether, 27)Show SMILES COCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C32H31F3O6S/c1-38-17-5-2-6-18-39-28-19-22(20-40-24-12-10-23(11-13-24)32(33,34)35)9-15-30(28)42-29-16-14-27(41-21-31(36)37)25-7-3-4-8-26(25)29/h9-16,19H,3-5,7-8,17-18,20-21H2,1H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29879
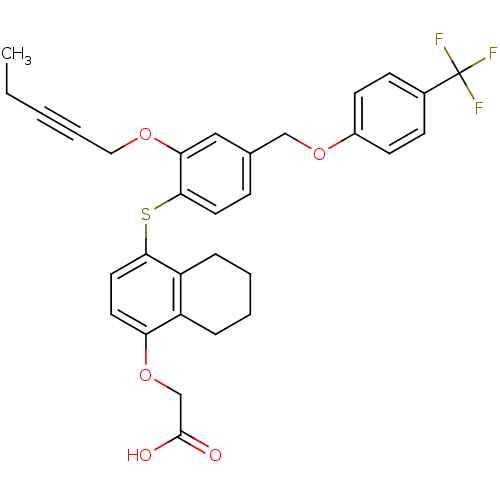
(alkynyl ether, 21)Show SMILES CCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O5S/c1-2-3-6-17-37-27-18-21(19-38-23-12-10-22(11-13-23)31(32,33)34)9-15-29(27)40-28-16-14-26(39-20-30(35)36)24-7-4-5-8-25(24)28/h9-16,18H,2,4-5,7-8,17,19-20H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29883

(alkynyl ether, 25)Show SMILES C[C@H](O)C#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 |r| Show InChI InChI=1S/C31H29F3O6S/c1-20(35)5-4-16-38-27-17-21(18-39-23-11-9-22(10-12-23)31(32,33)34)8-14-29(27)41-28-15-13-26(40-19-30(36)37)24-6-2-3-7-25(24)28/h8-15,17,20,35H,2-3,6-7,16,18-19H2,1H3,(H,36,37)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29878

(alkynyl ether, 20)Show SMILES CC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H27F3O5S/c1-2-3-16-36-26-17-20(18-37-22-11-9-21(10-12-22)30(31,32)33)8-14-28(26)39-27-15-13-25(38-19-29(34)35)23-6-4-5-7-24(23)27/h8-15,17H,4-7,16,18-19H2,1H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499200
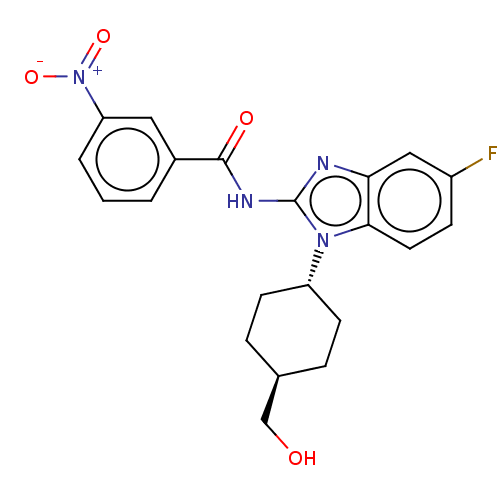
(CHEMBL3735044)Show SMILES OC[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wU:5.8,wD:2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C21H21FN4O4/c22-15-6-9-19-18(11-15)23-21(25(19)16-7-4-13(12-27)5-8-16)24-20(28)14-2-1-3-17(10-14)26(29)30/h1-3,6,9-11,13,16,27H,4-5,7-8,12H2,(H,23,24,28)/t13-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499210
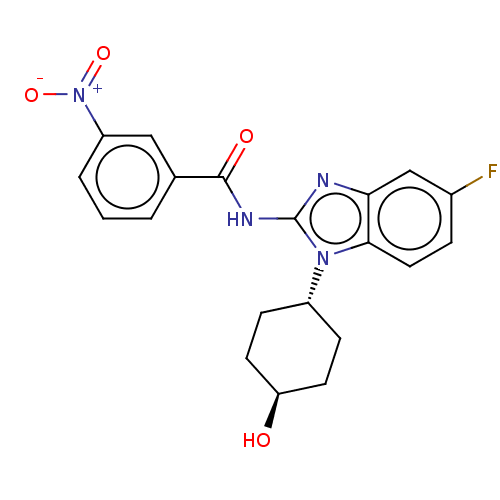
(CHEMBL3734846)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.45,1.38,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C20H19FN4O4/c21-13-4-9-18-17(11-13)22-20(24(18)14-5-7-16(26)8-6-14)23-19(27)12-2-1-3-15(10-12)25(28)29/h1-4,9-11,14,16,26H,5-8H2,(H,22,23,27)/t14-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29877

