 Found 776 hits with Last Name = 'borthwick' and Initial = 'ad'
Found 776 hits with Last Name = 'borthwick' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384817
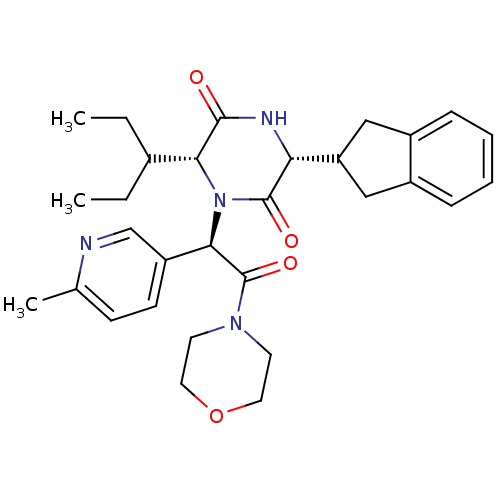
(CHEMBL2037514)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-4-20(5-2)26-28(35)32-25(24-16-21-8-6-7-9-22(21)17-24)29(36)34(26)27(23-11-10-19(3)31-18-23)30(37)33-12-14-38-15-13-33/h6-11,18,20,24-27H,4-5,12-17H2,1-3H3,(H,32,35)/t25-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384816

(CHEMBL2037516)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H38N4O3/c1-7-19(8-2)25-27(34)31-24(22-15-20-11-9-10-12-21(20)16-22)28(35)33(25)26(29(36)32(5)6)23-14-13-17(3)30-18(23)4/h9-14,19,22,24-26H,7-8,15-16H2,1-6H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384800
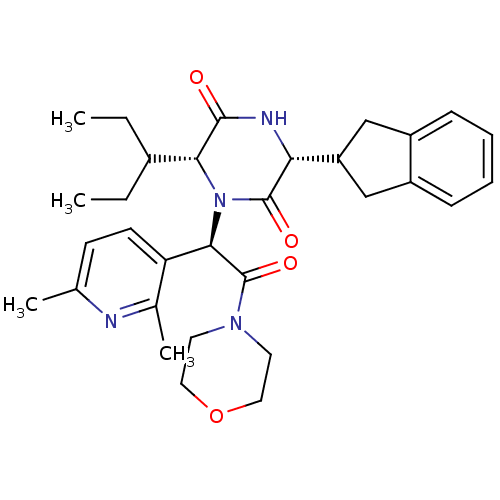
(CHEMBL2037517)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C31H40N4O4/c1-5-21(6-2)27-29(36)33-26(24-17-22-9-7-8-10-23(22)18-24)30(37)35(27)28(25-12-11-19(3)32-20(25)4)31(38)34-13-15-39-16-14-34/h7-12,21,24,26-28H,5-6,13-18H2,1-4H3,(H,33,36)/t26-,27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384823
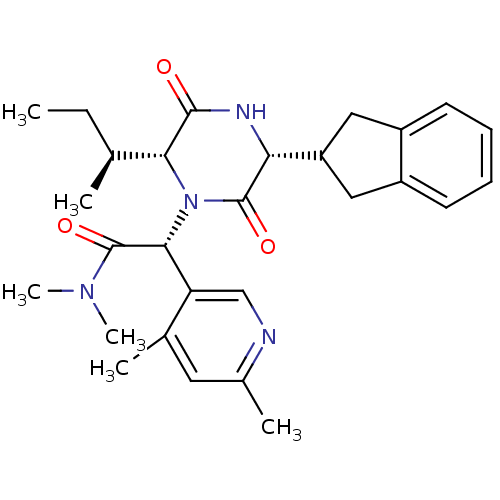
(CHEMBL2037507)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-15-29-18(4)12-17(22)3/h8-12,15-16,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384837

(CHEMBL2037515)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-27(34)31-23(21-14-19-10-8-9-11-20(19)15-21)28(35)32(24)25(26(33)29-5)22-13-12-16(3)30-17(22)4/h8-13,18,21,23-25H,6-7,14-15H2,1-5H3,(H,29,33)(H,31,34)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384822
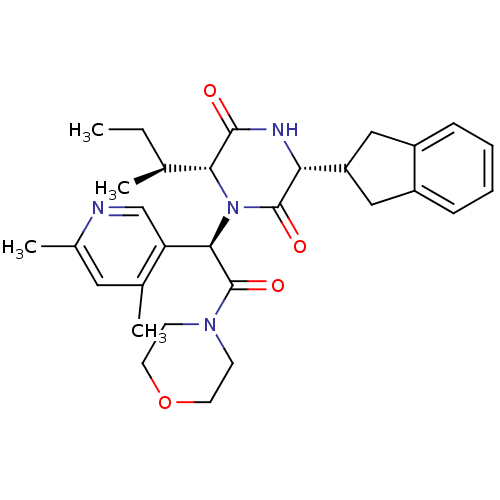
(CHEMBL2037508)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-17-31-20(4)14-19(24)3)30(37)33-10-12-38-13-11-33/h6-9,14,17-18,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384818
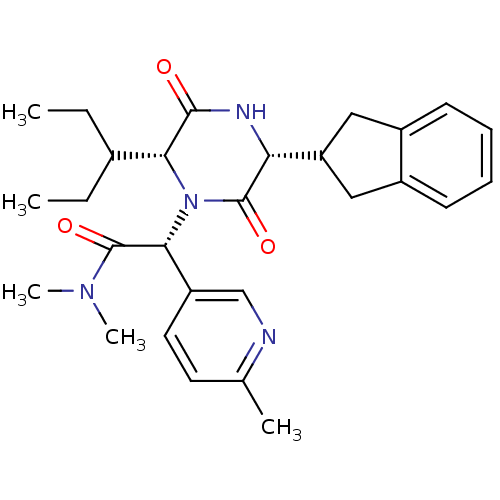
(CHEMBL2037513)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-6-18(7-2)24-26(33)30-23(22-14-19-10-8-9-11-20(19)15-22)27(34)32(24)25(28(35)31(4)5)21-13-12-17(3)29-16-21/h8-13,16,18,22-25H,6-7,14-15H2,1-5H3,(H,30,33)/t23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384815
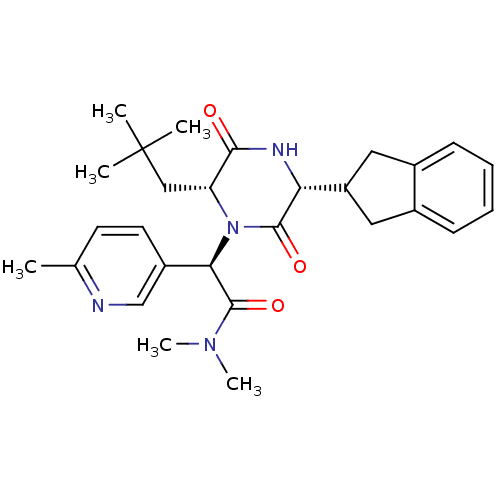
