 Found 360 hits with Last Name = 'pierce' and Initial = 'ac'
Found 360 hits with Last Name = 'pierce' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098242

(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2CCCCCc2c1 Show InChI InChI=1S/C27H39N5O3S/c1-32(23-9-5-6-10-23)27(33)25(17-19-11-13-21(14-12-19)26(28)30-29)31-36(34,35)24-16-15-20-7-3-2-4-8-22(20)18-24/h11-16,18,23,25-26,30-31H,2-10,17,28-29H2,1H3/t25-,26?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50146643
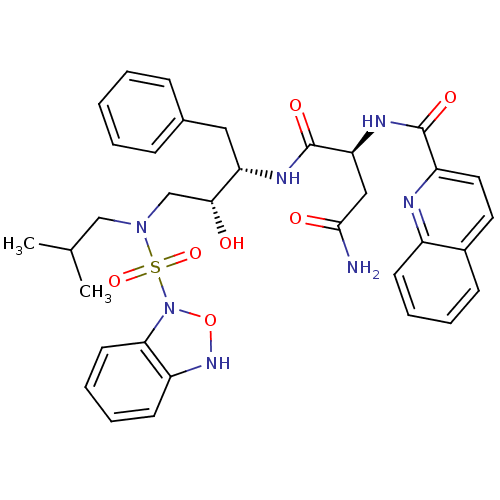
(CHEMBL94384 | N*1*-{(1S,2R)-3-[(3H-Benzo[1,2,5]oxa...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)S(=O)(=O)N1ONc2ccccc12 Show InChI InChI=1S/C34H39N7O7S/c1-22(2)20-40(49(46,47)41-30-15-9-8-14-26(30)39-48-41)21-31(42)28(18-23-10-4-3-5-11-23)37-34(45)29(19-32(35)43)38-33(44)27-17-16-24-12-6-7-13-25(24)36-27/h3-17,22,28-29,31,39,42H,18-21H2,1-2H3,(H2,35,43)(H,37,45)(H,38,44)/t28-,29-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-I protease was determined |
J Med Chem 47: 2768-75 (2004)
Article DOI: 10.1021/jm030543u
BindingDB Entry DOI: 10.7270/Q2319VB4 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098232

(3-[4-(Amino-hydrazino-methyl)-phenyl]-2-(anthracen...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2cc3ccccc3cc2c1 Show InChI InChI=1S/C30H35N5O3S/c1-35(26-8-4-5-9-26)30(36)28(16-20-10-12-21(13-11-20)29(31)33-32)34-39(37,38)27-15-14-24-17-22-6-2-3-7-23(22)18-25(24)19-27/h2-3,6-7,10-15,17-19,26,28-29,33-34H,4-5,8-9,16,31-32H2,1H3/t28-,29?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098243

(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2CCCCc2c1 Show InChI InChI=1S/C26H37N5O3S/c1-31(22-8-4-5-9-22)26(32)24(16-18-10-12-20(13-11-18)25(27)29-28)30-35(33,34)23-15-14-19-6-2-3-7-21(19)17-23/h10-15,17,22,24-25,29-30H,2-9,16,27-28H2,1H3/t24-,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098245
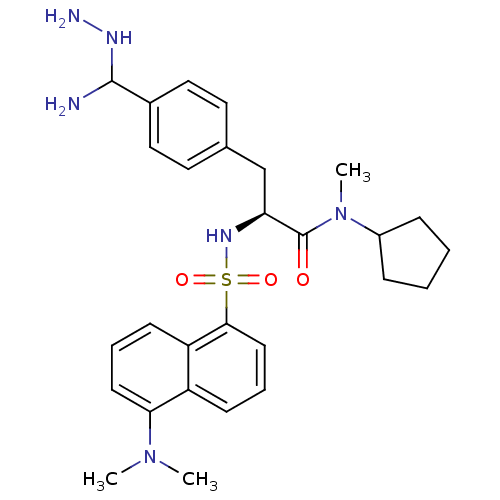
(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N[C@@H](Cc1ccc(cc1)C(N)NN)C(=O)N(C)C1CCCC1 Show InChI InChI=1S/C28H38N6O3S/c1-33(2)25-12-6-11-23-22(25)10-7-13-26(23)38(36,37)32-24(28(35)34(3)21-8-4-5-9-21)18-19-14-16-20(17-15-19)27(29)31-30/h6-7,10-17,21,24,27,31-32H,4-5,8-9,18,29-30H2,1-3H3/t24-,27?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098235