(alkynyl ether, 19)Show SMILES OC(=O)COc1ccc(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCC#C)c2CCCCc12 Show InChI InChI=1S/C29H25F3O5S/c1-2-15-35-25-16-19(17-36-21-10-8-20(9-11-21)29(30,31)32)7-13-27(25)38-26-14-12-24(37-18-28(33)34)22-5-3-4-6-23(22)26/h1,7-14,16H,3-6,15,17-18H2,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | 52 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499211
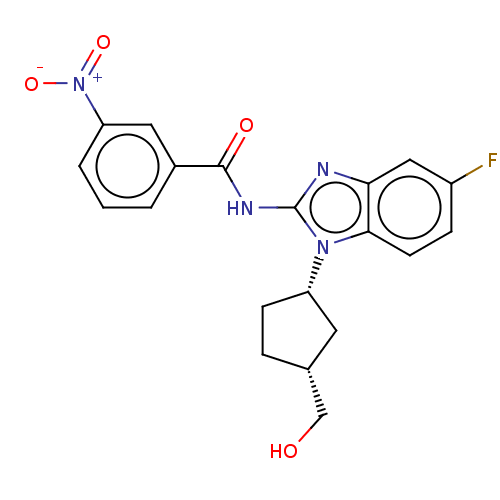
(CHEMBL3735247)Show SMILES OC[C@@H]1CC[C@@H](C1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r| Show InChI InChI=1S/C20H19FN4O4/c21-14-5-7-18-17(10-14)22-20(24(18)15-6-4-12(8-15)11-26)23-19(27)13-2-1-3-16(9-13)25(28)29/h1-3,5,7,9-10,12,15,26H,4,6,8,11H2,(H,22,23,27)/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29867

(phenoxyacetic acid-ether, 9)Show SMILES COCCCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c(C)c1C Show InChI InChI=1S/C28H29F3O6S/c1-18-19(2)25(12-10-23(18)37-17-27(32)33)38-26-11-5-20(15-24(26)35-14-4-13-34-3)16-36-22-8-6-21(7-9-22)28(29,30)31/h5-12,15H,4,13-14,16-17H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499204
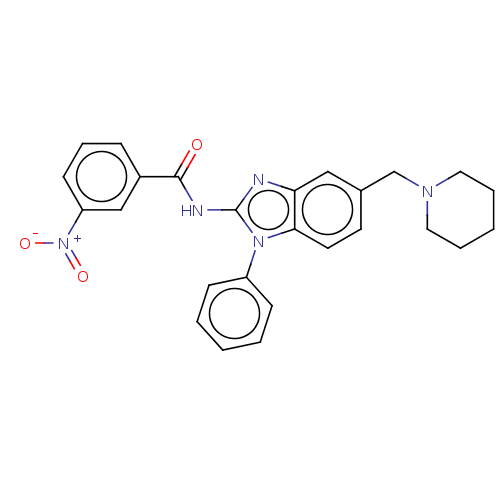
(CHEMBL3735719)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)Nc1nc2cc(CN3CCCCC3)ccc2n1-c1ccccc1 Show InChI InChI=1S/C26H25N5O3/c32-25(20-8-7-11-22(17-20)31(33)34)28-26-27-23-16-19(18-29-14-5-2-6-15-29)12-13-24(23)30(26)21-9-3-1-4-10-21/h1,3-4,7-13,16-17H,2,5-6,14-15,18H2,(H,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29884

