

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369539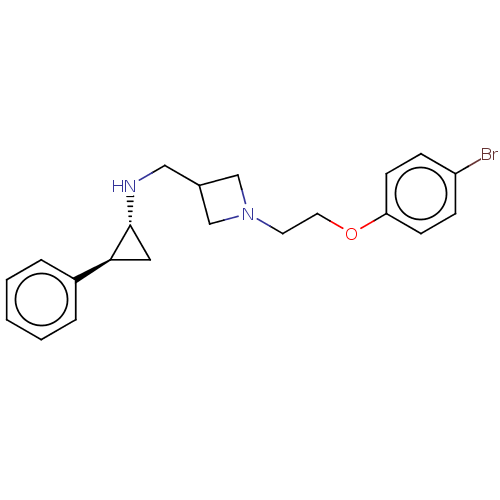 (US10233152, Example 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369535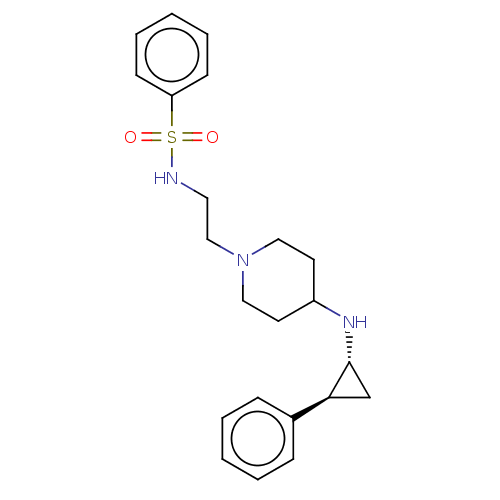 (US10233152, Example 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50303276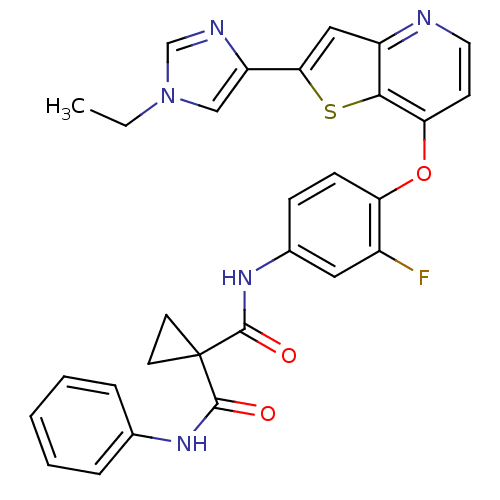 (CHEMBL565807 | N-(4-(2-(1-ethyl-1H-imidazole-4-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 assessed as inhibition of ERK phosphorylation | Bioorg Med Chem Lett 19: 6836-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.095 BindingDB Entry DOI: 10.7270/Q2GM87C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369495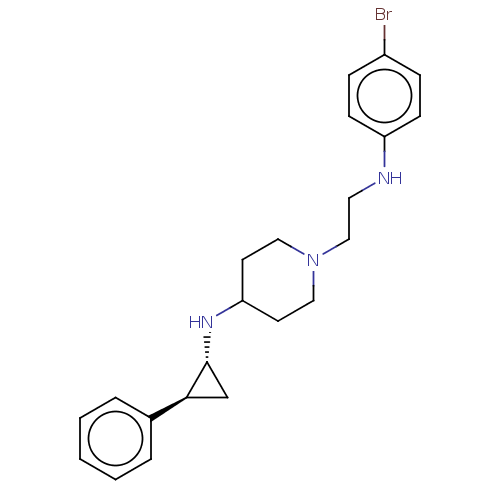 (US10233152, Example 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369532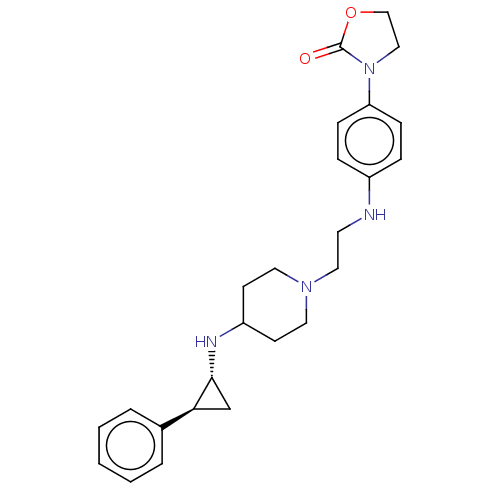 (US10233152, Example 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM93149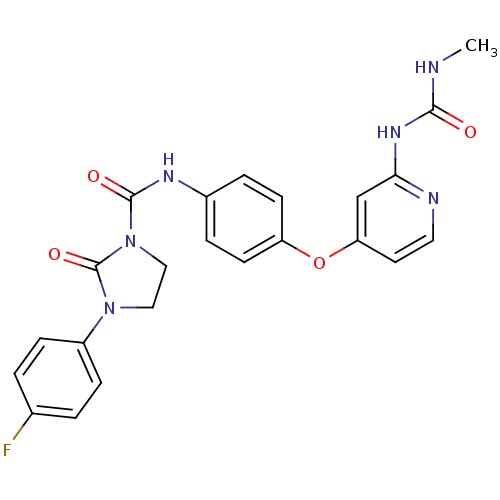 (2-oxoimidazolidine-1-carboxamide, 16) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
