 Found 413 hits with Last Name = 'nolting' and Initial = 'af'
Found 413 hits with Last Name = 'nolting' and Initial = 'af' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125979
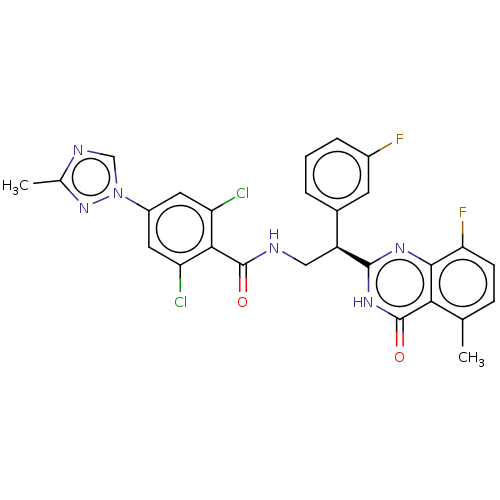
(CHEMBL3627899)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2nc3c(F)ccc(C)c3c(=O)[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2F2N6O2/c1-13-6-7-21(31)24-22(13)27(39)35-25(34-24)18(15-4-3-5-16(30)8-15)11-32-26(38)23-19(28)9-17(10-20(23)29)37-12-33-14(2)36-37/h3-10,12,18H,11H2,1-2H3,(H,32,38)(H,34,35,39)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125972
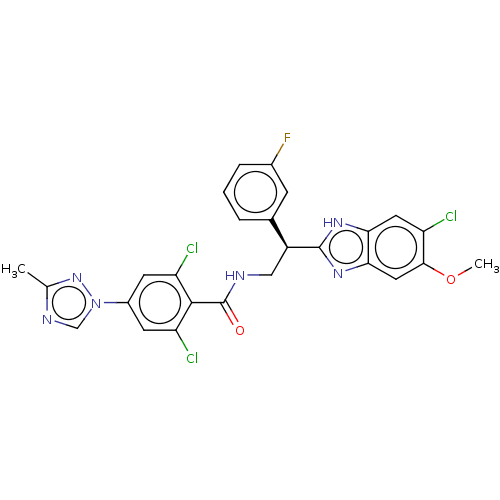
(CHEMBL3627894)Show SMILES COc1cc2nc([nH]c2cc1Cl)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O2/c1-13-32-12-36(35-13)16-7-19(28)24(20(29)8-16)26(37)31-11-17(14-4-3-5-15(30)6-14)25-33-21-9-18(27)23(38-2)10-22(21)34-25/h3-10,12,17H,11H2,1-2H3,(H,31,37)(H,33,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285852
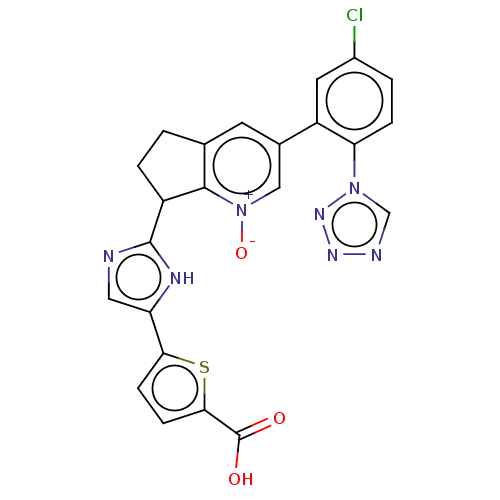
(5-(2-{3-[5-chloro-2- (1H-tetrazol-1- yl)phenyl]-1-...)Show SMILES OC(=O)c1ccc(s1)-c1cnc([nH]1)C1CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125980
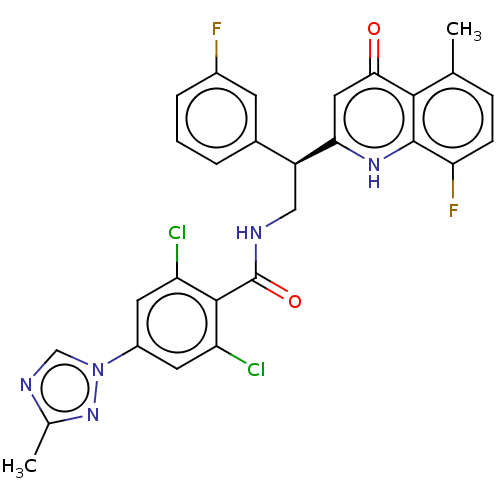
(CHEMBL3627900)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(=O)c3c(C)ccc(F)c3[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O2/c1-14-6-7-22(32)27-25(14)24(38)11-23(35-27)19(16-4-3-5-17(31)8-16)12-33-28(39)26-20(29)9-18(10-21(26)30)37-13-34-15(2)36-37/h3-11,13,19H,12H2,1-2H3,(H,33,39)(H,35,38)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125978
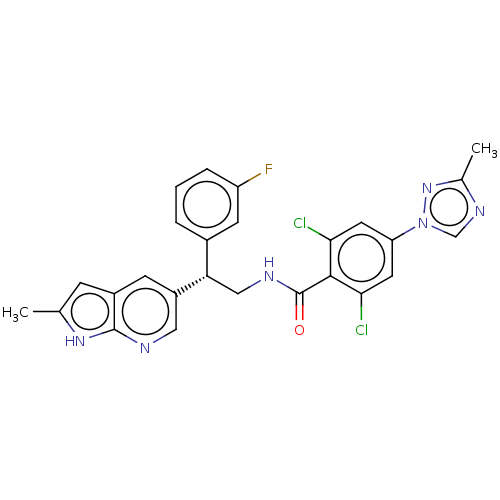
(CHEMBL3627898 | US10189819, Example 77)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cnc3[nH]c(C)cc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-6-17-7-18(11-30-25(17)33-14)21(16-4-3-5-19(29)8-16)12-31-26(36)24-22(27)9-20(10-23(24)28)35-13-32-15(2)34-35/h3-11,13,21H,12H2,1-2H3,(H,30,33)(H,31,36)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125960