(CHEMBL2037496)Show SMILES CN(C)C(=O)[C@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)c1ccc(C)nc1 |r| Show InChI InChI=1S/C28H36N4O3/c1-17-11-12-20(16-29-17)24(27(35)31(5)6)32-22(15-28(2,3)4)25(33)30-23(26(32)34)21-13-18-9-7-8-10-19(18)14-21/h7-12,16,21-24H,13-15H2,1-6H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384824
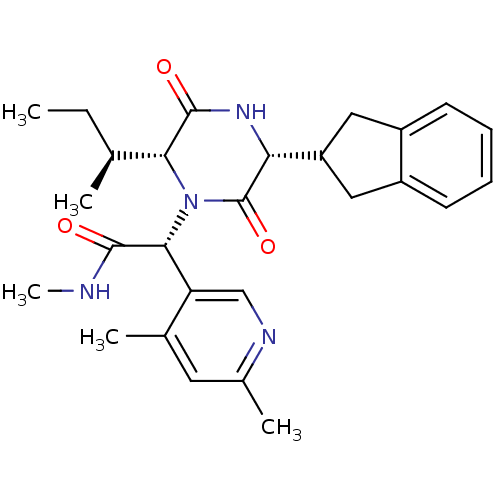
(CHEMBL2037506)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2cnc(C)cc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-12-18-9-7-8-10-19(18)13-20)27(34)31(23)24(25(32)28-5)21-14-29-17(4)11-16(21)3/h7-11,14-15,20,22-24H,6,12-13H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384834
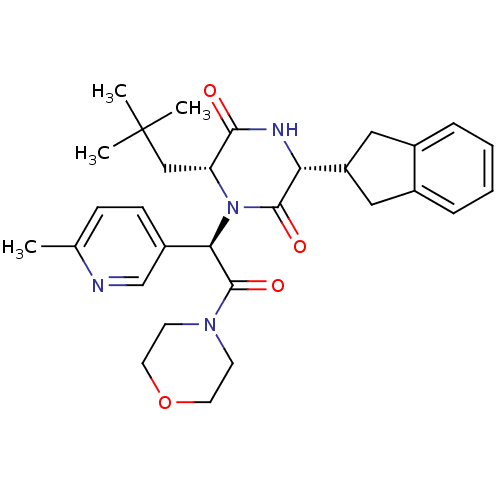
(CHEMBL2037497)Show SMILES Cc1ccc(cn1)[C@@H](N1[C@H](CC(C)(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C30H38N4O4/c1-19-9-10-22(18-31-19)26(29(37)33-11-13-38-14-12-33)34-24(17-30(2,3)4)27(35)32-25(28(34)36)23-15-20-7-5-6-8-21(20)16-23/h5-10,18,23-26H,11-17H2,1-4H3,(H,32,35)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384838
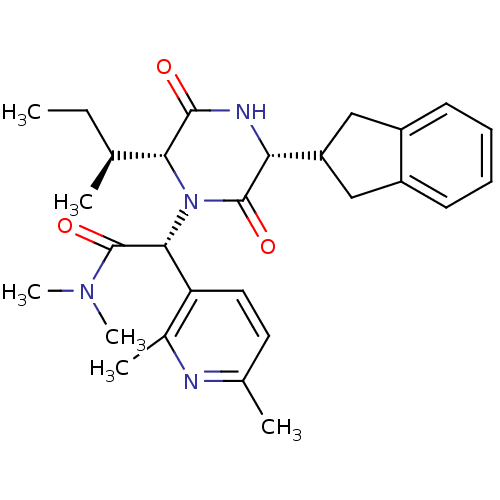
(CHEMBL2037510)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-16(2)24-26(33)30-23(21-14-19-10-8-9-11-20(19)15-21)27(34)32(24)25(28(35)31(5)6)22-13-12-17(3)29-18(22)4/h8-13,16,21,23-25H,7,14-15H2,1-6H3,(H,30,33)/t16-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50190528
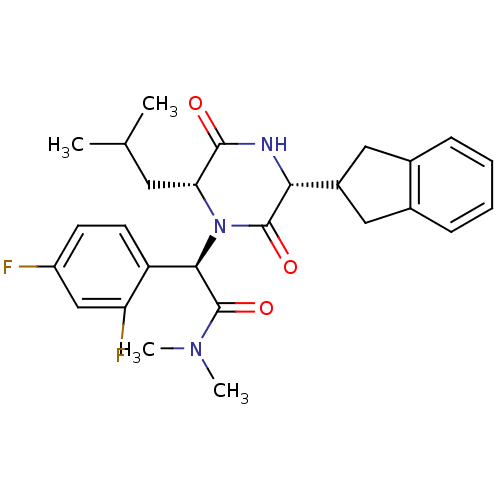
((2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihy...)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 Show InChI InChI=1S/C27H31F2N3O3/c1-15(2)11-22-25(33)30-23(18-12-16-7-5-6-8-17(16)13-18)26(34)32(22)24(27(35)31(3)4)20-10-9-19(28)14-21(20)29/h5-10,14-15,18,22-24H,11-13H2,1-4H3,(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228930
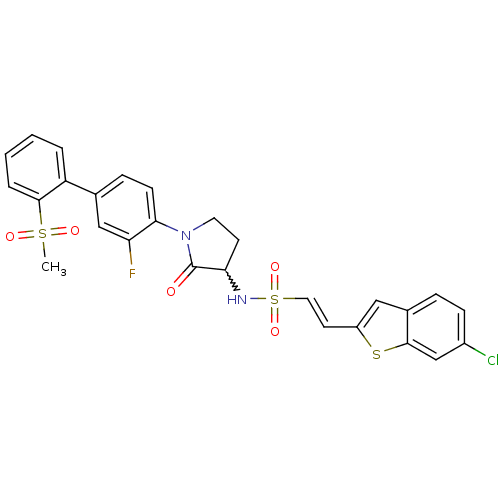
((E)-2-(6-chloro-benzo[b]thiophen-2-yl)-ethenesulfo...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3cc4ccc(Cl)cc4s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C27H22ClFN2O5S3/c1-38(33,34)26-5-3-2-4-21(26)17-7-9-24(22(29)15-17)31-12-10-23(27(31)32)30-39(35,36)13-11-20-14-18-6-8-19(28)16-25(18)37-20/h2-9,11,13-16,23,30H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384819