(alkynyl ether, 26)Show SMILES COCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O6S/c1-37-16-4-5-17-38-27-18-21(19-39-23-11-9-22(10-12-23)31(32,33)34)8-14-29(27)41-28-15-13-26(40-20-30(35)36)24-6-2-3-7-25(24)28/h8-15,18H,2-3,6-7,16-17,19-20H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29869
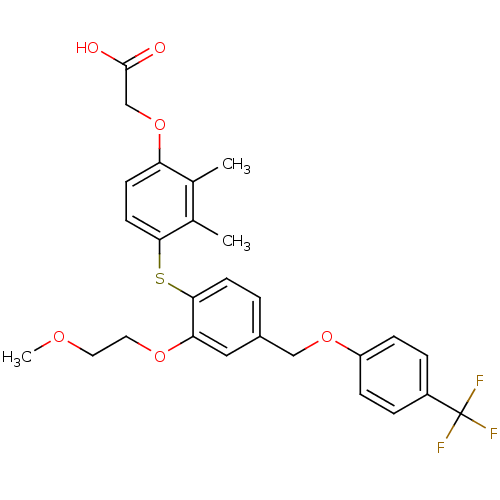
(phenoxyacetic acid-ether, 11)Show SMILES COCCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c(C)c1C Show InChI InChI=1S/C27H27F3O6S/c1-17-18(2)24(11-9-22(17)36-16-26(31)32)37-25-10-4-19(14-23(25)34-13-12-33-3)15-35-21-7-5-20(6-8-21)27(28,29)30/h4-11,14H,12-13,15-16H2,1-3H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499201

(CHEMBL3736469)Show SMILES OC[C@H]1CC[C@@H](C1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 |r| Show InChI InChI=1S/C20H19FN4O4/c21-14-5-7-18-17(10-14)22-20(24(18)15-6-4-12(8-15)11-26)23-19(27)13-2-1-3-16(9-13)25(28)29/h1-3,5,7,9-10,12,15,26H,4,6,8,11H2,(H,22,23,27)/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29891
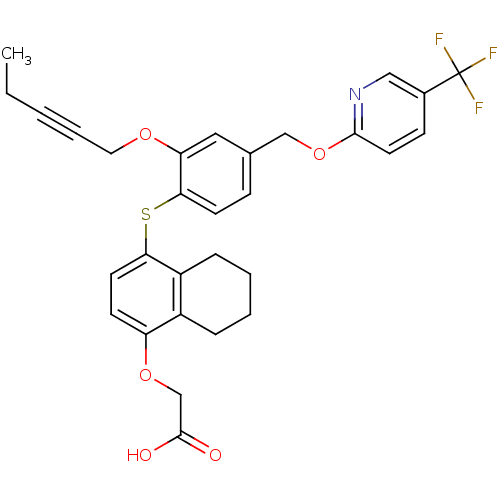
(alkynyl ether, 33)Show SMILES CCC#CCOc1cc(COc2ccc(cn2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H28F3NO5S/c1-2-3-6-15-37-25-16-20(18-39-28-14-10-21(17-34-28)30(31,32)33)9-12-27(25)40-26-13-11-24(38-19-29(35)36)22-7-4-5-8-23(22)26/h9-14,16-17H,2,4-5,7-8,15,18-19H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499207

(CHEMBL3735975)Show SMILES OCc1ccc2n([C@H]3CC[C@H](O)CC3)c(NC(=O)c3cccc(c3)[N+]([O-])=O)nc2c1 |r,wU:7.6,wD:10.10,(-4.78,.9,;-3.72,1.53,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.24,-2.7,;3.72,-3.12,;4.09,-4.62,;2.98,-5.68,;3.28,-6.88,;1.5,-5.26,;1.13,-3.76,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,)| Show InChI InChI=1S/C21H22N4O5/c26-12-13-4-9-19-18(10-13)22-21(24(19)15-5-7-17(27)8-6-15)23-20(28)14-2-1-3-16(11-14)25(29)30/h1-4,9-11,15,17,26-27H,5-8,12H2,(H,22,23,28)/t15-,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499197