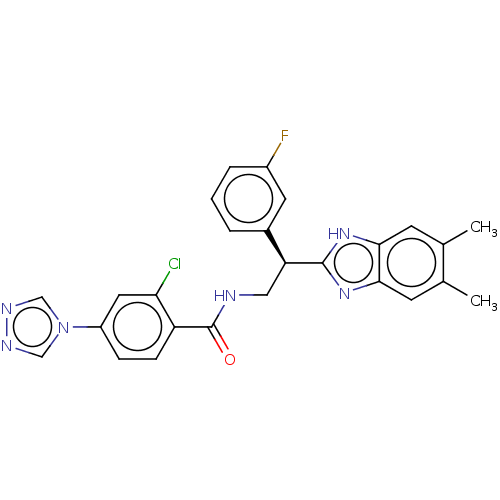
(CHEMBL3627871)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-8-23-24(9-16(15)2)33-25(32-23)21(17-4-3-5-18(28)10-17)12-29-26(35)20-7-6-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125976
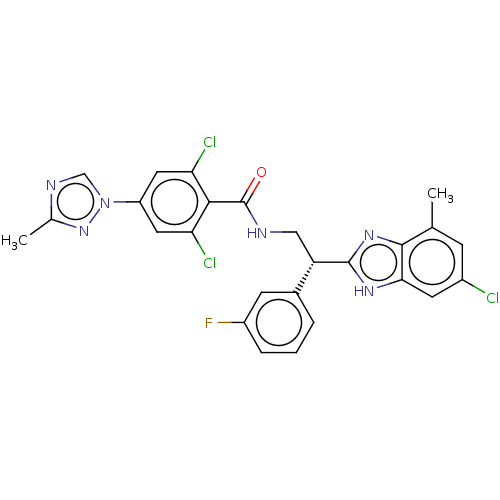
(CHEMBL3627896)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3c(C)cc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O/c1-13-6-16(27)8-22-24(13)34-25(33-22)19(15-4-3-5-17(30)7-15)11-31-26(37)23-20(28)9-18(10-21(23)29)36-12-32-14(2)35-36/h3-10,12,19H,11H2,1-2H3,(H,31,37)(H,33,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125965
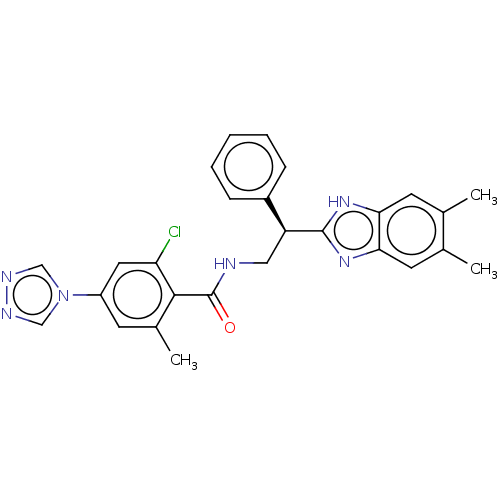
(CHEMBL3627866)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(C)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C27H25ClN6O/c1-16-10-23-24(11-17(16)2)33-26(32-23)21(19-7-5-4-6-8-19)13-29-27(35)25-18(3)9-20(12-22(25)28)34-14-30-31-15-34/h4-12,14-15,21H,13H2,1-3H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125973
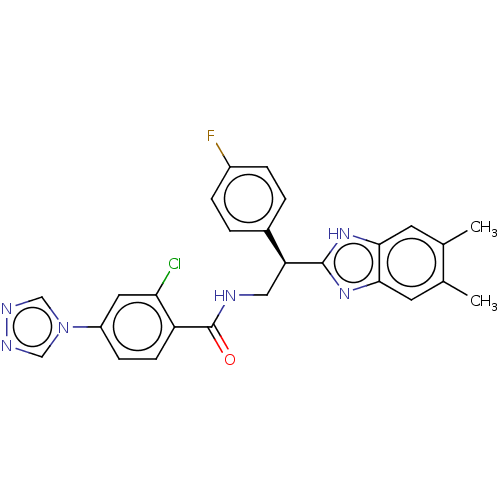
(CHEMBL3627872)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-9-23-24(10-16(15)2)33-25(32-23)21(17-3-5-18(28)6-4-17)12-29-26(35)20-8-7-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125977
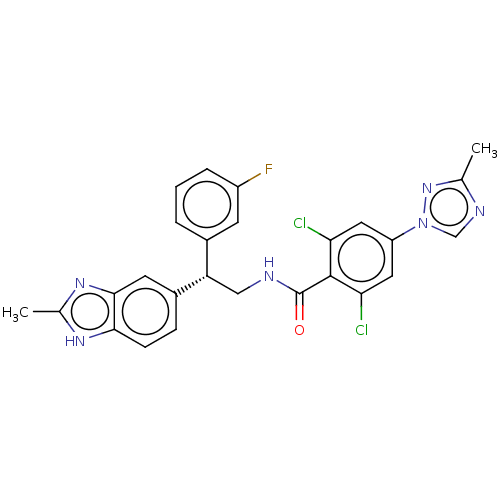
(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125974
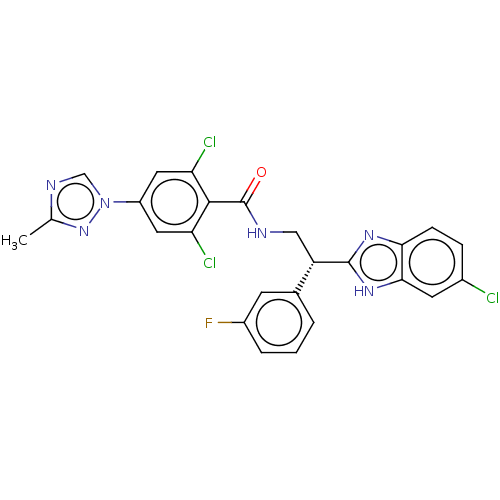
(CHEMBL3627895 | US10189819, Example 46)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3ccc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C25H18Cl3FN6O/c1-13-31-12-35(34-13)17-9-19(27)23(20(28)10-17)25(36)30-11-18(14-3-2-4-16(29)7-14)24-32-21-6-5-15(26)8-22(21)33-24/h2-10,12,18H,11H2,1H3,(H,30,36)(H,32,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125981