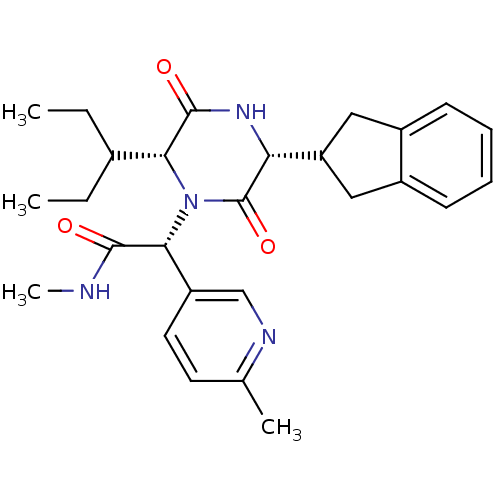
(CHEMBL2037512)Show SMILES CCC(CC)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-5-17(6-2)23-26(33)30-22(21-13-18-9-7-8-10-19(18)14-21)27(34)31(23)24(25(32)28-4)20-12-11-16(3)29-15-20/h7-12,15,17,21-24H,5-6,13-14H2,1-4H3,(H,28,32)(H,30,33)/t22-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384820
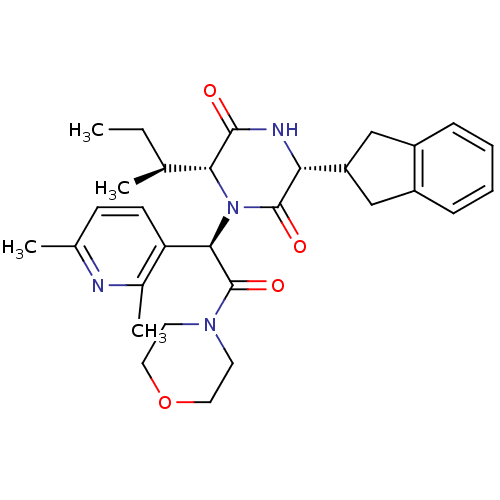
(EPELSIBAN | GSK557296B)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-18(2)26-28(35)32-25(23-16-21-8-6-7-9-22(21)17-23)29(36)34(26)27(24-11-10-19(3)31-20(24)4)30(37)33-12-14-38-15-13-33/h6-11,18,23,25-27H,5,12-17H2,1-4H3,(H,32,35)/t18-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384803
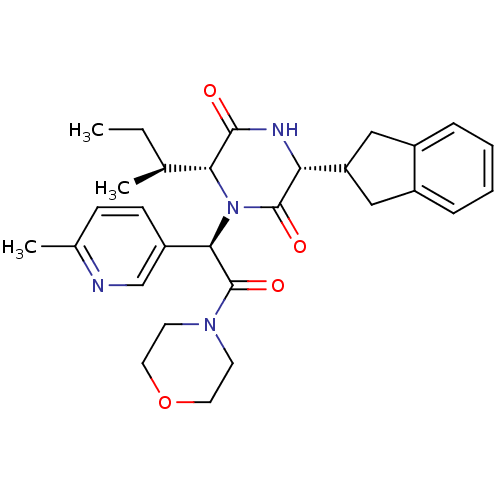
(CHEMBL2037501)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24+,25+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676
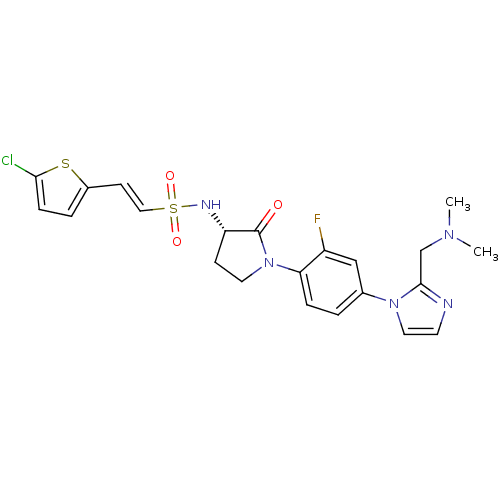
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228950
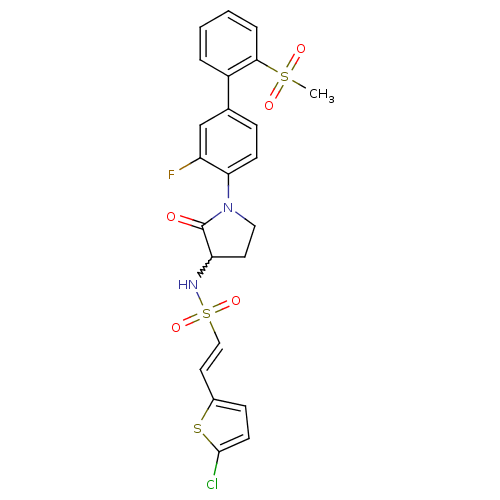
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374259
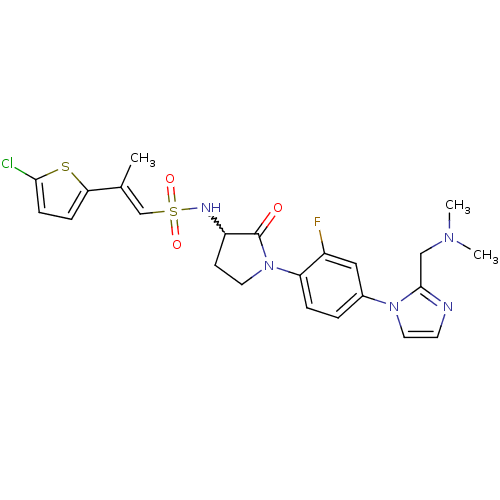
(CHEMBL257741)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C(/C)c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-15(20-6-7-21(24)34-20)14-35(32,33)27-18-8-10-30(23(18)31)19-5-4-16(12-17(19)25)29-11-9-26-22(29)13-28(2)3/h4-7,9,11-12,14,18,27H,8,10,13H2,1-3H3/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384805
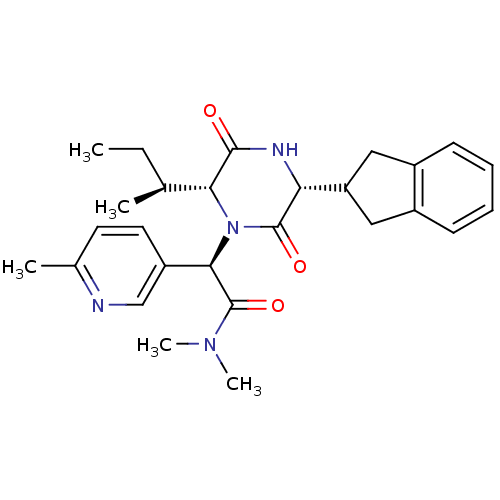
(CHEMBL2037499)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-16(2)23-25(32)29-22(21-13-18-9-7-8-10-19(18)14-21)26(33)31(23)24(27(34)30(4)5)20-12-11-17(3)28-15-20/h7-12,15-16,21-24H,6,13-14H2,1-5H3,(H,29,32)/t16-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306153
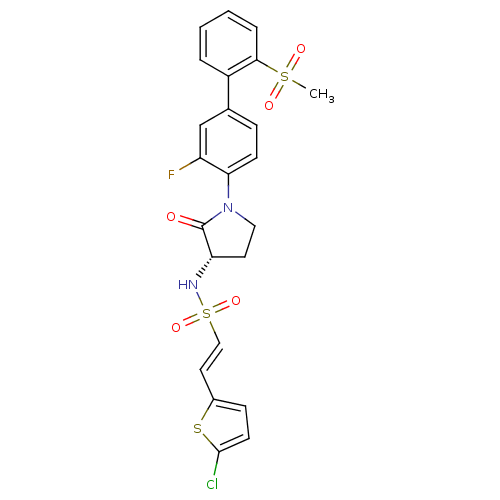
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(3-fluoro-2'-(m...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384836
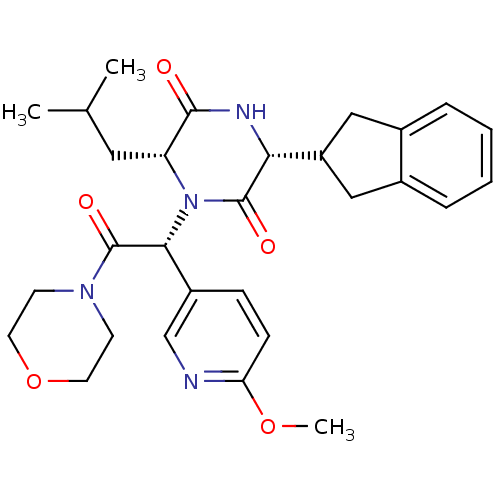
(CHEMBL2037487)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C29H36N4O5/c1-18(2)14-23-27(34)31-25(22-15-19-6-4-5-7-20(19)16-22)28(35)33(23)26(21-8-9-24(37-3)30-17-21)29(36)32-10-12-38-13-11-32/h4-9,17-18,22-23,25-26H,10-16H2,1-3H3,(H,31,34)/t23-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384812