(CHEMBL3736465)Show SMILES OCCc1ccc(cc1)-n1c(NC(=O)c2cccc(c2)C(F)(F)F)nc2cc(CN3CCCCC3)ccc12 Show InChI InChI=1S/C29H29F3N4O2/c30-29(31,32)23-6-4-5-22(18-23)27(38)34-28-33-25-17-21(19-35-14-2-1-3-15-35)9-12-26(25)36(28)24-10-7-20(8-11-24)13-16-37/h4-12,17-18,37H,1-3,13-16,19H2,(H,33,34,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29870
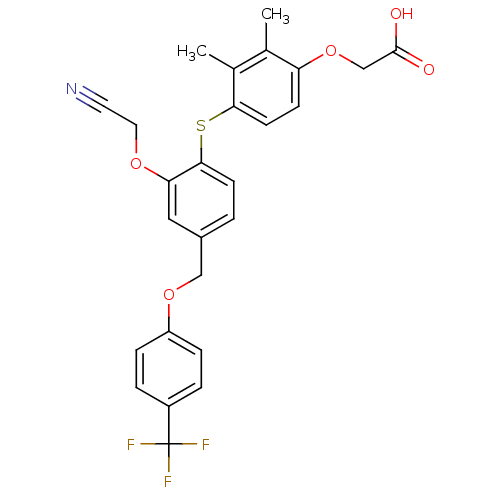
(phenoxyacetic acid-ether, 12)Show SMILES Cc1c(C)c(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCC#N)ccc1OCC(O)=O Show InChI InChI=1S/C26H22F3NO5S/c1-16-17(2)23(10-8-21(16)35-15-25(31)32)36-24-9-3-18(13-22(24)33-12-11-30)14-34-20-6-4-19(5-7-20)26(27,28)29/h3-10,13H,12,14-15H2,1-2H3,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29886

(alkynyl ether, 28)Show SMILES OC(=O)COc1ccc(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCC#Cc2cccnc2)c2CCCCc12 Show InChI InChI=1S/C34H28F3NO5S/c35-34(36,37)25-10-12-26(13-11-25)42-21-24-9-15-32(30(19-24)41-18-4-6-23-5-3-17-38-20-23)44-31-16-14-29(43-22-33(39)40)27-7-1-2-8-28(27)31/h3,5,9-17,19-20H,1-2,7-8,18,21-22H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | 680 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 1
(Homo sapiens (Human)) | BDBM50499199

(CHEMBL3735673)Show SMILES O[C@H]1CC[C@@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wU:4.7,wD:1.0,(3.28,-6.88,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C26H31N5O4/c32-22-10-8-20(9-11-22)30-24-12-7-18(17-29-13-2-1-3-14-29)15-23(24)27-26(30)28-25(33)19-5-4-6-21(16-19)31(34)35/h4-7,12,15-16,20,22,32H,1-3,8-11,13-14,17H2,(H,27,28,33)/t20-,22- | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of IRAK-1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29884

(alkynyl ether, 26)Show SMILES COCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O6S/c1-37-16-4-5-17-38-27-18-21(19-39-23-11-9-22(10-12-23)31(32,33)34)8-14-29(27)41-28-15-13-26(40-20-30(35)36)24-6-2-3-7-25(24)28/h8-15,18H,2-3,6-7,16-17,19-20H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29880

(alkynyl ether, 22)Show SMILES CC(C)(O)C#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C32H31F3O6S/c1-31(2,38)16-5-17-39-27-18-21(19-40-23-11-9-22(10-12-23)32(33,34)35)8-14-29(27)42-28-15-13-26(41-20-30(36)37)24-6-3-4-7-25(24)28/h8-15,18,38H,3-4,6-7,17,19-20H2,1-2H3,(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29886