(CHEMBL3627901 | US10189819, Example 113)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3c(C)ccc(F)c3n2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O/c1-15-6-8-24(32)27-20(15)7-9-25(35-27)21(17-4-3-5-18(31)10-17)13-33-28(38)26-22(29)11-19(12-23(26)30)37-14-34-16(2)36-37/h3-12,14,21H,13H2,1-2H3,(H,33,38)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125971
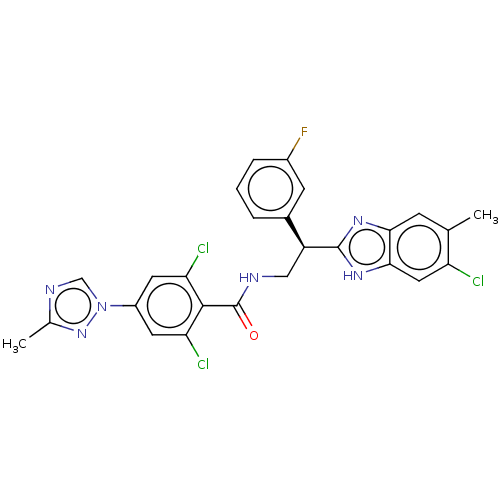
(CHEMBL3627893)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3cc(C)c(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O/c1-13-6-22-23(10-19(13)27)34-25(33-22)18(15-4-3-5-16(30)7-15)11-31-26(37)24-20(28)8-17(9-21(24)29)36-12-32-14(2)35-36/h3-10,12,18H,11H2,1-2H3,(H,31,37)(H,33,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125970
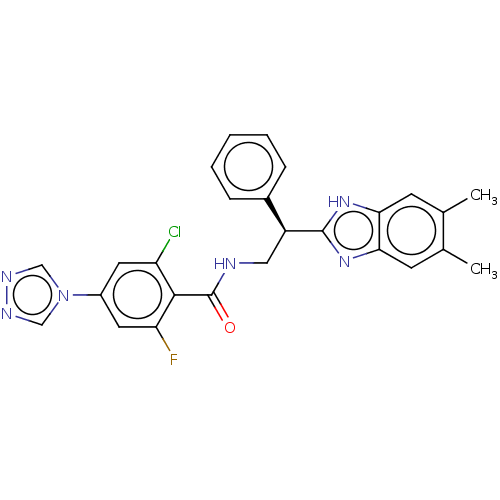
(CHEMBL3627865)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(F)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125963
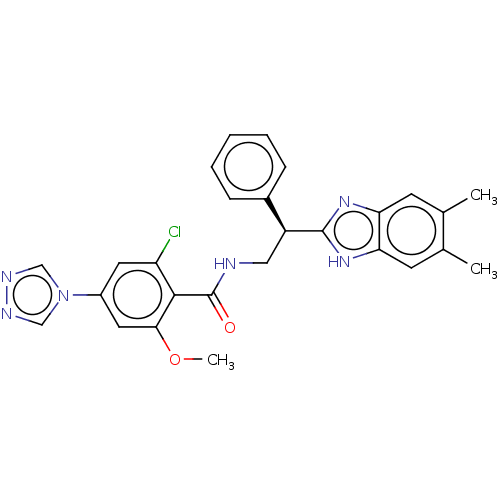
(CHEMBL3627868)Show SMILES COc1cc(cc(Cl)c1C(=O)NC[C@H](c1nc2cc(C)c(C)cc2[nH]1)c1ccccc1)-n1cnnc1 |r| Show InChI InChI=1S/C27H25ClN6O2/c1-16-9-22-23(10-17(16)2)33-26(32-22)20(18-7-5-4-6-8-18)13-29-27(35)25-21(28)11-19(12-24(25)36-3)34-14-30-31-15-34/h4-12,14-15,20H,13H2,1-3H3,(H,29,35)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125975
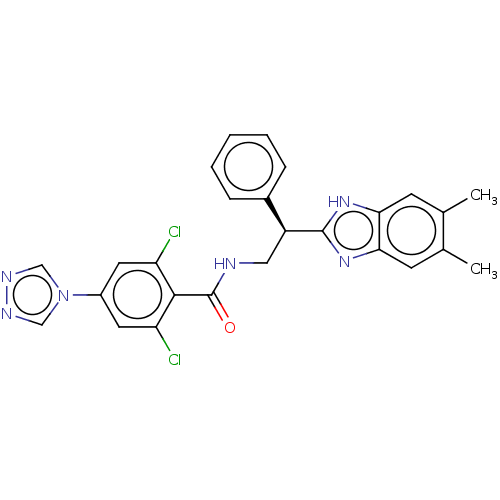
(CHEMBL3627873)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22Cl2N6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125975