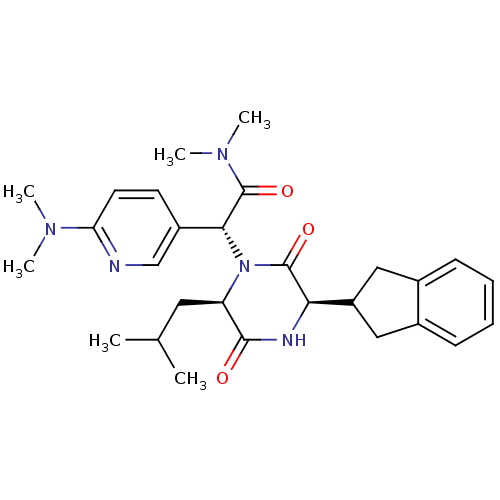
(CHEMBL2037489)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H37N5O3/c1-17(2)13-22-26(34)30-24(21-14-18-9-7-8-10-19(18)15-21)27(35)33(22)25(28(36)32(5)6)20-11-12-23(29-16-20)31(3)4/h7-12,16-17,21-22,24-25H,13-15H2,1-6H3,(H,30,34)/t22-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Capsid scaffolding protein
(Human cytomegalovirus (strain AD169) (HHV-5) (Huma...) | BDBM50133948

((S)-2-[(3aS,6S,6aR)-4-(6-Hydroxymethyl-benzothiazo...)Show SMILES CC(C)c1ccc(NC(=O)N2CCC[C@H]2C(=O)N2CC[C@H]3[C@H]2[C@H](C)C(=O)N3c2nc3ccc(CO)cc3s2)cc1 Show InChI InChI=1S/C30H35N5O4S/c1-17(2)20-7-9-21(10-8-20)31-29(39)33-13-4-5-24(33)28(38)34-14-12-23-26(34)18(3)27(37)35(23)30-32-22-11-6-19(16-36)15-25(22)40-30/h6-11,15,17-18,23-24,26,36H,4-5,12-14,16H2,1-3H3,(H,31,39)/t18-,23-,24-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Potency against human cytomegalovirus protease in HCMV pNA assay |
J Med Chem 46: 4428-49 (2003)
Article DOI: 10.1021/jm030810w
BindingDB Entry DOI: 10.7270/Q2WH2PD8 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384811
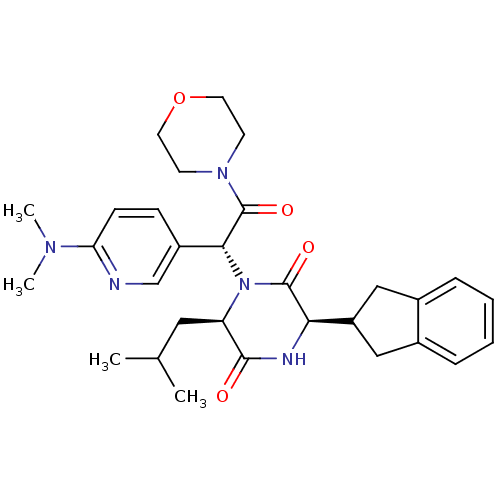
(CHEMBL2037490)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(nc2)N(C)C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H39N5O4/c1-19(2)15-24-28(36)32-26(23-16-20-7-5-6-8-21(20)17-23)29(37)35(24)27(30(38)34-11-13-39-14-12-34)22-9-10-25(31-18-22)33(3)4/h5-10,18-19,23-24,26-27H,11-17H2,1-4H3,(H,32,36)/t24-,26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384835
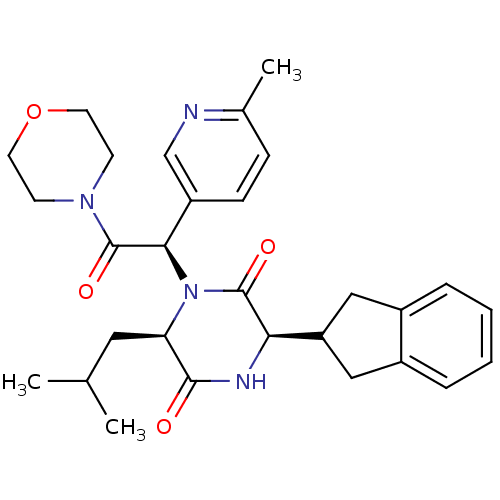
(CHEMBL2037492)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)14-24-27(34)31-25(23-15-20-6-4-5-7-21(20)16-23)28(35)33(24)26(22-9-8-19(3)30-17-22)29(36)32-10-12-37-13-11-32/h4-9,17-18,23-26H,10-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384821
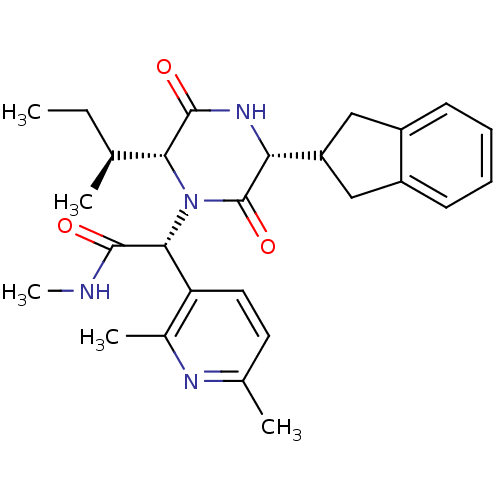
(CHEMBL2037509)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)NC)c2ccc(C)nc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C27H34N4O3/c1-6-15(2)23-26(33)30-22(20-13-18-9-7-8-10-19(18)14-20)27(34)31(23)24(25(32)28-5)21-12-11-16(3)29-17(21)4/h7-12,15,20,22-24H,6,13-14H2,1-5H3,(H,28,32)(H,30,33)/t15-,22+,23+,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384814