MethylGene Inc. | Assay Description Inhibition assay using kinase inhibitors. | International Journal of Medicinal Chemistry 2012: 1-6 (2012) BindingDB Entry DOI: 10.7270/Q2X065P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50114813 (8-(4'-Bromo-biphenyl-4-yl)-8-oxo-octanoic acid hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369621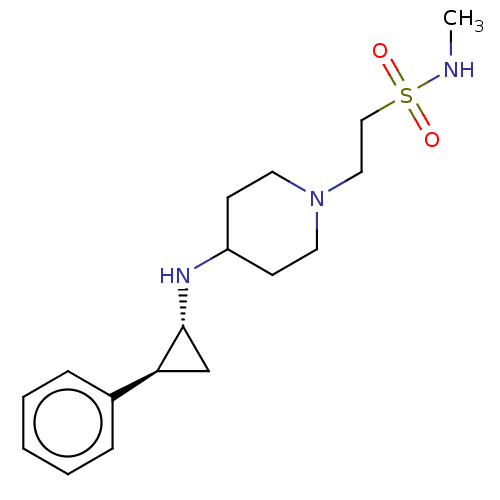 (US10233152, Example 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369496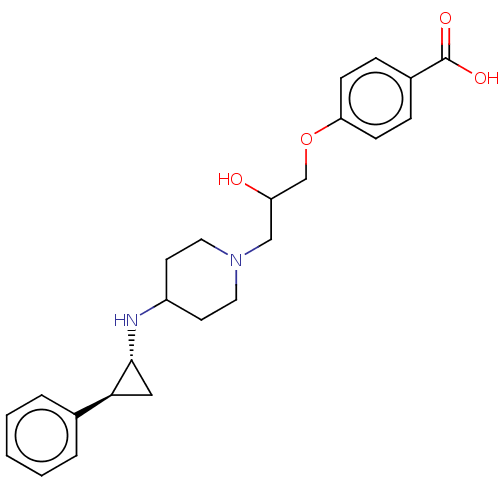 (US10233152, Example 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369512 (US10233152, Example 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369533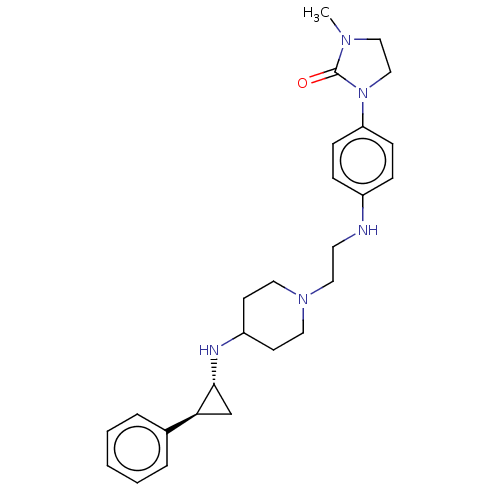 (US10233152, Example 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369478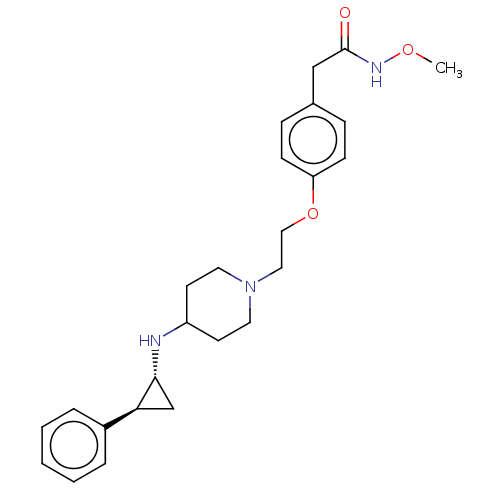 (N,N-Dimethyl-2-(4-(2-(4-(((1R,2S)-2-phenylcyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM93147 (2-oxoimidazolidine-1-carboxamide, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
MethylGene Inc. | Assay Description Inhibition assay using kinase inhibitors. | International Journal of Medicinal Chemistry 2012: 1-6 (2012) BindingDB Entry DOI: 10.7270/Q2X065P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369484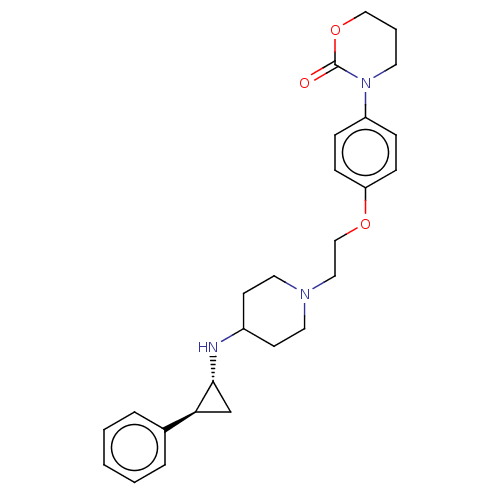 (US10233152, Example 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50248054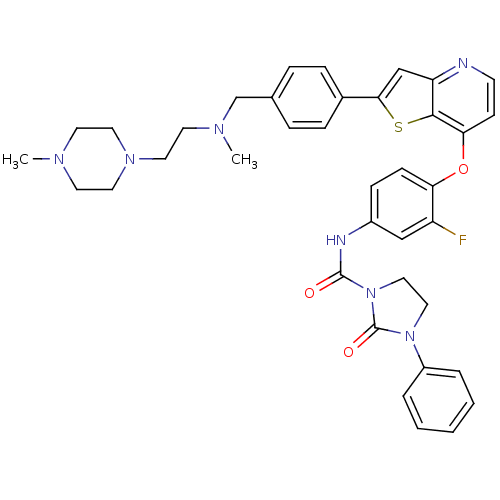 (CHEMBL508395 | N-(3-fluoro-4-(2-(4-((methyl(2-(4-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused recombinant VGFR2 (unknown origin) catalytic domain expressed in baculovirus-infected Sf9 cells after 10 mins by DELFIA assay | Bioorg Med Chem Lett 19: 1323-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.068 BindingDB Entry DOI: 10.7270/Q23F4PHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50248089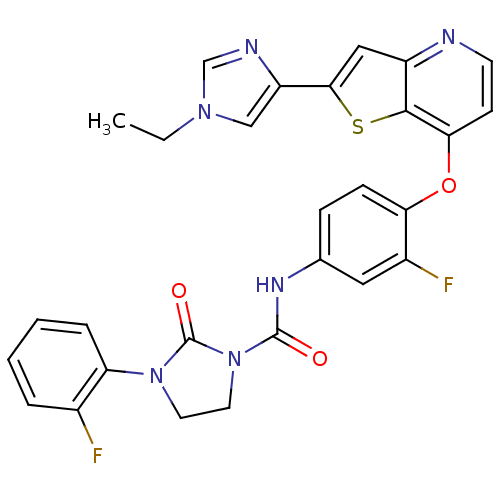 (CHEMBL517469 | N-(4-(2-(1-ethyl-1H-imidazol-4-yl)t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused recombinant VGFR2 (unknown origin) catalytic domain expressed in baculovirus-infected Sf9 cells after 10 mins by DELFIA assay | Bioorg Med Chem Lett 19: 1323-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.068 BindingDB Entry DOI: 10.7270/Q23F4PHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369650 (US10233152, Example 157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369530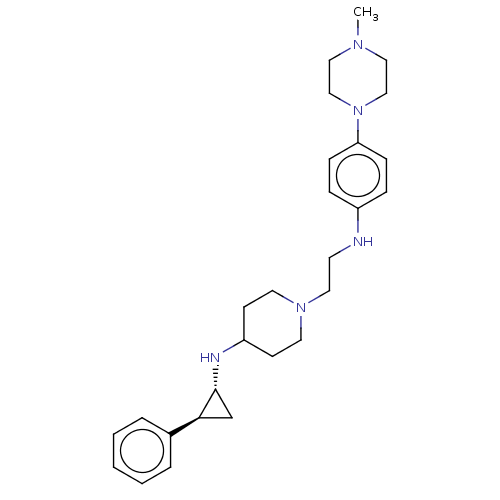 (US10233152, Example 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM93150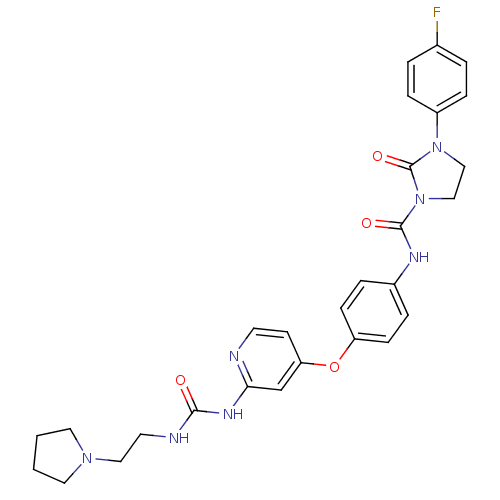 (2-oxoimidazolidine-1-carboxamide, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
