 Found 293 hits with Last Name = 'bax' and Initial = 'bd'
Found 293 hits with Last Name = 'bax' and Initial = 'bd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50172920
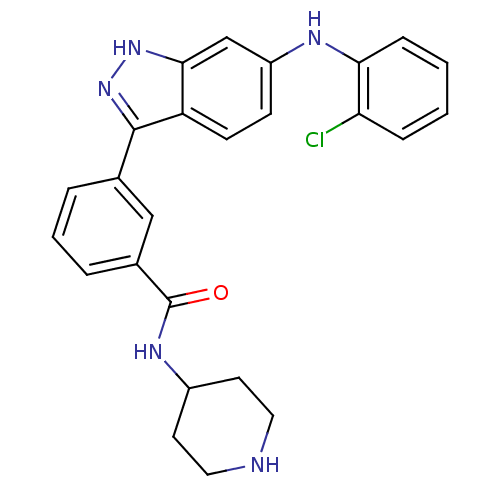
(3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NC1CCNCC1 Show InChI InChI=1S/C25H24ClN5O/c26-21-6-1-2-7-22(21)28-19-8-9-20-23(15-19)30-31-24(20)16-4-3-5-17(14-16)25(32)29-18-10-12-27-13-11-18/h1-9,14-15,18,27-28H,10-13H2,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50172920

(3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NC1CCNCC1 Show InChI InChI=1S/C25H24ClN5O/c26-21-6-1-2-7-22(21)28-19-8-9-20-23(15-19)30-31-24(20)16-4-3-5-17(14-16)25(32)29-18-10-12-27-13-11-18/h1-9,14-15,18,27-28H,10-13H2,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50178832
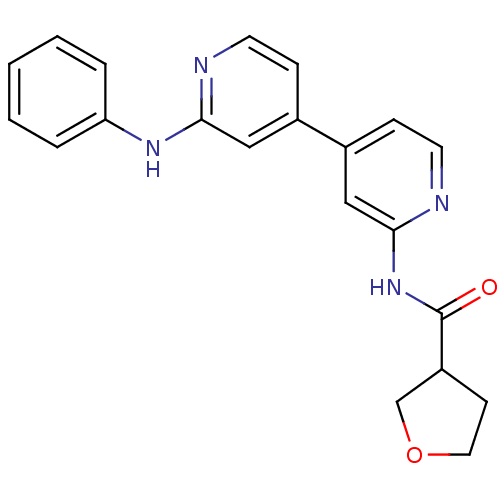
(CHEMBL203535 | N-(2'-(phenylamino)-4,4'-bipyridin-...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)C1CCOC1 Show InChI InChI=1S/C21H20N4O2/c26-21(17-8-11-27-14-17)25-20-13-16(7-10-23-20)15-6-9-22-19(12-15)24-18-4-2-1-3-5-18/h1-7,9-10,12-13,17H,8,11,14H2,(H,22,24)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510295
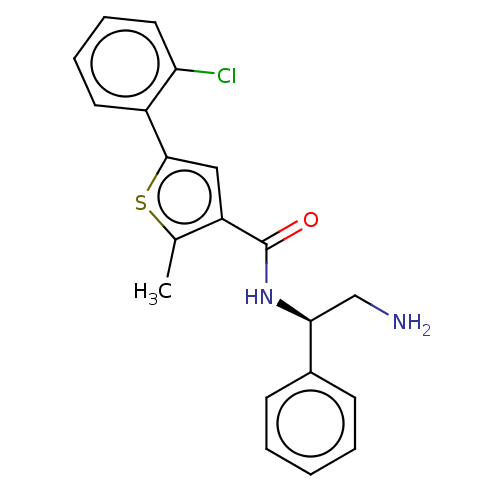
(CHEMBL4443422)Show SMILES Cc1sc(cc1C(=O)N[C@@H](CN)c1ccccc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C20H19ClN2OS/c1-13-16(11-19(25-13)15-9-5-6-10-17(15)21)20(24)23-18(12-22)14-7-3-2-4-8-14/h2-11,18H,12,22H2,1H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415616
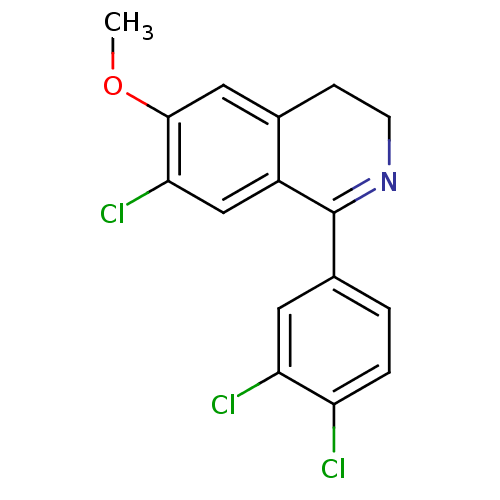
(CHEMBL1087421)Show SMILES COc1cc2CCN=C(c3ccc(Cl)c(Cl)c3)c2cc1Cl |t:7| Show InChI InChI=1S/C16H12Cl3NO/c1-21-15-7-9-4-5-20-16(11(9)8-14(15)19)10-2-3-12(17)13(18)6-10/h2-3,6-8H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal histidine-tagged human full length JNK3 by radiometric filter binding assay |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50172920