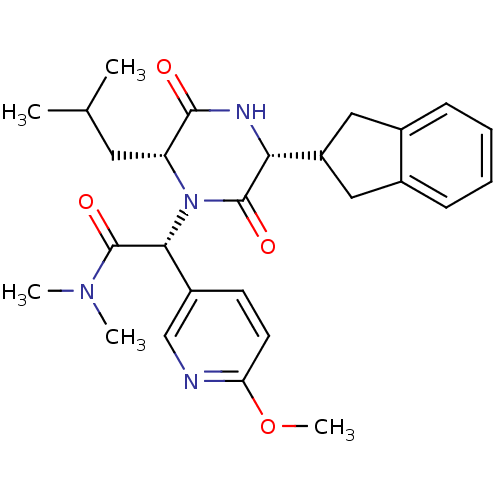
(CHEMBL2037486)Show SMILES COc1ccc(cn1)[C@@H](N1[C@H](CC(C)C)C(=O)N[C@H](C2Cc3ccccc3C2)C1=O)C(=O)N(C)C |r| Show InChI InChI=1S/C27H34N4O4/c1-16(2)12-21-25(32)29-23(20-13-17-8-6-7-9-18(17)14-20)26(33)31(21)24(27(34)30(3)4)19-10-11-22(35-5)28-15-19/h6-11,15-16,20-21,23-24H,12-14H2,1-5H3,(H,29,32)/t21-,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374262
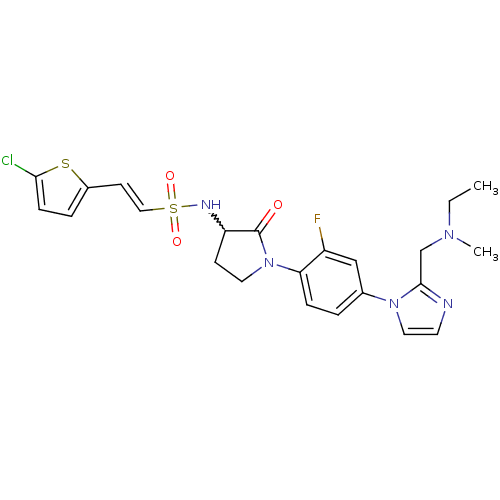
(CHEMBL272779)Show SMILES CCN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-3-28(2)15-22-26-10-12-29(22)16-4-6-20(18(25)14-16)30-11-8-19(23(30)31)27-35(32,33)13-9-17-5-7-21(24)34-17/h4-7,9-10,12-14,19,27H,3,8,11,15H2,1-2H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228954
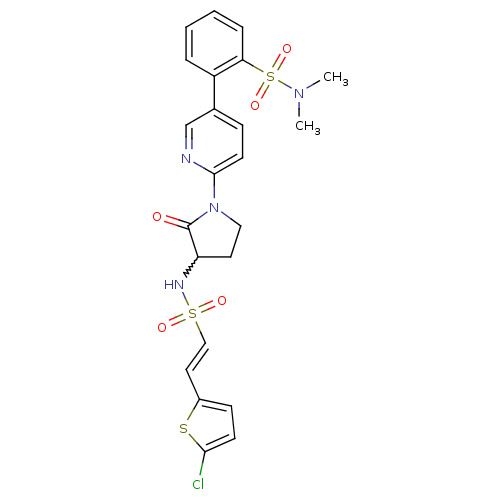
((E)-2-(6-(3-(2-(5-chlorothiophen-2-yl)vinylsulfona...)Show SMILES CN(C)S(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:21.23| Show InChI InChI=1S/C23H23ClN4O5S3/c1-27(2)36(32,33)20-6-4-3-5-18(20)16-7-10-22(25-15-16)28-13-11-19(23(28)29)26-35(30,31)14-12-17-8-9-21(24)34-17/h3-10,12,14-15,19,26H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374264

(CHEMBL257863)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)-n1ccnc1CN1CCCC1 |w:10.11| Show InChI InChI=1S/C24H25ClFN5O3S2/c25-22-6-4-18(35-22)8-14-36(33,34)28-20-7-12-31(24(20)32)21-5-3-17(15-19(21)26)30-13-9-27-23(30)16-29-10-1-2-11-29/h3-6,8-9,13-15,20,28H,1-2,7,10-12,16H2/b14-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326716
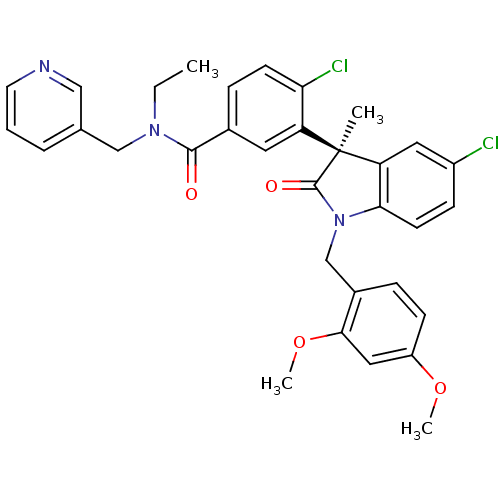
((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...)Show SMILES CCN(Cc1cccnc1)C(=O)c1ccc(Cl)c(c1)[C@]1(C)C(=O)N(Cc2ccc(OC)cc2OC)c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C33H31Cl2N3O4/c1-5-37(19-21-7-6-14-36-18-21)31(39)22-9-12-28(35)26(15-22)33(2)27-16-24(34)10-13-29(27)38(32(33)40)20-23-8-11-25(41-3)17-30(23)42-4/h6-18H,5,19-20H2,1-4H3/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human oxytocin receptor expressed in CHO cells |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374270
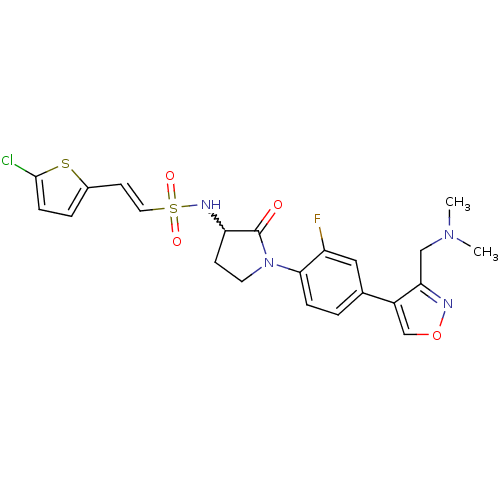