(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H33N5O3S/c1-31(22-8-4-5-9-22)26(32)24(16-18-10-12-20(13-11-18)25(27)29-28)30-35(33,34)23-15-14-19-6-2-3-7-21(19)17-23/h2-3,6-7,10-15,17,22,24-25,29-30H,4-5,8-9,16,27-28H2,1H3/t24-,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305149
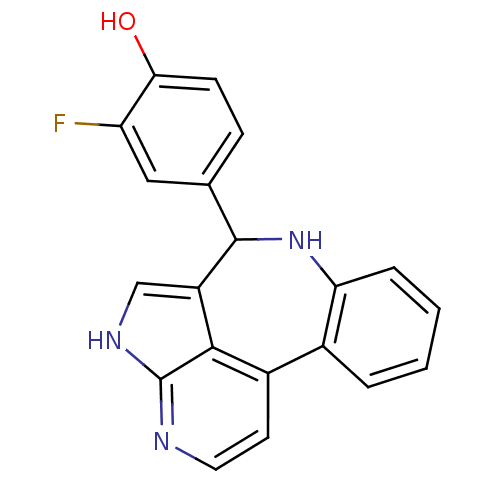
(2-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show InChI InChI=1S/C20H14FN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305150
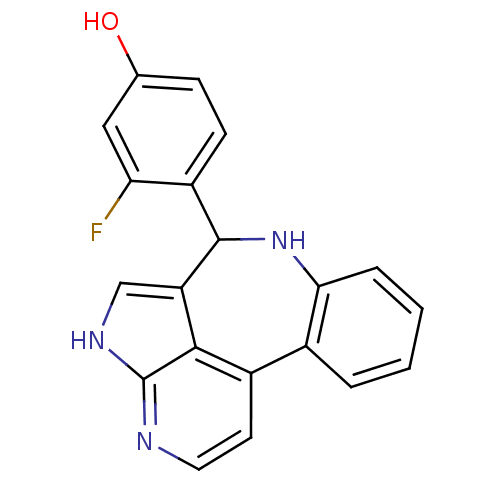
(3-fluoro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C20H14FN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305157
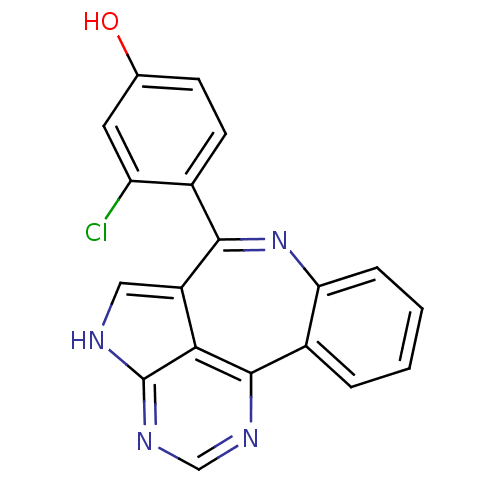
(3-chloro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(c(Cl)c1)-c1nc2ccccc2c2ncnc3[nH]cc1c23 Show InChI InChI=1S/C19H11ClN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355479
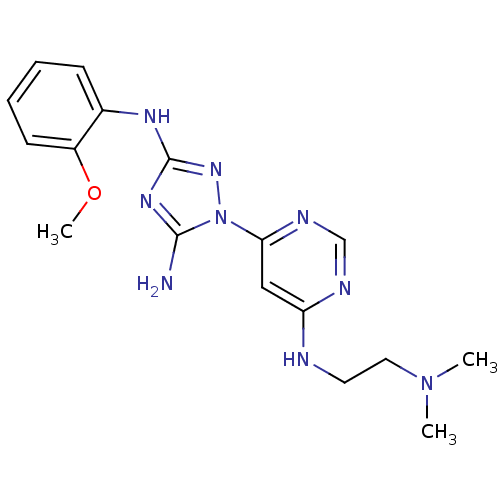
(CHEMBL1835746)Show InChI InChI=1S/C17H23N9O/c1-25(2)9-8-19-14-10-15(21-11-20-14)26-16(18)23-17(24-26)22-12-6-4-5-7-13(12)27-3/h4-7,10-11H,8-9H2,1-3H3,(H,19,20,21)(H3,18,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305155
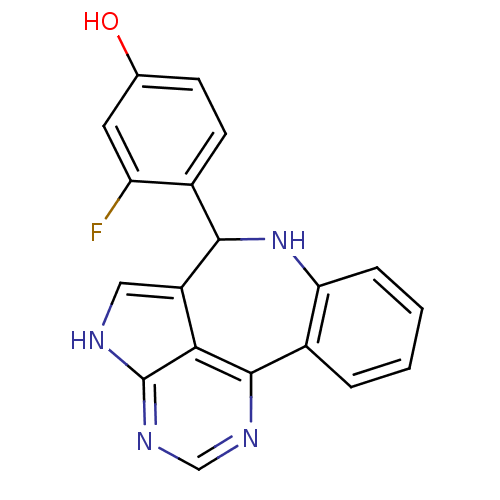
(3-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ncnc4[nH]cc2c34)c(F)c1 Show InChI InChI=1S/C19H13FN4O/c20-14-7-10(25)5-6-11(14)17-13-8-21-19-16(13)18(22-9-23-19)12-3-1-2-4-15(12)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305156
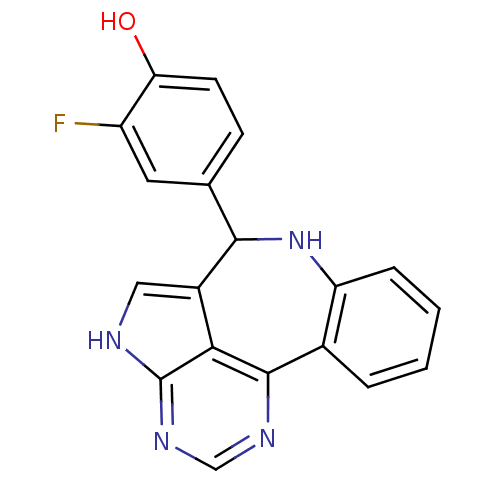
(2-fluoro-4-{8,12,14,16-tetraazatetracyclo[8.6.1.0^...)Show InChI InChI=1S/C19H13FN4O/c20-13-7-10(5-6-15(13)25)17-12-8-21-19-16(12)18(22-9-23-19)11-3-1-2-4-14(11)24-17/h1-9,17,24-25H,(H,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50098235

(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H33N5O3S/c1-31(22-8-4-5-9-22)26(32)24(16-18-10-12-20(13-11-18)25(27)29-28)30-35(33,34)23-15-14-19-6-2-3-7-21(19)17-23/h2-3,6-7,10-15,17,22,24-25,29-30H,4-5,8-9,16,27-28H2,1H3/t24-,25?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against bovine thrombin. |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50098236