(alkynyl ether, 28)Show SMILES OC(=O)COc1ccc(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCC#Cc2cccnc2)c2CCCCc12 Show InChI InChI=1S/C34H28F3NO5S/c35-34(36,37)25-10-12-26(13-11-25)42-21-24-9-15-32(30(19-24)41-18-4-6-23-5-3-17-38-20-23)44-31-16-14-29(43-22-33(39)40)27-7-1-2-8-28(27)31/h3,5,9-17,19-20H,1-2,7-8,18,21-22H2,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29890

(alkynyl ether, 32)Show SMILES CCC#CCOc1cc(COc2ccc(Cl)cc2C)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H31ClO5S/c1-3-4-7-16-35-28-18-22(19-36-26-12-11-23(32)17-21(26)2)10-14-30(28)38-29-15-13-27(37-20-31(33)34)24-8-5-6-9-25(24)29/h10-15,17-18H,3,5-6,8-9,16,19-20H2,1-2H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29887
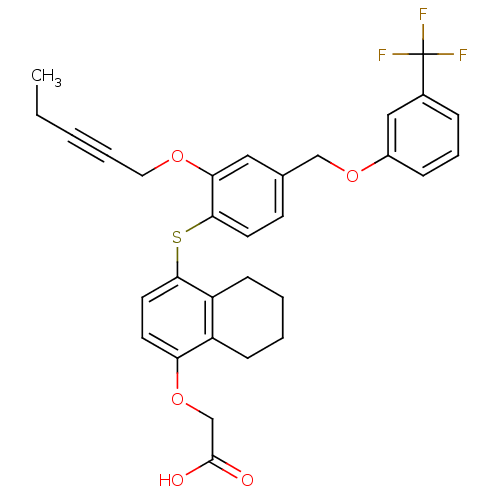
(alkynyl ether, 29)Show SMILES CCC#CCOc1cc(COc2cccc(c2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O5S/c1-2-3-6-16-37-27-17-21(19-38-23-9-7-8-22(18-23)31(32,33)34)12-14-29(27)40-28-15-13-26(39-20-30(35)36)24-10-4-5-11-25(24)28/h7-9,12-15,17-18H,2,4-5,10-11,16,19-20H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7
(Homo sapiens (Human)) | BDBM50499194

(CHEMBL3734854)Show SMILES OC[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(CN3CCCCC3)ccc12 |r,wD:5.8,2.1,(4.54,-7.52,;3.36,-7.18,;2.98,-5.68,;4.09,-4.62,;3.72,-3.12,;2.24,-2.7,;1.13,-3.76,;1.5,-5.26,;1.76,-1.24,;2.66,.02,;4.2,.04,;4.95,1.38,;4.32,2.44,;6.49,1.4,;7.24,2.75,;8.78,2.76,;9.57,1.44,;8.82,.1,;7.28,.08,;9.6,-1.23,;10.83,-1.22,;8.99,-2.3,;1.76,1.24,;.3,.77,;-1.03,1.55,;-2.38,.77,;-3.72,1.53,;-5.05,.74,;-6.39,1.5,;-7.71,.72,;-7.7,-.82,;-6.36,-1.58,;-5.03,-.8,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,)| Show InChI InChI=1S/C27H33N5O4/c33-18-19-7-10-22(11-8-19)31-25-12-9-20(17-30-13-2-1-3-14-30)15-24(25)28-27(31)29-26(34)21-5-4-6-23(16-21)32(35)36/h4-6,9,12,15-16,19,22,33H,1-3,7-8,10-11,13-14,17-18H2,(H,28,29,34)/t19-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of TAK1 (unknown origin) |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29879