(CHEMBL3627873)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22Cl2N6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50083254

(CHEMBL3423058)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H23ClN6O/c1-16-10-23-24(11-17(16)2)32-25(31-23)21(18-6-4-3-5-7-18)13-28-26(34)20-9-8-19(12-22(20)27)33-14-29-30-15-33/h3-12,14-15,21H,13H2,1-2H3,(H,28,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125961
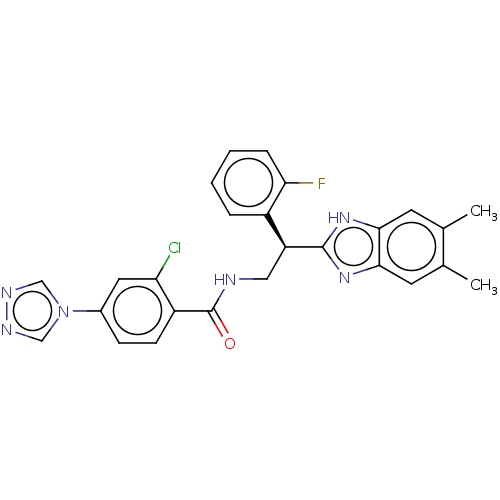
(CHEMBL3627870)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1F |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-9-23-24(10-16(15)2)33-25(32-23)20(18-5-3-4-6-22(18)28)12-29-26(35)19-8-7-17(11-21(19)27)34-13-30-31-14-34/h3-11,13-14,20H,12H2,1-2H3,(H,29,35)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125962
(CHEMBL3627869)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3cc(C)c(C)cc3[nH]2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C27H24Cl2N6O/c1-15-9-23-24(10-16(15)2)33-26(32-23)20(18-7-5-4-6-8-18)13-30-27(36)25-21(28)11-19(12-22(25)29)35-14-31-17(3)34-35/h4-12,14,20H,13H2,1-3H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125964