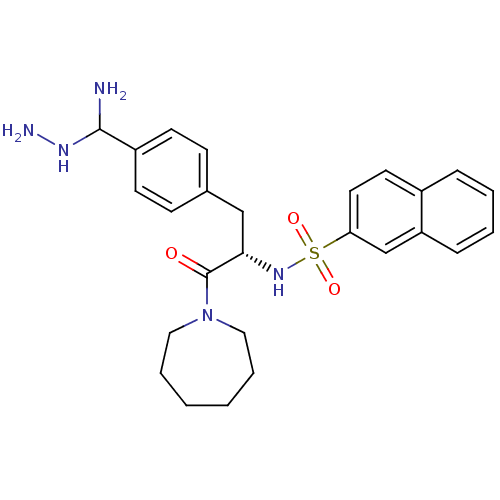
(CHEMBL9415 | Naphthalene-2-sulfonic acid {1-[4-(am...)Show SMILES NNC(N)c1ccc(C[C@H](NS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCCC2)cc1 Show InChI InChI=1S/C26H33N5O3S/c27-25(29-28)21-11-9-19(10-12-21)17-24(26(32)31-15-5-1-2-6-16-31)30-35(33,34)23-14-13-20-7-3-4-8-22(20)18-23/h3-4,7-14,18,24-25,29-30H,1-2,5-6,15-17,27-28H2/t24-,25?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against bovine thrombin. |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305148
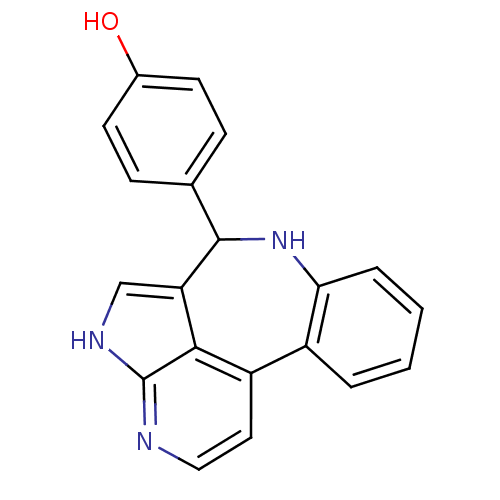
(4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,17...)Show InChI InChI=1S/C20H15N3O/c24-13-7-5-12(6-8-13)19-16-11-22-20-18(16)15(9-10-21-20)14-3-1-2-4-17(14)23-19/h1-11,19,23-24H,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50044287
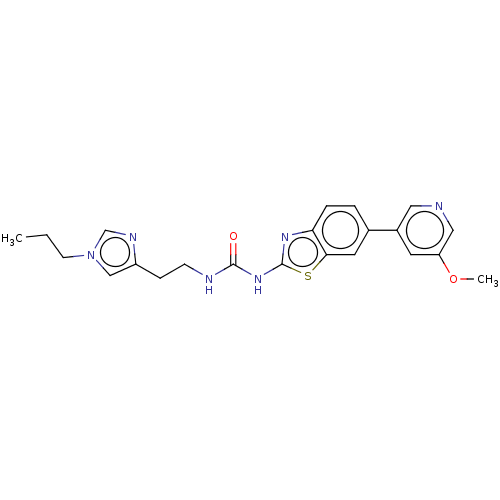
(CHEMBL3356900)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3ccc(cc3s2)-c2cncc(OC)c2)c1 Show InChI InChI=1S/C22H24N6O2S/c1-3-8-28-13-17(25-14-28)6-7-24-21(29)27-22-26-19-5-4-15(10-20(19)31-22)16-9-18(30-2)12-23-11-16/h4-5,9-14H,3,6-8H2,1-2H3,(H2,24,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305153
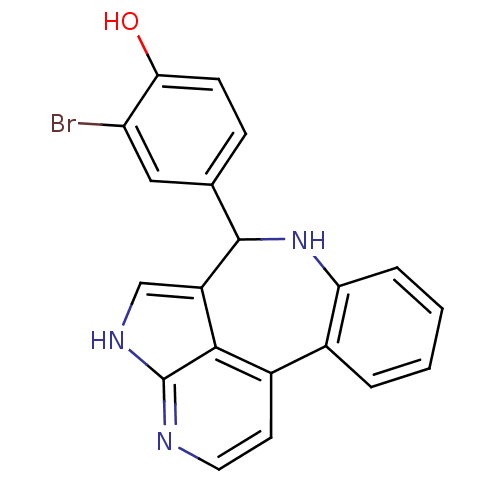
(2-bromo-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}....)Show InChI InChI=1S/C20H14BrN3O/c21-15-9-11(5-6-17(15)25)19-14-10-23-20-18(14)13(7-8-22-20)12-3-1-2-4-16(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355491

(CHEMBL1835870)Show SMILES Nc1nc(Nc2ccc(cc2)[C@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,wD:14.18,(-9.05,.18,;-7.58,.64,;-7.09,2.1,;-5.55,2.08,;-4.8,3.43,;-3.26,3.44,;-2.48,2.12,;-.94,2.14,;-.18,3.48,;-.98,4.81,;-2.52,4.79,;1.35,3.5,;2.1,4.85,;3.65,4.86,;4.44,3.53,;3.68,2.19,;2.14,2.18,;5.97,3.55,;6.75,2.22,;8.29,2.23,;9.05,3.57,;8.27,4.9,;6.73,4.89,;-5.09,.62,;-6.35,-.28,;-6.36,-1.81,;-5.04,-2.59,;-5.05,-4.13,;-6.39,-4.89,;-7.72,-4.11,;-7.71,-2.57,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355489
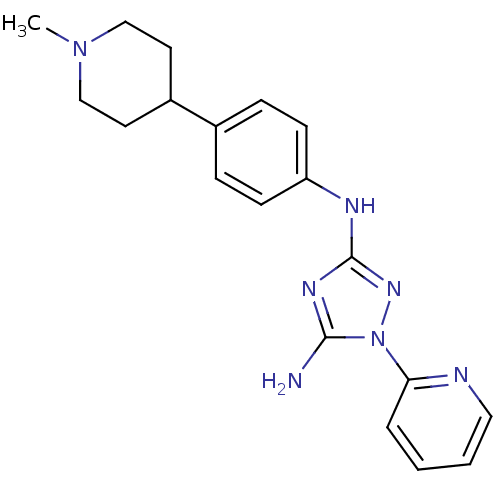