MethylGene Inc. | Assay Description Inhibition assay using kinase inhibitors. | International Journal of Medicinal Chemistry 2012: 1-6 (2012) BindingDB Entry DOI: 10.7270/Q2X065P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369551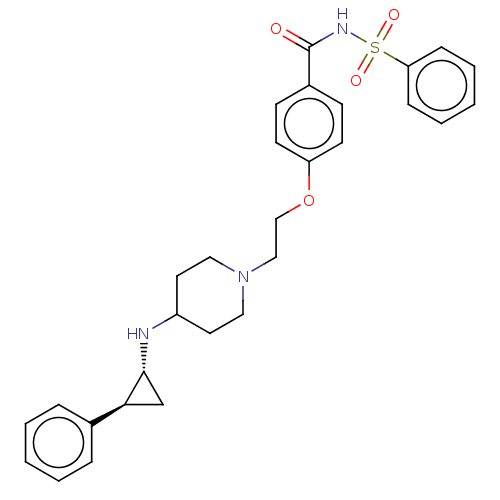 (US10233152, Example 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369574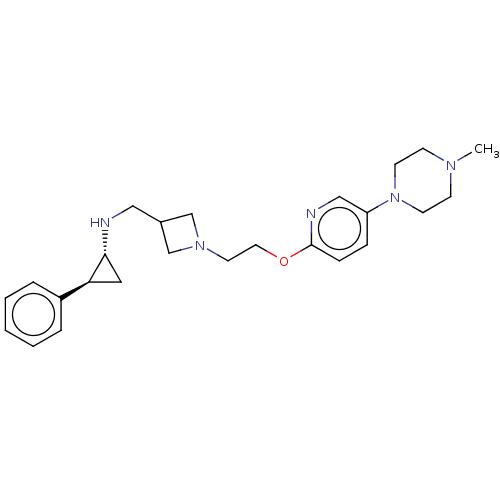 (US10233152, Example 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369576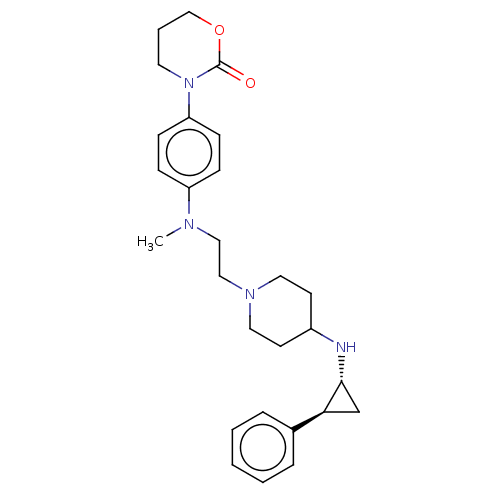 (US10233152, Example 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369476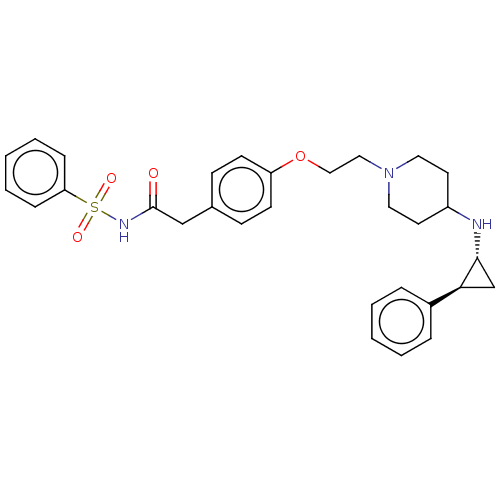 (N-(Methylsulfonyl)-2-(4-(2-(4-(((1R,2S)-2-phenylcy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369483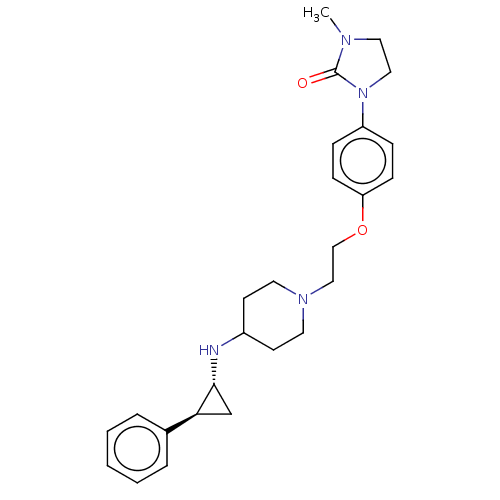 (US10233152, Example 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369491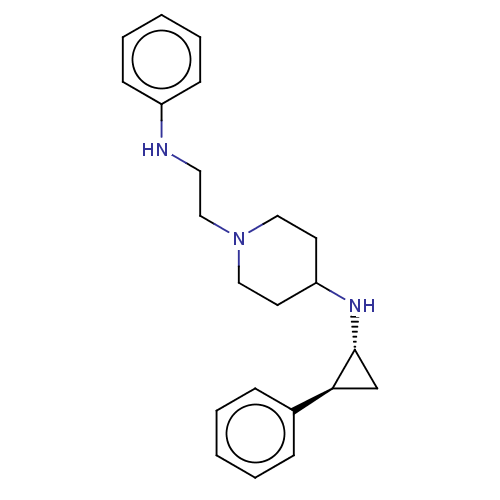 (US10233152, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM270491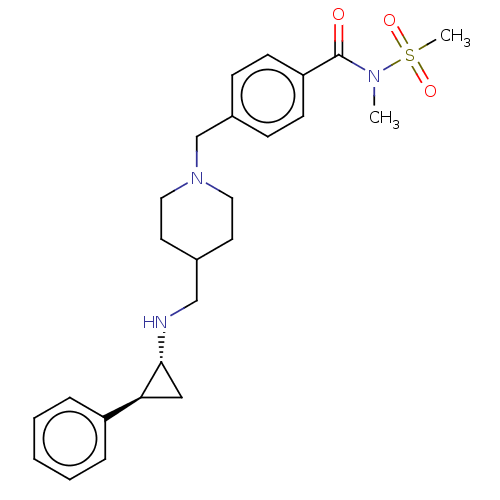 (US10059668, Example 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Mirati Therapeutics, Inc. US Patent | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | US Patent US10059668 (2018) BindingDB Entry DOI: 