(CHEMBL3627867)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1C(F)(F)F)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C27H22ClF3N6O/c1-15-8-22-23(9-16(15)2)36-25(35-22)19(17-6-4-3-5-7-17)12-32-26(38)24-20(27(29,30)31)10-18(11-21(24)28)37-13-33-34-14-37/h3-11,13-14,19H,12H2,1-2H3,(H,32,38)(H,35,36)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125975

(CHEMBL3627873)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22Cl2N6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125975

(CHEMBL3627873)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22Cl2N6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125965

(CHEMBL3627866)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(C)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C27H25ClN6O/c1-16-10-23-24(11-17(16)2)33-26(32-23)21(19-7-5-4-6-8-19)13-29-27(35)25-18(3)9-20(12-22(25)28)34-14-30-31-15-34/h4-12,14-15,21H,13H2,1-3H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125963

(CHEMBL3627868)Show SMILES COc1cc(cc(Cl)c1C(=O)NC[C@H](c1nc2cc(C)c(C)cc2[nH]1)c1ccccc1)-n1cnnc1 |r| Show InChI InChI=1S/C27H25ClN6O2/c1-16-9-22-23(10-17(16)2)33-26(32-22)20(18-7-5-4-6-8-18)13-29-27(35)25-21(28)11-19(12-24(25)36-3)34-14-30-31-15-34/h4-12,14-15,20H,13H2,1-3H3,(H,29,35)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125964

(CHEMBL3627867)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(Cl)cc(cc1C(F)(F)F)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C27H22ClF3N6O/c1-15-8-22-23(9-16(15)2)36-25(35-22)19(17-6-4-3-5-7-17)12-32-26(38)24-20(27(29,30)31)10-18(11-21(24)28)37-13-33-34-14-37/h3-11,13-14,19H,12H2,1-2H3,(H,32,38)(H,35,36)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50083254

(CHEMBL3423058)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H23ClN6O/c1-16-10-23-24(11-17(16)2)32-25(31-23)21(18-6-4-3-5-7-18)13-28-26(34)20-9-8-19(12-22(20)27)33-14-29-30-15-33/h3-12,14-15,21H,13H2,1-2H3,(H,28,34)(H,31,32)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125961

(CHEMBL3627870)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccccc1F |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-9-23-24(10-16(15)2)33-25(32-23)20(18-5-3-4-6-22(18)28)12-29-26(35)19-8-7-17(11-21(19)27)34-13-30-31-14-34/h3-11,13-14,20H,12H2,1-2H3,(H,29,35)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 412 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125970

(CHEMBL3627865)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1c(F)cc(cc1Cl)-n1cnnc1)c1ccccc1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-8-22-23(9-16(15)2)33-25(32-22)19(17-6-4-3-5-7-17)12-29-26(35)24-20(27)10-18(11-21(24)28)34-13-30-31-14-34/h3-11,13-14,19H,12H2,1-2H3,(H,29,35)(H,32,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 544 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125960

(CHEMBL3627871)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-8-23-24(9-16(15)2)33-25(32-23)21(17-4-3-5-18(28)10-17)12-29-26(35)20-7-6-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125973