(CHEMBL1835867)Show SMILES CN1CCC(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H23N7/c1-25-12-9-15(10-13-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-11-21-17/h2-8,11,15H,9-10,12-13H2,1H3,(H3,20,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355464
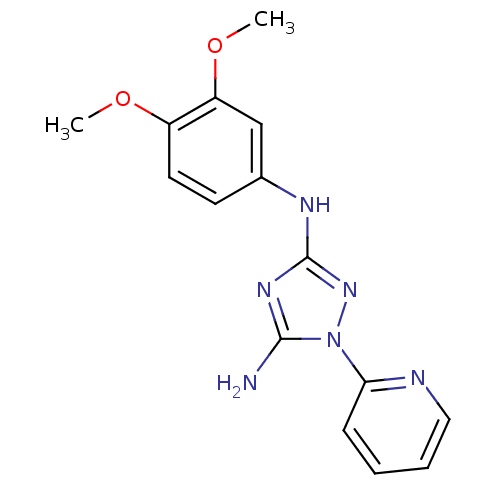
(CHEMBL1835740)Show InChI InChI=1S/C15H16N6O2/c1-22-11-7-6-10(9-12(11)23-2)18-15-19-14(16)21(20-15)13-5-3-4-8-17-13/h3-9H,1-2H3,(H3,16,18,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305154
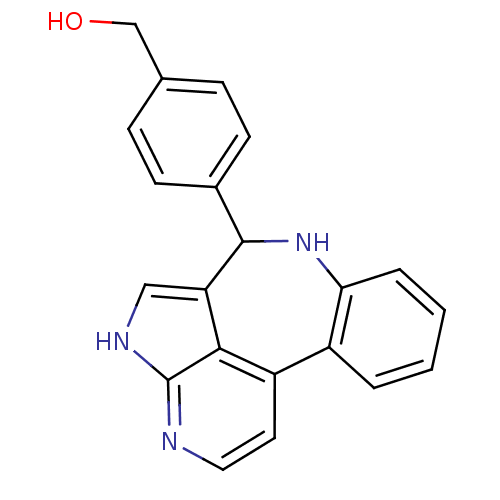
((4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}.0^{13,1...)Show InChI InChI=1S/C21H17N3O/c25-12-13-5-7-14(8-6-13)20-17-11-23-21-19(17)16(9-10-22-21)15-3-1-2-4-18(15)24-20/h1-11,20,24-25H,12H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274571
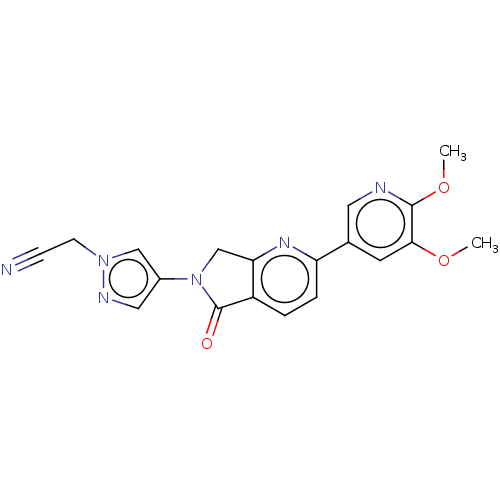
(CHEMBL4127784)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#N)c1 Show InChI InChI=1S/C19H16N6O3/c1-27-17-7-12(8-21-18(17)28-2)15-4-3-14-16(23-15)11-25(19(14)26)13-9-22-24(10-13)6-5-20/h3-4,7-10H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50098237
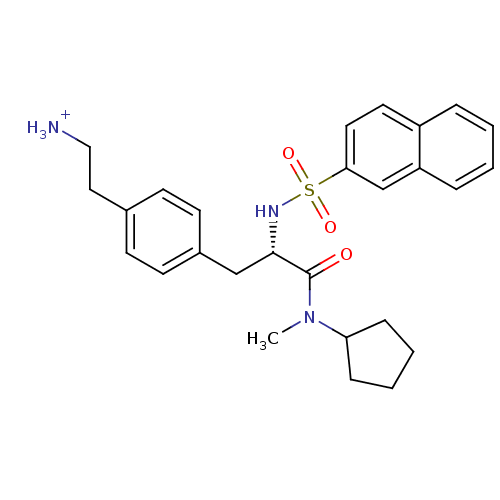
(2-{4-[2-(Cyclopentyl-methyl-carbamoyl)-2-(naphthal...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(CC[NH3+])cc1)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C27H33N3O3S/c1-30(24-8-4-5-9-24)27(31)26(18-21-12-10-20(11-13-21)16-17-28)29-34(32,33)25-15-14-22-6-2-3-7-23(22)19-25/h2-3,6-7,10-15,19,24,26,29H,4-5,8-9,16-18,28H2,1H3/p+1/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098244
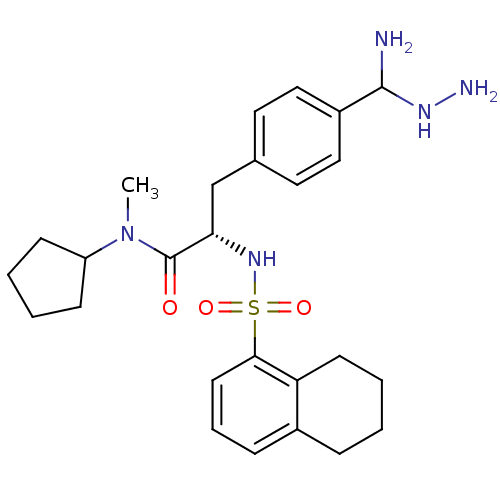
(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1cccc2CCCCc12 Show InChI InChI=1S/C26H37N5O3S/c1-31(21-9-3-4-10-21)26(32)23(17-18-13-15-20(16-14-18)25(27)29-28)30-35(33,34)24-12-6-8-19-7-2-5-11-22(19)24/h6,8,12-16,21,23,25,29-30H,2-5,7,9-11,17,27-28H2,1H3/t23-,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274538
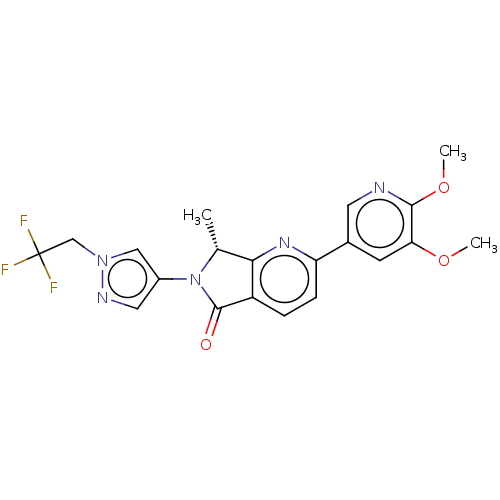
(CHEMBL4126773)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50305152
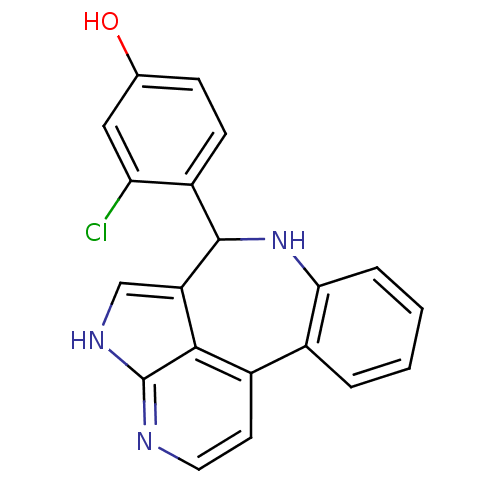