(CHEMBL271015)Show SMILES CN(C)Cc1nocc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C22H22ClFN4O4S2/c1-27(2)12-19-16(13-32-25-19)14-3-5-20(17(24)11-14)28-9-7-18(22(28)29)26-34(30,31)10-8-15-4-6-21(23)33-15/h3-6,8,10-11,13,18,26H,7,9,12H2,1-2H3/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384804
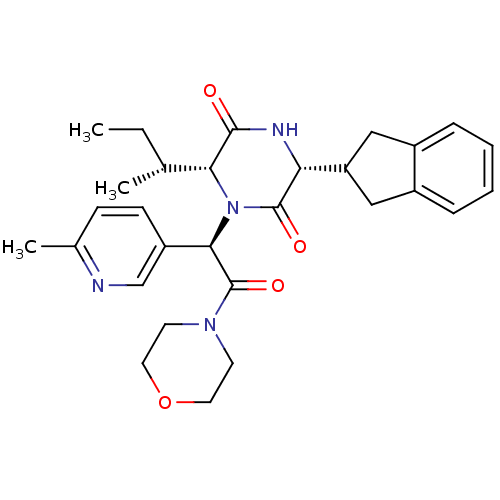
(CHEMBL2037500)Show SMILES CC[C@@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O4/c1-4-18(2)25-27(34)31-24(23-15-20-7-5-6-8-21(20)16-23)28(35)33(25)26(22-10-9-19(3)30-17-22)29(36)32-11-13-37-14-12-32/h5-10,17-18,23-26H,4,11-16H2,1-3H3,(H,31,34)/t18-,24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384809

(CHEMBL2037493)Show SMILES CC(C)C[C@H]1N([C@@H](C(=O)N2CCCC2)c2ccc(C)nc2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C29H36N4O3/c1-18(2)14-24-27(34)31-25(23-15-20-8-4-5-9-21(20)16-23)28(35)33(24)26(22-11-10-19(3)30-17-22)29(36)32-12-6-7-13-32/h4-5,8-11,17-18,23-26H,6-7,12-16H2,1-3H3,(H,31,34)/t24-,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374263
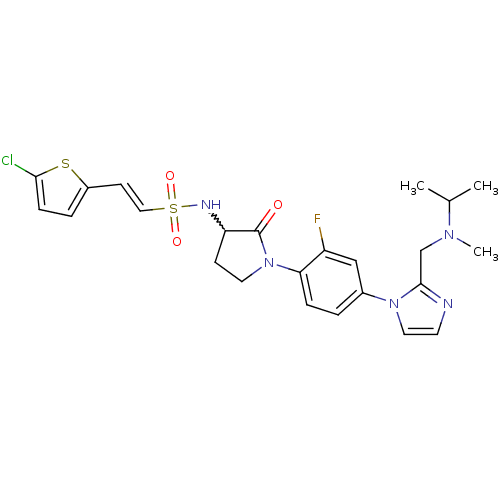
(CHEMBL437097)Show SMILES CC(C)N(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:18.19| Show InChI InChI=1S/C24H27ClFN5O3S2/c1-16(2)29(3)15-23-27-10-12-30(23)17-4-6-21(19(26)14-17)31-11-8-20(24(31)32)28-36(33,34)13-9-18-5-7-22(25)35-18/h4-7,9-10,12-14,16,20,28H,8,11,15H2,1-3H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328712
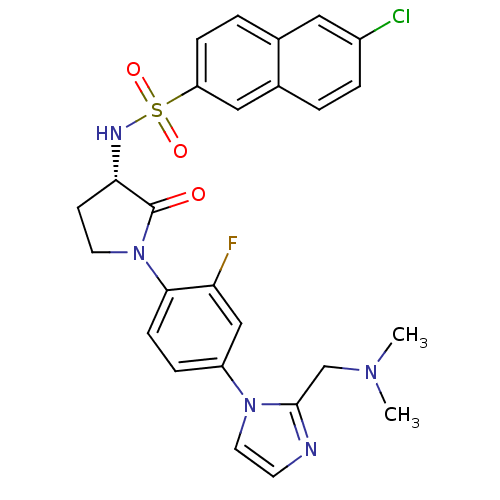
((S)-6-chloro-N-(1-(4-(2-((dimethylamino)methyl)-1H...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C26H25ClFN5O3S/c1-31(2)16-25-29-10-12-32(25)20-6-8-24(22(28)15-20)33-11-9-23(26(33)34)30-37(35,36)21-7-4-17-13-19(27)5-3-18(17)14-21/h3-8,10,12-15,23,30H,9,11,16H2,1-2H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326714

((3R,6R)-6-sec-butyl-3-(2,3-dihydro-1H-inden-2-yl)-...)Show SMILES CC[C@H](C)[C@@]1(C)N([C@@H](C(=O)N2CCOCC2)c2coc(C)n2)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O5/c1-5-17(2)28(4)27(35)30-23(21-14-19-8-6-7-9-20(19)15-21)25(33)32(28)24(22-16-37-18(3)29-22)26(34)31-10-12-36-13-11-31/h6-9,16-17,21,23-24H,5,10-15H2,1-4H3,(H,30,35)/t17-,23+,24+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from oxytocin receptor in human uterus tissue |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50326722