(CHEMBL3627872)Show SMILES Cc1cc2nc([nH]c2cc1C)[C@@H](CNC(=O)c1ccc(cc1Cl)-n1cnnc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H22ClFN6O/c1-15-9-23-24(10-16(15)2)33-25(32-23)21(17-3-5-18(28)6-4-17)12-29-26(35)20-8-7-19(11-22(20)27)34-13-30-31-14-34/h3-11,13-14,21H,12H2,1-2H3,(H,29,35)(H,32,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125972

(CHEMBL3627894)Show SMILES COc1cc2nc([nH]c2cc1Cl)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O2/c1-13-32-12-36(35-13)16-7-19(28)24(20(29)8-16)26(37)31-11-17(14-4-3-5-15(30)6-14)25-33-21-9-18(27)23(38-2)10-22(21)34-25/h3-10,12,17H,11H2,1-2H3,(H,31,37)(H,33,34)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125962

(CHEMBL3627869)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3cc(C)c(C)cc3[nH]2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C27H24Cl2N6O/c1-15-9-23-24(10-16(15)2)33-26(32-23)20(18-7-5-4-6-8-18)13-30-27(36)25-21(28)11-19(12-22(25)29)35-14-31-17(3)34-35/h4-12,14,20H,13H2,1-3H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125977

(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125971

(CHEMBL3627893)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3cc(C)c(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O/c1-13-6-22-23(10-19(13)27)34-25(33-22)18(15-4-3-5-16(30)7-15)11-31-26(37)24-20(28)8-17(9-21(24)29)36-12-32-14(2)35-36/h3-10,12,18H,11H2,1-2H3,(H,31,37)(H,33,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125974

(CHEMBL3627895 | US10189819, Example 46)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3ccc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C25H18Cl3FN6O/c1-13-31-12-35(34-13)17-9-19(27)23(20(28)10-17)25(36)30-11-18(14-3-2-4-16(29)7-14)24-32-21-6-5-15(26)8-22(21)33-24/h2-10,12,18H,11H2,1H3,(H,30,36)(H,32,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125978

(CHEMBL3627898 | US10189819, Example 77)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cnc3[nH]c(C)cc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-6-17-7-18(11-30-25(17)33-14)21(16-4-3-5-19(29)8-16)12-31-26(36)24-22(27)9-20(10-23(24)28)35-13-32-15(2)34-35/h3-11,13,21H,12H2,1-2H3,(H,30,33)(H,31,36)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125980

(CHEMBL3627900)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(=O)c3c(C)ccc(F)c3[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O2/c1-14-6-7-22(32)27-25(14)24(38)11-23(35-27)19(16-4-3-5-17(31)8-16)12-33-28(39)26-20(29)9-18(10-21(26)30)37-13-34-15(2)36-37/h3-11,13,19H,12H2,1-2H3,(H,33,39)(H,35,38)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125981

(CHEMBL3627901 | US10189819, Example 113)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3c(C)ccc(F)c3n2)c(Cl)c1 |r| Show InChI InChI=1S/C28H21Cl2F2N5O/c1-15-6-8-24(32)27-20(15)7-9-25(35-27)21(17-4-3-5-18(31)10-17)13-33-28(38)26-22(29)11-19(12-23(26)30)37-14-34-16(2)36-37/h3-12,14,21H,13H2,1-2H3,(H,33,38)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125979

(CHEMBL3627899)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2nc3c(F)ccc(C)c3c(=O)[nH]2)c(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2F2N6O2/c1-13-6-7-21(31)24-22(13)27(39)35-25(34-24)18(15-4-3-5-16(30)8-15)11-32-26(38)23-19(28)9-17(10-20(23)29)37-12-33-14(2)36-37/h3-10,12,18H,11H2,1-2H3,(H,32,38)(H,34,35,39)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125976