(3-chloro-4-{8,12,14-triazatetracyclo[8.6.1.0^{2,7}...)Show SMILES Oc1ccc(C2Nc3ccccc3-c3ccnc4[nH]cc2c34)c(Cl)c1 Show InChI InChI=1S/C20H14ClN3O/c21-16-9-11(25)5-6-14(16)19-15-10-23-20-18(15)13(7-8-22-20)12-3-1-2-4-17(12)24-19/h1-10,19,24-25H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 20: 153-6 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.021
BindingDB Entry DOI: 10.7270/Q2PN95RQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274537
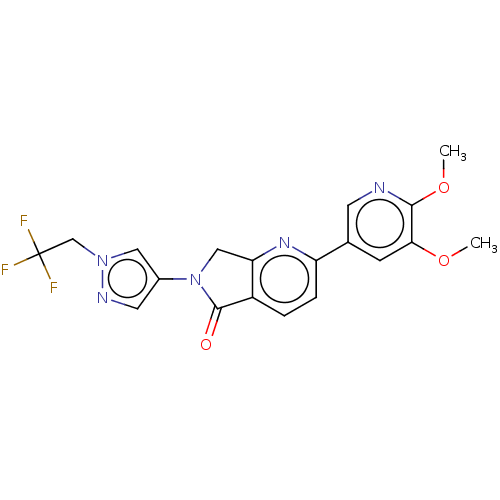
(CHEMBL4129974)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)(F)F)c1 Show InChI InChI=1S/C19H16F3N5O3/c1-29-16-5-11(6-23-17(16)30-2)14-4-3-13-15(25-14)9-27(18(13)28)12-7-24-26(8-12)10-19(20,21)22/h3-8H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
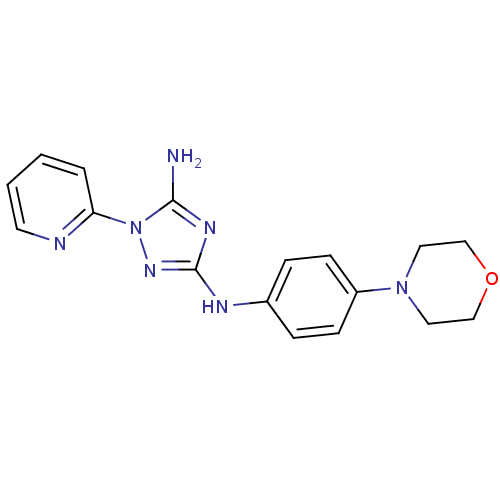
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355480
(CHEMBL1835747)Show InChI InChI=1S/C17H19N7O/c18-16-21-17(22-24(16)15-3-1-2-8-19-15)20-13-4-6-14(7-5-13)23-9-11-25-12-10-23/h1-8H,9-12H2,(H3,18,20,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
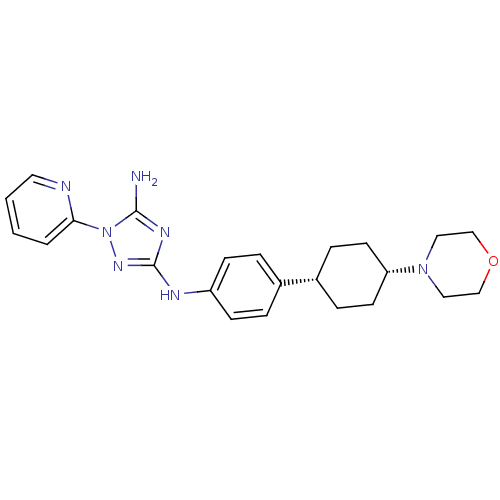
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355492
(CHEMBL1835871)Show SMILES Nc1nc(Nc2ccc(cc2)[C@@H]2CC[C@@H](CC2)N2CCOCC2)nn1-c1ccccn1 |r,wU:11.11,14.18,(2.59,-31.18,;4.06,-30.71,;4.55,-29.26,;6.09,-29.27,;6.84,-27.92,;8.38,-27.91,;9.16,-29.23,;10.7,-29.21,;11.46,-27.87,;10.67,-26.54,;9.13,-26.56,;13,-27.85,;13.78,-29.18,;15.32,-29.16,;16.08,-27.82,;15.29,-26.49,;13.75,-26.5,;17.62,-27.8,;18.4,-29.13,;19.93,-29.12,;20.7,-27.78,;19.92,-26.45,;18.37,-26.46,;6.55,-30.74,;5.3,-31.63,;5.28,-33.17,;6.6,-33.94,;6.59,-35.48,;5.25,-36.25,;3.92,-35.46,;3.93,-33.92,)| Show InChI InChI=1S/C23H29N7O/c24-22-27-23(28-30(22)21-3-1-2-12-25-21)26-19-8-4-17(5-9-19)18-6-10-20(11-7-18)29-13-15-31-16-14-29/h1-5,8-9,12,18,20H,6-7,10-11,13-16H2,(H3,24,26,27,28)/t18-,20+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
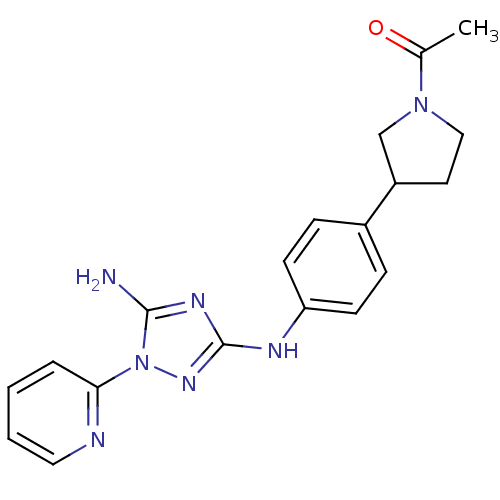
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355490
(CHEMBL1835869)Show SMILES CC(=O)N1CCC(C1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C19H21N7O/c1-13(27)25-11-9-15(12-25)14-5-7-16(8-6-14)22-19-23-18(20)26(24-19)17-4-2-3-10-21-17/h2-8,10,15H,9,11-12H2,1H3,(H3,20,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355482
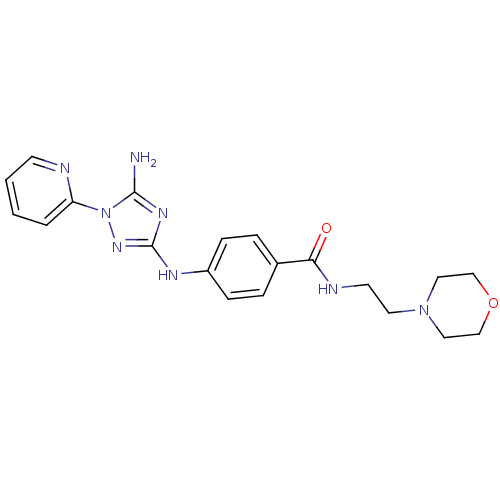