10.7270/Q2ZC84XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50303276 (CHEMBL565807 | N-(4-(2-(1-ethyl-1H-imidazole-4-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of TPR-met autophosphorylation expressed in HEK293T cells after 150 mins by ELISA | Bioorg Med Chem Lett 19: 6836-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.095 BindingDB Entry DOI: 10.7270/Q2GM87C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50303262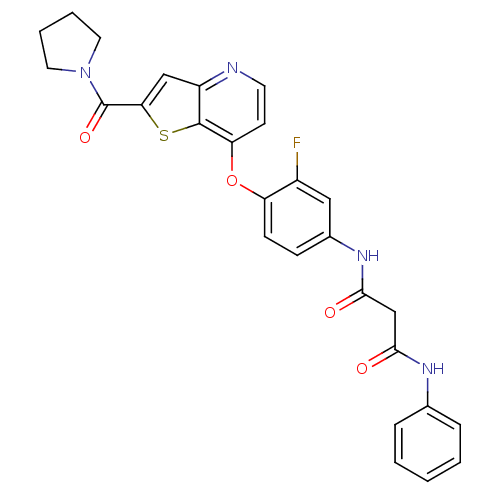 (CHEMBL566010 | N1-(3-fluoro-4-(2-(pyrrolidine-1-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused recombinant VEGFR2 expressed in baculovirus infected Sf9 cells after 10 mins by DELFIA assay | Bioorg Med Chem Lett 19: 6836-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.095 BindingDB Entry DOI: 10.7270/Q2GM87C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369541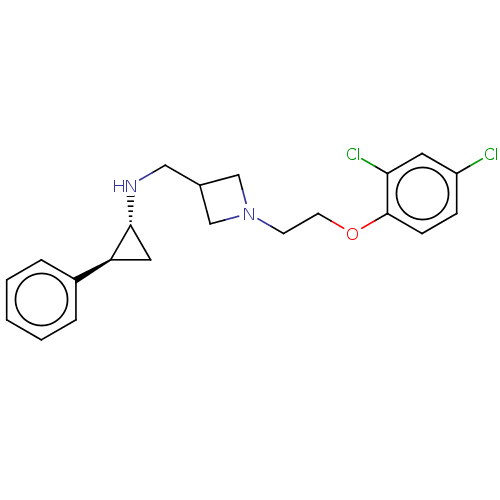 (US10233152, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50114835 ((E)-8-(biphenyl-4-yl)-N-hydroxy-8-(hydroxyimino)oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM93145 (2-oxoimidazolidine-1-carboxamide, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