(alkynyl ether, 21)Show SMILES CCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O5S/c1-2-3-6-17-37-27-18-21(19-38-23-12-10-22(11-13-23)31(32,33)34)9-15-29(27)40-28-16-14-26(39-20-30(35)36)24-7-4-5-8-25(24)28/h9-16,18H,2,4-5,7-8,17,19-20H2,1H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 33 | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29871
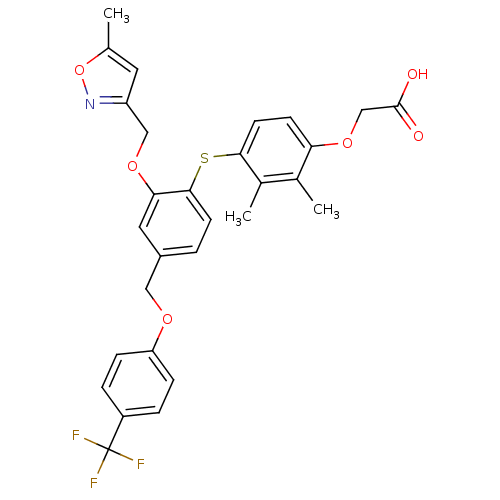
(phenoxyacetic acid-ether, 13)Show SMILES Cc1cc(COc2cc(COc3ccc(cc3)C(F)(F)F)ccc2Sc2ccc(OCC(O)=O)c(C)c2C)no1 Show InChI InChI=1S/C29H26F3NO6S/c1-17-12-22(33-39-17)15-37-25-13-20(14-36-23-7-5-21(6-8-23)29(30,31)32)4-10-27(25)40-26-11-9-24(18(2)19(26)3)38-16-28(34)35/h4-13H,14-16H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29887

(alkynyl ether, 29)Show SMILES CCC#CCOc1cc(COc2cccc(c2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O5S/c1-2-3-6-16-37-27-17-21(19-38-23-9-7-8-22(18-23)31(32,33)34)12-14-29(27)40-28-15-13-26(39-20-30(35)36)24-10-4-5-11-25(24)28/h7-9,12-15,17-18H,2,4-5,10-11,16,19-20H2,1H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29889
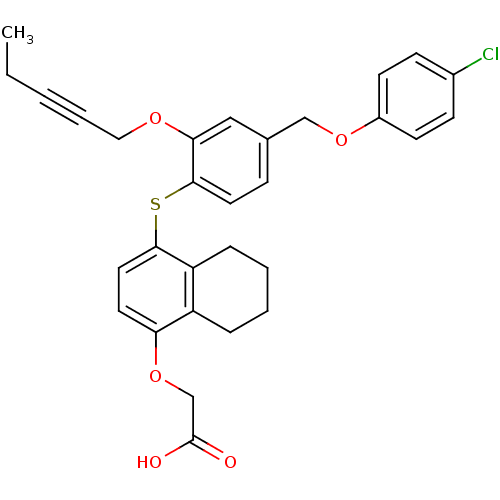
(alkynyl ether, 31)Show SMILES CCC#CCOc1cc(COc2ccc(Cl)cc2)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H29ClO5S/c1-2-3-6-17-34-27-18-21(19-35-23-12-10-22(31)11-13-23)9-15-29(27)37-28-16-14-26(36-20-30(32)33)24-7-4-5-8-25(24)28/h9-16,18H,2,4-5,7-8,17,19-20H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM29872
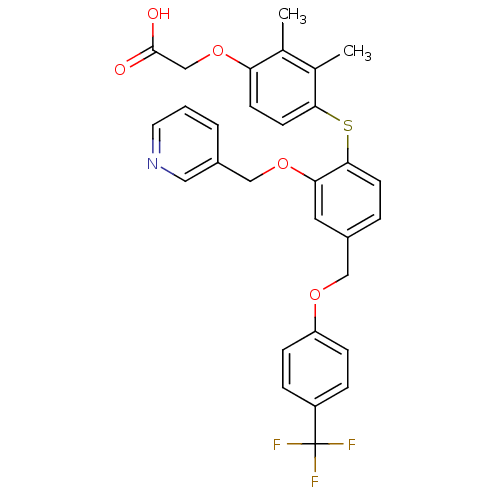
(phenoxyacetic acid-ether, 14)Show SMILES Cc1c(C)c(Sc2ccc(COc3ccc(cc3)C(F)(F)F)cc2OCc2cccnc2)ccc1OCC(O)=O Show InChI InChI=1S/C30H26F3NO5S/c1-19-20(2)27(12-10-25(19)39-18-29(35)36)40-28-11-5-21(14-26(28)38-17-22-4-3-13-34-15-22)16-37-24-8-6-23(7-9-24)30(31,32)33/h3-15H,16-18H2,1-2H3,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARdelta ligand binding was directly measured using a scintillation proximity assay. Plates were read on a Packard TopCount. IC50 values f... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50499209
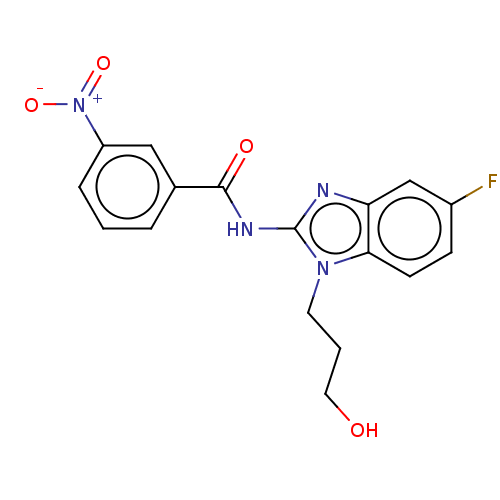
(CHEMBL3735096)Show SMILES OCCCn1c(NC(=O)c2cccc(c2)[N+]([O-])=O)nc2cc(F)ccc12 Show InChI InChI=1S/C17H15FN4O4/c18-12-5-6-15-14(10-12)19-17(21(15)7-2-8-23)20-16(24)11-3-1-4-13(9-11)22(25)26/h1,3-6,9-10,23H,2,7-8H2,(H,19,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length IRAK-4 (unknown origin) using the biotinylated substrate RRRVTSPARRS by chemiluminescent ELISA |
Bioorg Med Chem Lett 25: 5546-50 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.060
BindingDB Entry DOI: 10.7270/Q29W0JG8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29889