(CHEMBL1835749)Show SMILES Nc1nc(Nc2ccc(cc2)C(=O)NCCN2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C20H24N8O2/c21-19-25-20(26-28(19)17-3-1-2-8-22-17)24-16-6-4-15(5-7-16)18(29)23-9-10-27-11-13-30-14-12-27/h1-8H,9-14H2,(H,23,29)(H3,21,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274572
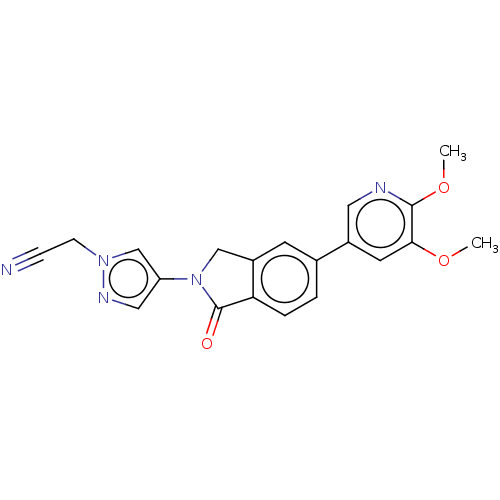
(CHEMBL4129180)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2c1)c1cnn(CC#N)c1 Show InChI InChI=1S/C20H17N5O3/c1-27-18-8-14(9-22-19(18)28-2)13-3-4-17-15(7-13)11-25(20(17)26)16-10-23-24(12-16)6-5-21/h3-4,7-10,12H,6,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355467
(CHEMBL1833992)Show InChI InChI=1S/C18H21N7O2/c1-26-15-6-5-13(24-8-10-27-11-9-24)12-14(15)21-18-22-17(19)25(23-18)16-4-2-3-7-20-16/h2-7,12H,8-11H2,1H3,(H3,19,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Bos taurus (Bovine)) | BDBM50098230
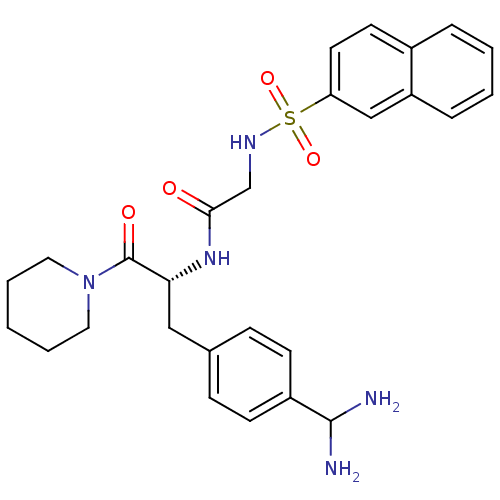
(CHEMBL9491 | N-[1-(4-Diaminomethyl-benzyl)-2-oxo-2...)Show SMILES NC(N)c1ccc(C[C@@H](NC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C27H33N5O4S/c28-26(29)21-10-8-19(9-11-21)16-24(27(34)32-14-4-1-5-15-32)31-25(33)18-30-37(35,36)23-13-12-20-6-2-3-7-22(20)17-23/h2-3,6-13,17,24,26,30H,1,4-5,14-16,18,28-29H2,(H,31,33)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against bovine thrombin. |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50378535
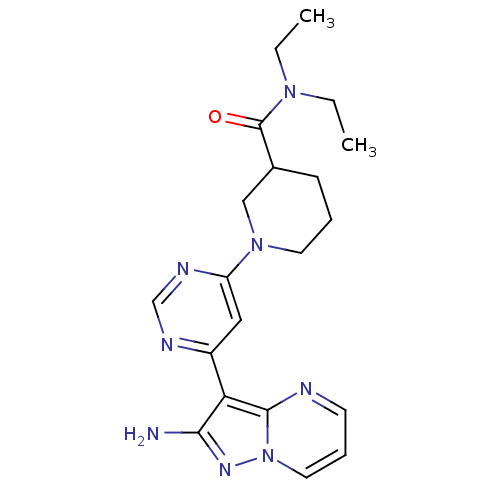
(CHEMBL1204012)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1cc(ncn1)-c1c(N)nn2cccnc12 Show InChI InChI=1S/C20H26N8O/c1-3-26(4-2)20(29)14-7-5-9-27(12-14)16-11-15(23-13-24-16)17-18(21)25-28-10-6-8-22-19(17)28/h6,8,10-11,13-14H,3-5,7,9,12H2,1-2H3,(H2,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 by radiometric assay |
Bioorg Med Chem Lett 19: 6529-33 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.053
BindingDB Entry DOI: 10.7270/Q2SJ1MKQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355483
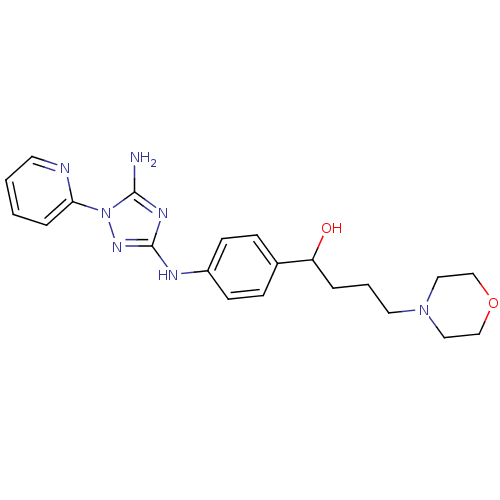
(CHEMBL1835750)Show SMILES Nc1nc(Nc2ccc(cc2)C(O)CCCN2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C21H27N7O2/c22-20-25-21(26-28(20)19-5-1-2-10-23-19)24-17-8-6-16(7-9-17)18(29)4-3-11-27-12-14-30-15-13-27/h1-2,5-10,18,29H,3-4,11-15H2,(H3,22,24,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274541