MethylGene Inc. | Assay Description Inhibition assay using kinase inhibitors. | International Journal of Medicinal Chemistry 2012: 1-6 (2012) BindingDB Entry DOI: 10.7270/Q2X065P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50114811 (8-Oxo-8-[4-(4-phenyl-piperazin-1-yl)-phenyl]-octan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369531 (US10233152, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM93148 (2-oxoimidazolidine-1-carboxamide, 15) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
MethylGene Inc. | Assay Description Inhibition assay using kinase inhibitors. | International Journal of Medicinal Chemistry 2012: 1-6 (2012) BindingDB Entry DOI: 10.7270/Q2X065P9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369580 (US10233152, Example 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369593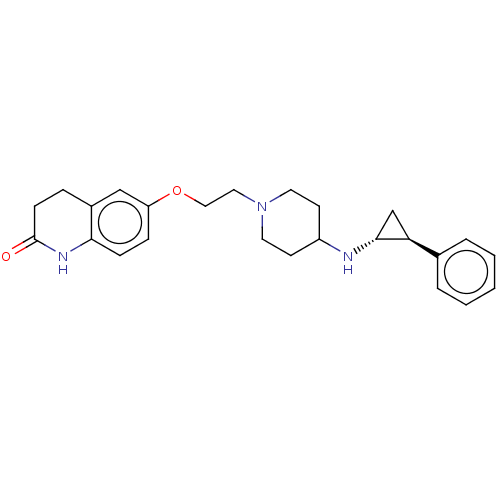 (US10233152, Example 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369472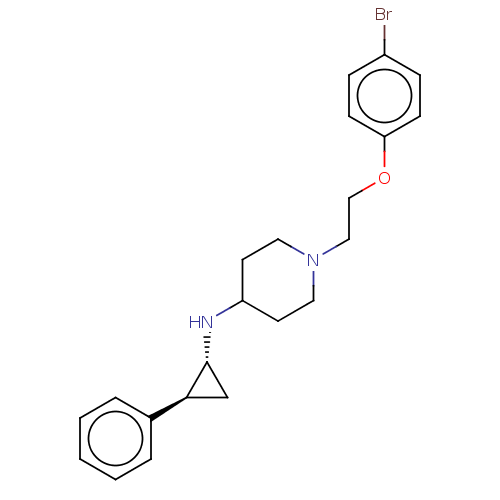 (1-[2-(4-bromophenoxy)ethyl]-N-[(1R,2S)-2-phenylcyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369480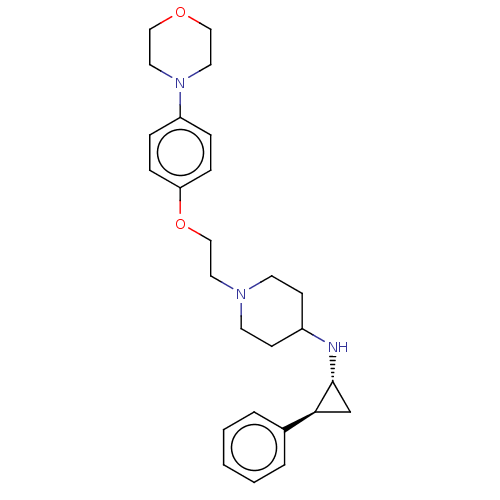 (1-[2-(4-morpholinophenoxy)ethyl]-N—[(R, 2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369643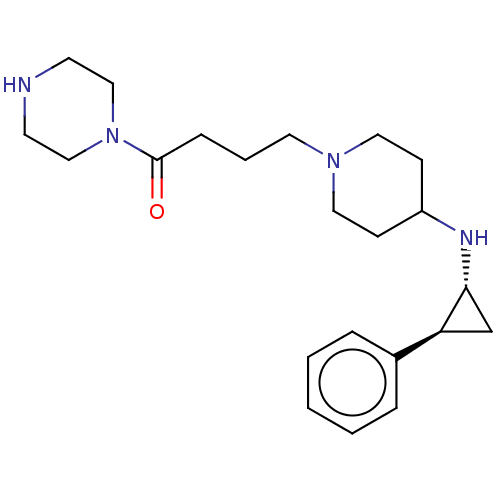 (US10233152, Example 