(alkynyl ether, 31)Show SMILES CCC#CCOc1cc(COc2ccc(Cl)cc2)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C30H29ClO5S/c1-2-3-6-17-34-27-18-21(19-35-23-12-10-22(31)11-13-23)9-15-29(27)37-28-16-14-26(36-20-30(32)33)24-7-4-5-8-25(24)28/h9-16,18H,2,4-5,7-8,17,19-20H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29882

(alkynyl ether, 24)Show SMILES OCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C31H29F3O6S/c32-31(33,34)22-9-11-23(12-10-22)39-19-21-8-14-29(27(18-21)38-17-5-1-4-16-35)41-28-15-13-26(40-20-30(36)37)24-6-2-3-7-25(24)28/h8-15,18,35H,2-4,6-7,16-17,19-20H2,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM29885

(alkynyl ether, 27)Show SMILES COCCC#CCOc1cc(COc2ccc(cc2)C(F)(F)F)ccc1Sc1ccc(OCC(O)=O)c2CCCCc12 Show InChI InChI=1S/C32H31F3O6S/c1-38-17-5-2-6-18-39-28-19-22(20-40-24-12-10-23(11-13-24)32(33,34)35)9-15-30(28)42-29-16-14-27(41-21-31(36)37)25-7-3-4-8-26(25)29/h9-16,19H,3-5,7-8,17-18,20-21H2,1H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The human PPARgamma ligand binding was directly measured using a filtration assay in a UniFilter 350 96-well assay plate (Polyfiltronics). The reacti... |
Bioorg Med Chem Lett 19: 3550-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.151
BindingDB Entry DOI: 10.7270/Q2NK3CC0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data