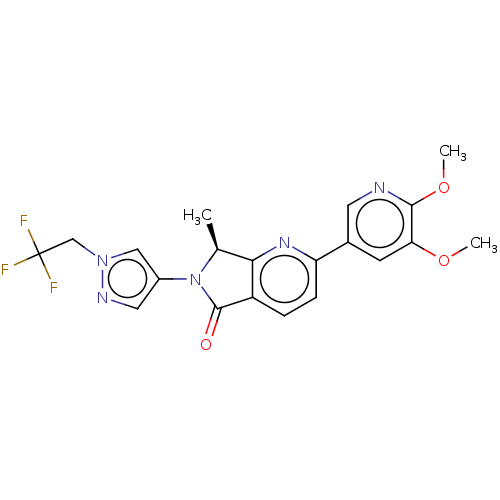
(CHEMBL4130036)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N([C@@H](C)c2n1)c1cnn(CC(F)(F)F)c1 |r| Show InChI InChI=1S/C20H18F3N5O3/c1-11-17-14(19(29)28(11)13-8-25-27(9-13)10-20(21,22)23)4-5-15(26-17)12-6-16(30-2)18(31-3)24-7-12/h4-9,11H,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098241
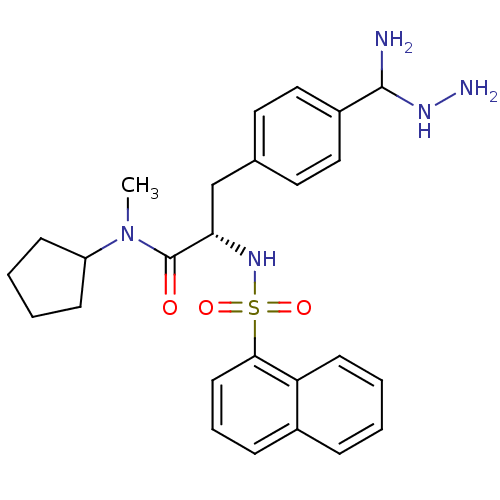
(3-[4-(Amino-hydrazino-methyl)-phenyl]-N-cyclopenty...)Show SMILES CN(C1CCCC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H33N5O3S/c1-31(21-9-3-4-10-21)26(32)23(17-18-13-15-20(16-14-18)25(27)29-28)30-35(33,34)24-12-6-8-19-7-2-5-11-22(19)24/h2,5-8,11-16,21,23,25,29-30H,3-4,9-10,17,27-28H2,1H3/t23-,25?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355481
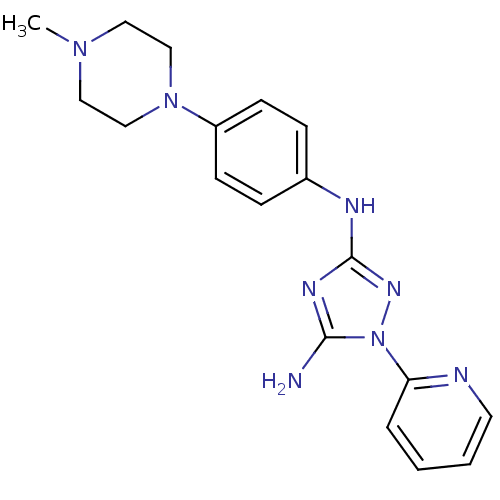
(CHEMBL1835748)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(N)n(n2)-c2ccccn2)cc1 Show InChI InChI=1S/C18H22N8/c1-24-10-12-25(13-11-24)15-7-5-14(6-8-15)21-18-22-17(19)26(23-18)16-4-2-3-9-20-16/h2-9H,10-13H2,1H3,(H3,19,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274559

(CHEMBL4126707)Show SMILES CCn1cc(cn1)N1Cc2nc(ccc2C1=O)-c1cnc(OC)c(OC)c1 Show InChI InChI=1S/C19H19N5O3/c1-4-23-10-13(9-21-23)24-11-16-14(19(24)25)5-6-15(22-16)12-7-17(26-2)18(27-3)20-8-12/h5-10H,4,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274542

(CHEMBL4127853)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC#C)c1 Show InChI InChI=1S/C20H17N5O3/c1-4-7-24-11-14(10-22-24)25-12-17-15(20(25)26)5-6-16(23-17)13-8-18(27-2)19(28-3)21-9-13/h1,5-6,8-11H,7,12H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355488
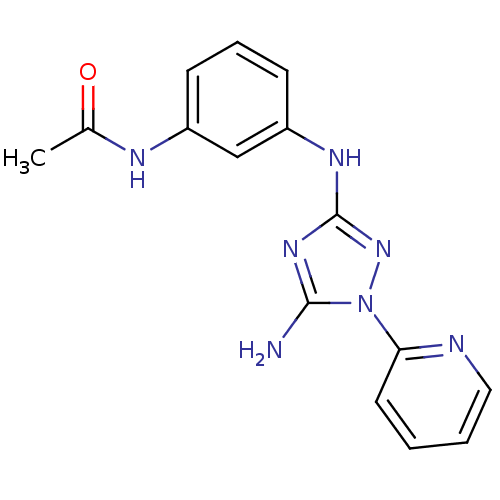
(CHEMBL1835866)Show InChI InChI=1S/C15H15N7O/c1-10(23)18-11-5-4-6-12(9-11)19-15-20-14(16)22(21-15)13-7-2-3-8-17-13/h2-9H,1H3,(H,18,23)(H3,16,19,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355464

(CHEMBL1835740)Show InChI InChI=1S/C15H16N6O2/c1-22-11-7-6-10(9-12(11)23-2)18-15-19-14(16)21(20-15)13-5-3-4-8-17-13/h3-9H,1-2H3,(H3,16,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant c-KIT domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50274543