150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369482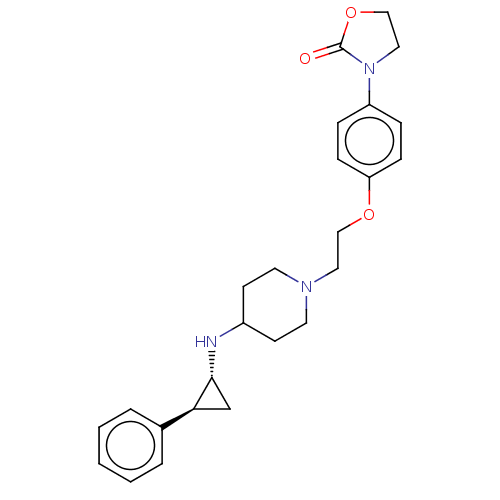 (US10233152, Example 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369481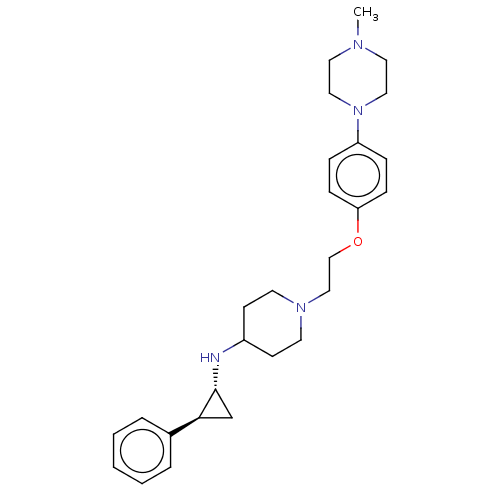 (US10233152, Example 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM270481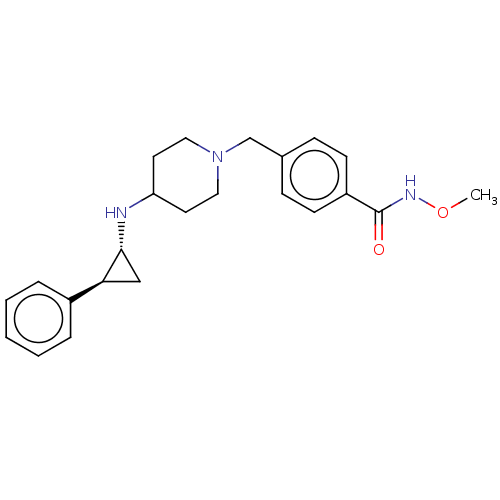 (US10059668, Example 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Mirati Therapeutics, Inc. US Patent | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | US Patent US10059668 (2018) BindingDB Entry DOI: 10.7270/Q2ZC84XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50303272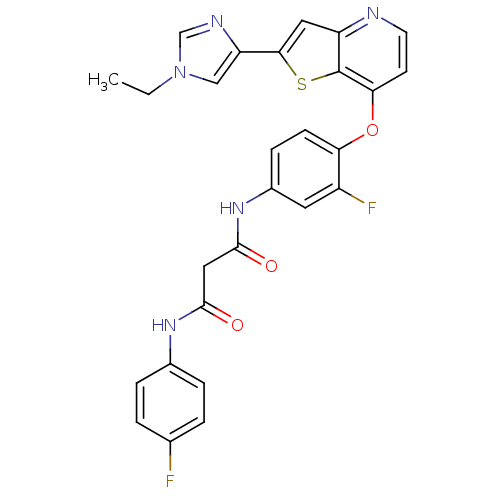 (CHEMBL567475 | N1-(4-(2-(1-ethyl-1H-imidazole-4-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused recombinant VEGFR2 expressed in baculovirus infected Sf9 cells after 10 mins by DELFIA assay | Bioorg Med Chem Lett 19: 6836-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.095 BindingDB Entry DOI: 10.7270/Q2GM87C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50303266 (CHEMBL565990 | N1-(4-(2-(1-ethyl-1H-imidazole-4-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition of GST-fused recombinant VEGFR2 expressed in baculovirus infected