(CHEMBL3627896)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@H](c2nc3c(C)cc(Cl)cc3[nH]2)c2cccc(F)c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O/c1-13-6-16(27)8-22-24(13)34-25(33-22)19(15-4-3-5-17(30)7-15)11-31-26(37)23-20(28)9-18(10-21(23)29)36-12-32-14(2)35-36/h3-10,12,19H,11H2,1-2H3,(H,31,37)(H,33,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 10a using fluorescent peptide nAcetyl-KPR-AFC as substrate |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285900
(7-(5-(5-carboxythiophen-3-yl)-4-chloro-1H-imidazol...)Show SMILES COC(=O)c1cc(cs1)-c1[nH]c(nc1Cl)C1(O)CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1NC(=O)OC(C)(C)C | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285973
((7S)-7-[5-(4-aminophenyl)-4- chloro-1H-imidazol-2-...)Show SMILES Nc1ccc(cc1)-c1[nH]c(nc1Cl)[C@]1(O)CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.207 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285883
((S)-methyl [4-(2-{3-[5- chloro-2-(1H-tetrazol-1- y...)Show SMILES COC(=O)Nc1ccc(-c2cnc([nH]2)[C@]2(O)CCc3cc(c[n+]([O-])c23)-c2cc(Cl)ccc2-n2cnnn2)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285860
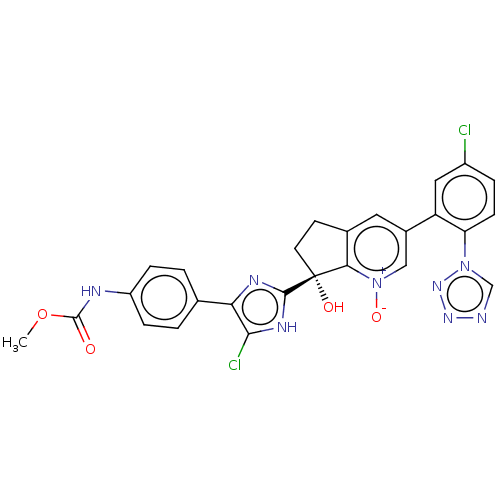
(US10081617, Example 9)Show SMILES COC(=O)Nc1ccc(cc1)-c1nc([nH]c1Cl)[C@@]1(O)CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285898
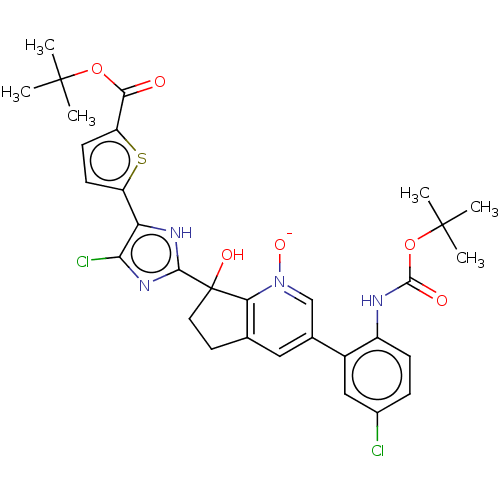
(7-(5-(5-carboxythiophen-2-yl)-4-chloro-1H-imidazol...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(Cl)cc1C1=CC2=C([N]([O-])=C1)C(O)(CC2)c1nc(Cl)c([nH]1)-c1ccc(s1)C(=O)OC(C)(C)C |c:18,21,t:16| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285953
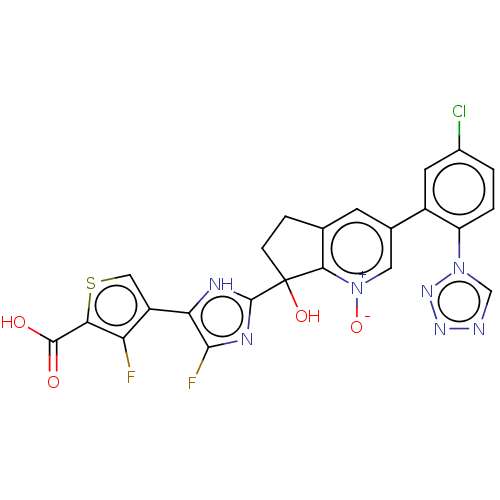
(4-(2-{3-[5-chloro-2-(1H- tetrazol-1-yl)phenyl]-7- ...)Show SMILES OC(=O)c1scc(c1F)-c1[nH]c(nc1F)C1(O)CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285959