(CHEMBL4129251)Show SMILES COc1cc(cnc1OC)-c1ccc2C(=O)N(Cc2n1)c1cnn(CC(F)F)c1 Show InChI InChI=1S/C19H17F2N5O3/c1-28-16-5-11(6-22-18(16)29-2)14-4-3-13-15(24-14)9-26(19(13)27)12-7-23-25(8-12)10-17(20)21/h3-8,17H,9-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate after 15 mins in presence of [33P-ATP] by liquid scintillation counting method |
J Med Chem 61: 5245-5256 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00085
BindingDB Entry DOI: 10.7270/Q2V98BK8 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50355486
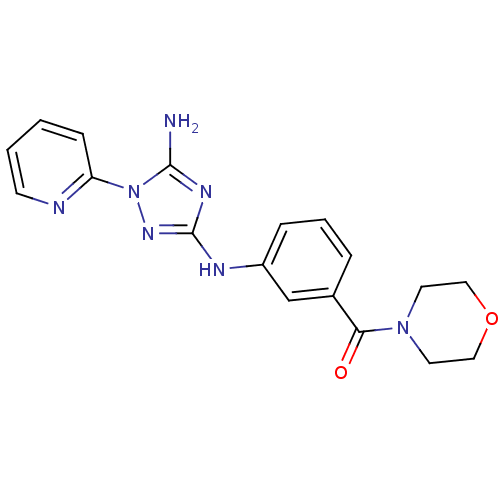
(CHEMBL1835864)Show SMILES Nc1nc(Nc2cccc(c2)C(=O)N2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C18H19N7O2/c19-17-22-18(23-25(17)15-6-1-2-7-20-15)21-14-5-3-4-13(12-14)16(26)24-8-10-27-11-9-24/h1-7,12H,8-11H2,(H3,19,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant c-KIT domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50355486

(CHEMBL1835864)Show SMILES Nc1nc(Nc2cccc(c2)C(=O)N2CCOCC2)nn1-c1ccccn1 Show InChI InChI=1S/C18H19N7O2/c19-17-22-18(23-25(17)15-6-1-2-7-20-15)21-14-5-3-4-13(12-14)16(26)24-8-10-27-11-9-24/h1-7,12H,8-11H2,(H3,19,21,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [33P]ATP from human recombinant FLT3 domain after 20 mins by scintillation counting |
J Med Chem 54: 7184-92 (2011)
Article DOI: 10.1021/jm200712h
BindingDB Entry DOI: 10.7270/Q2DZ08QQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50098233
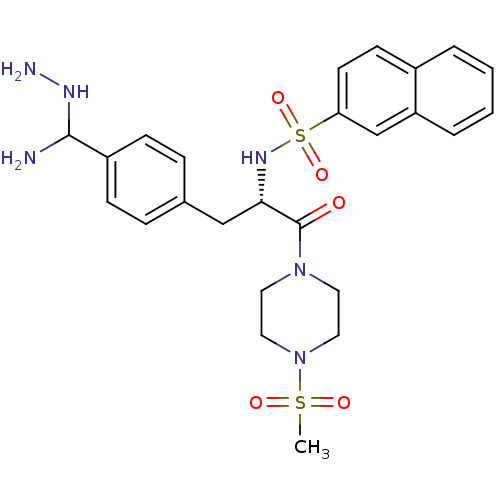
(CHEMBL9461 | Naphthalene-2-sulfonic acid [1-[4-(am...)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)[C@H](Cc1ccc(cc1)C(N)NN)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C25H32N6O5S2/c1-37(33,34)31-14-12-30(13-15-31)25(32)23(16-18-6-8-20(9-7-18)24(26)28-27)29-38(35,36)22-11-10-19-4-2-3-5-21(19)17-22/h2-11,17,23-24,28-29H,12-16,26-27H2,1H3/t23-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Binding affinity was determined against human thrombin |
J Med Chem 44: 1043-50 (2001)
BindingDB Entry DOI: 10.7270/Q2KS6S86 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data