Sf9 cells after 10 mins by DELFIA assay | Bioorg Med Chem Lett 19: 6836-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.095 BindingDB Entry DOI: 10.7270/Q2GM87C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50114814 (8-Naphthalen-2-yl-8-oxo-octanoic acid hydroxyamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50114816 (8-(biphenyl-4-yl)-N-hydroxy-8-oxooctanamide | 8-Bi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Inhibition against partially purified human histone deacetylase 1 (HDAC-1) | J Med Chem 45: 2877-85 (2002) BindingDB Entry DOI: 10.7270/Q29W0DT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369550 (US10233152, Example 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369513 (US10233152, Example 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM369514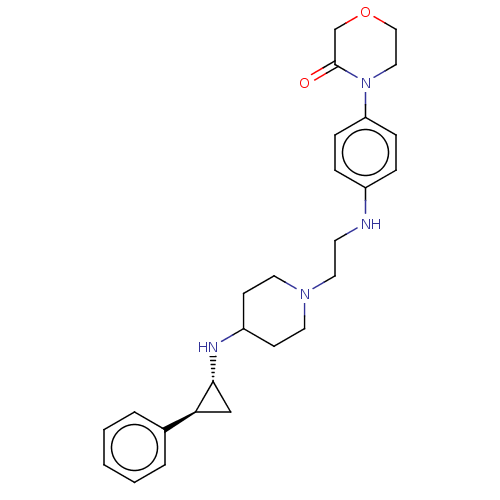 (US10233152, Example 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description Briefly, compounds of the present invention were solubilized in DMSO and a series of 10, three-fold serial dilutions were made for each compound in 1... | J Med Chem 50: 641-62 (2007) BindingDB Entry DOI: 10.7270/Q2GX4DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 950 total ) | Next | Last >> |