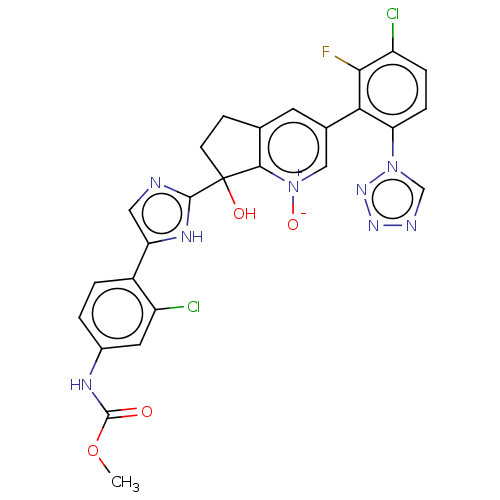
(US10081617, Example 106 | US10081617, Example 107 ...)Show SMILES COC(=O)Nc1ccc(-c2cnc([nH]2)C2(O)CCc3cc(c[n+]([O-])c23)-c2c(F)c(Cl)ccc2-n2cnnn2)c(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285853
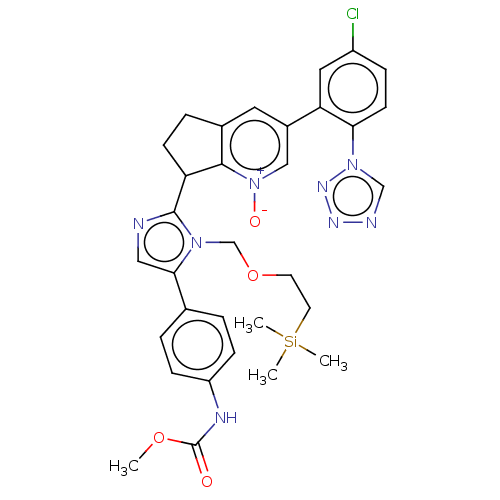
(Methyl [4-(2-{3-[5-chloro-2-(1H-tetrazol-1-yl)phen...)Show SMILES C[Si](C)(C)CCOCCl.OC(=O)C1CCc2cc(Br)cnc12.COC(=O)Nc1ccc(cc1)C(=O)CCl.CC1(C)OB(OC1(C)C)c1cc(Cl)ccc1N.COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C1CCc2cc(Br)cnc12.COC(=O)Nc1ccc(cc1)C(=O)COC(=O)C1CCc2cc(Br)cnc12.COC(=O)Nc1ccc(cc1)-c1cnc(C2CCc3cc(Br)cnc23)n1COCC[Si](C)(C)C.COC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C1CCc2cc(cnc12)-c1cc(Cl)ccc1-n1cnnn1.COC(=O)Nc1ccc(cc1)-c1cnc(C2CCc3cc(cnc23)-c2cc(Cl)ccc2N)n1COCC[Si](C)(C)C.COC(=O)Nc1ccc(cc1)-c1cnc(C2CCc3cc(cnc23)-c2cc(Cl)ccc2-n2cnnn2)n1COCC[Si](C)(C)C.COC(=O)Nc1ccc(cc1)-c1cnc(C2CCc3cc(c[n+]([O-])c23)-c2cc(Cl)ccc2-n2cnnn2)n1COCC[Si](C)(C)C |$;;;;;;;;;;;;;;;;;;;;;;;;;;HN;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;HN;;;;;;;;;;;HN;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;HN;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;$| Show InChI InChI=1S/C32H35ClN8O4Si/c1-44-32(42)36-25-9-5-21(6-10-25)29-17-34-31(39(29)20-45-13-14-46(2,3)4)26-11-7-22-15-23(18-41(43)30(22)26)27-16-24(33)8-12-28(27)40-19-35-37-38-40/h5-6,8-10,12,15-19,26H,7,11,13-14,20H2,1-4H3,(H,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM285890
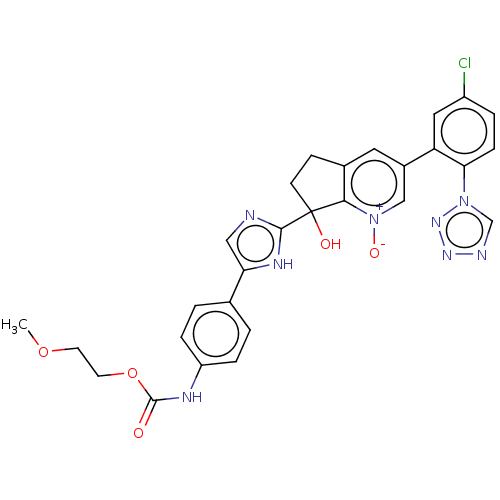
(2-methoxyethyl [4-(2-{3- [5-chloro-2-(1H-tetrazol-...)Show SMILES COCCOC(=O)Nc1ccc(cc1)-c1cnc([nH]1)C1(O)CCc2cc(c[n+]([O-])c12)-c1cc(Cl)ccc1-n1cnnn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US10081617 (2018)
BindingDB Entry DOI: 10.7270/Q2CR5WDN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data