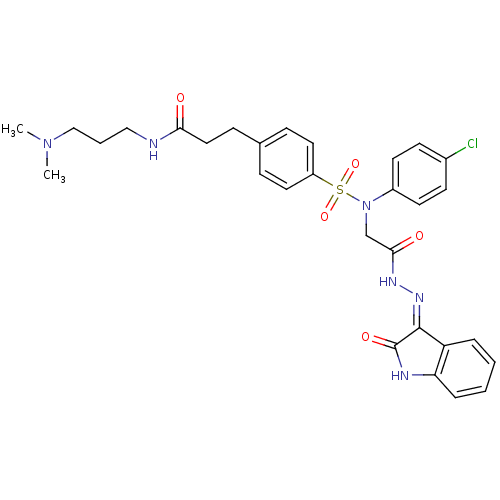
((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from vasopressin V1a receptor in human liver tissue |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326722

((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from human oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50326722

((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50326722

((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...)Show SMILES CN(C)CCCNC(=O)CCc1ccc(cc1)S(=O)(=O)N(CC(=O)N\N=C1/C(=O)Nc2ccccc12)c1ccc(Cl)cc1 Show InChI InChI=1S/C30H33ClN6O5S/c1-36(2)19-5-18-32-27(38)17-10-21-8-15-24(16-9-21)43(41,42)37(23-13-11-22(31)12-14-23)20-28(39)34-35-29-25-6-3-4-7-26(25)33-30(29)40/h3-4,6-9,11-16H,5,10,17-20H2,1-2H3,(H,32,38)(H,34,39)(H,33,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from vasopressin V2 receptor in human kidney tissue |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50326717
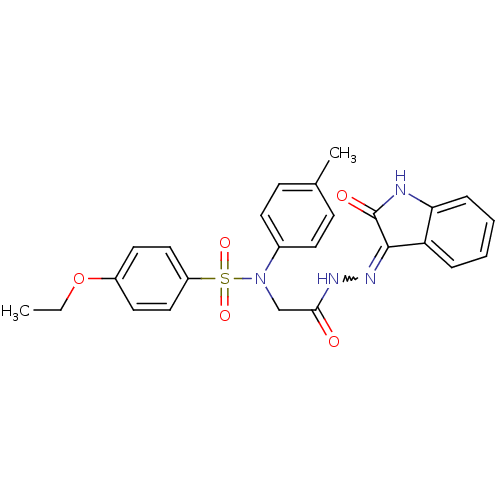
(4-ethoxy-N-(2-oxo-2-(2-(2-oxoindolin-3-ylidene)hyd...)Show SMILES CCOc1ccc(cc1)S(=O)(=O)N(CC(=O)NN=C1C(=O)Nc2ccccc12)c1ccc(C)cc1 |w:17.17| Show InChI InChI=1S/C25H24N4O5S/c1-3-34-19-12-14-20(15-13-19)35(32,33)29(18-10-8-17(2)9-11-18)16-23(30)27-28-24-21-6-4-5-7-22(21)26-25(24)31/h4-15H,3,16H2,1-2H3,(H,27,30)(H,26,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-oxytocin from rat oxytocin receptor expressed in HEK293-EBNA cells |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228940
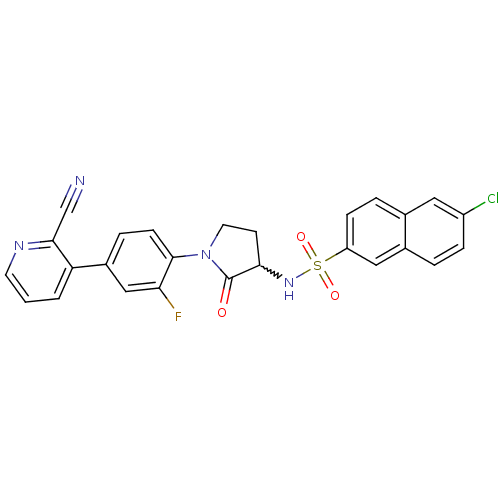
(6-chloro-N-(1-(4-(2-cyanopyridin-3-yl)-2-fluorophe...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-c1cccnc1C#N |w:10.11| Show InChI InChI=1S/C26H18ClFN4O3S/c27-19-6-3-17-13-20(7-4-16(17)12-19)36(34,35)31-23-9-11-32(26(23)33)25-8-5-18(14-22(25)28)21-2-1-10-30-24(21)15-29/h1-8,10,12-14,23,31H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50064711
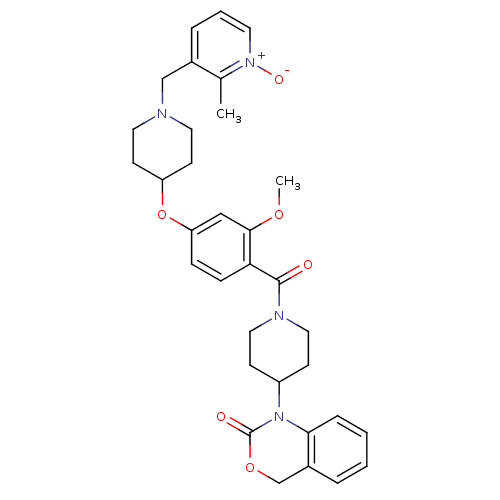
(1-(1-{2-Methoxy-4-[1-(2-methyl-1-oxy-pyridin-3-ylm...)Show SMILES COc1cc(OC2CCN(Cc3ccc[n+]([O-])c3C)CC2)ccc1C(=O)N1CCC(CC1)N1C(=O)OCc2ccccc12 Show InChI InChI=1S/C33H38N4O6/c1-23-24(7-5-15-36(23)40)21-34-16-13-27(14-17-34)43-28-9-10-29(31(20-28)41-2)32(38)35-18-11-26(12-19-35)37-30-8-4-3-6-25(30)22-42-33(37)39/h3-10,15,20,26-27H,11-14,16-19,21-22H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign
Curated by ChEMBL
| Assay Description
Displacement of [3H]-vasopressin from vasopressin V1a receptor in rat liver tissue |
J Med Chem 53: 6525-38 (2010)
Article DOI: 10.1021/jm901812z
BindingDB Entry DOI: 10.7270/Q20R9PMZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384839
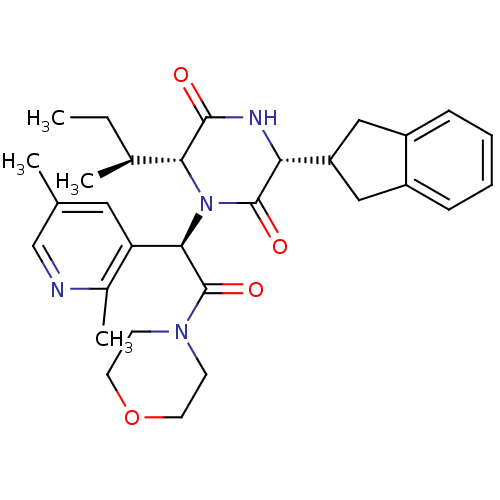
(CHEMBL2037505)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N2CCOCC2)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C30H38N4O4/c1-5-19(3)26-28(35)32-25(23-15-21-8-6-7-9-22(21)16-23)29(36)34(26)27(24-14-18(2)17-31-20(24)4)30(37)33-10-12-38-13-11-33/h6-9,14,17,19,23,25-27H,5,10-13,15-16H2,1-4H3,(H,32,35)/t19-,25+,26+,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50384801

(CHEMBL2037504)Show SMILES CC[C@H](C)[C@H]1N([C@@H](C(=O)N(C)C)c2cc(C)cnc2C)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1 |r| Show InChI InChI=1S/C28H36N4O3/c1-7-17(3)24-26(33)30-23(21-13-19-10-8-9-11-20(19)14-21)27(34)32(24)25(28(35)31(5)6)22-12-16(2)15-29-18(22)4/h8-12,15,17,21,23-25H,7,13-14H2,1-6H3,(H,30,33)/t17-,23+,24+,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
J Med Chem 55: 783-96 (2012)
Article DOI: 10.1021/jm201287w
BindingDB Entry DOI: 10.7270/Q2MS3TSH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
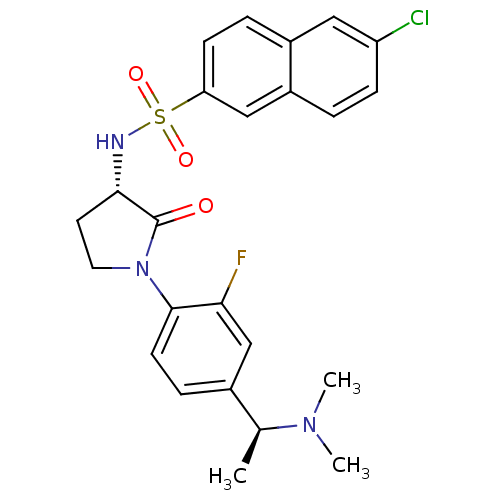
(Homo sapiens (Human)) | BDBM50306143
(6-chloro-N-((S)-1-(4-((S)-1-(dimethylamino)ethyl)-...)Show SMILES C[C@H](N(C)C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C24H25ClFN3O3S/c1-15(28(2)3)16-6-9-23(21(26)14-16)29-11-10-22(24(29)30)27-33(31,32)20-8-5-17-12-19(25)7-4-18(17)13-20/h4-9,12-15,22,27H,10-11H2,1-3H3/t15-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
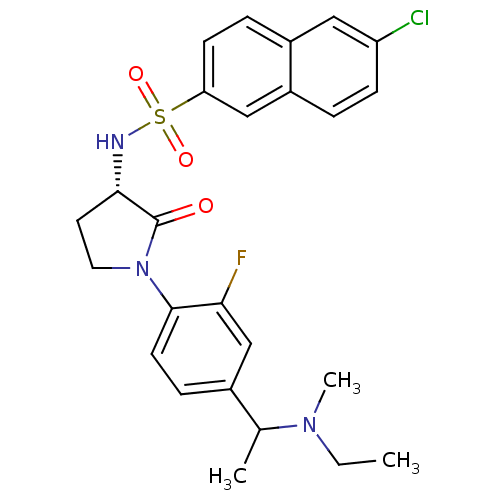
(Homo sapiens (Human)) | BDBM50306142
(6-chloro-N-((3S)-1-(4-(1-(ethyl(methyl)amino)ethyl...)Show SMILES CCN(C)C(C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H27ClFN3O3S/c1-4-29(3)16(2)17-7-10-24(22(27)15-17)30-12-11-23(25(30)31)28-34(32,33)21-9-6-18-13-20(26)8-5-19(18)14-21/h5-10,13-16,23,28H,4,11-12H2,1-3H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data