(3-(6-(2-chlorophenylamino)-1H-indazol-3-yl)-N-(pip...)Show SMILES Clc1ccccc1Nc1ccc2c(n[nH]c2c1)-c1cccc(c1)C(=O)NC1CCNCC1 Show InChI InChI=1S/C25H24ClN5O/c26-21-6-1-2-7-22(21)28-19-8-9-20-23(15-19)30-31-24(20)16-4-3-5-17(14-16)25(32)29-18-10-12-27-13-11-18/h1-9,14-15,18,27-28H,10-13H2,(H,29,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334942

(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C16H15F3N2O/c1-10(22)11-6-8-12(9-7-11)21-14-5-3-2-4-13(14)15(20-21)16(17,18)19/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8370
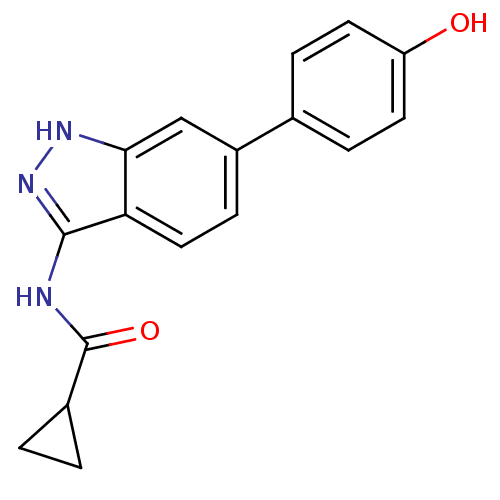
(N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...)Show InChI InChI=1S/C17H15N3O2/c21-13-6-3-10(4-7-13)12-5-8-14-15(9-12)19-20-16(14)18-17(22)11-1-2-11/h3-9,11,21H,1-2H2,(H2,18,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50279327
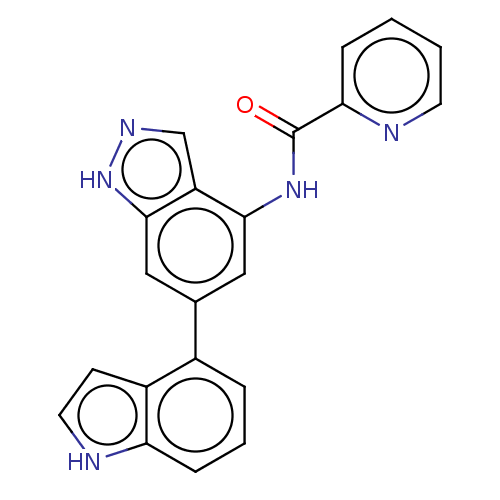
(CHEMBL4165288)Show SMILES O=C(Nc1cc(cc2[nH]ncc12)-c1cccc2[nH]ccc12)c1ccccn1 Show InChI InChI=1S/C21H15N5O/c27-21(18-5-1-2-8-22-18)25-19-10-13(11-20-16(19)12-24-26-20)14-4-3-6-17-15(14)7-9-23-17/h1-12,23H,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415620

(CHEMBL1088633)Show InChI InChI=1S/C16H13Cl2NO/c1-20-15-8-10-5-6-19-16(13(10)9-14(15)18)11-3-2-4-12(17)7-11/h2-4,7-9H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal histidine-tagged human full length JNK3 by radiometric filter binding assay |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50279329
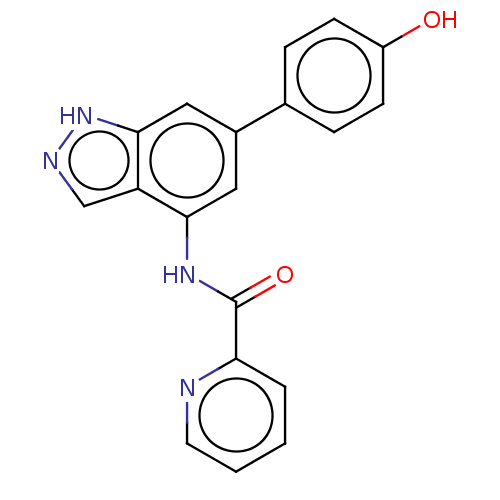
(CHEMBL4161596)Show SMILES Oc1ccc(cc1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-6-4-12(5-7-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-3-1-2-8-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510288
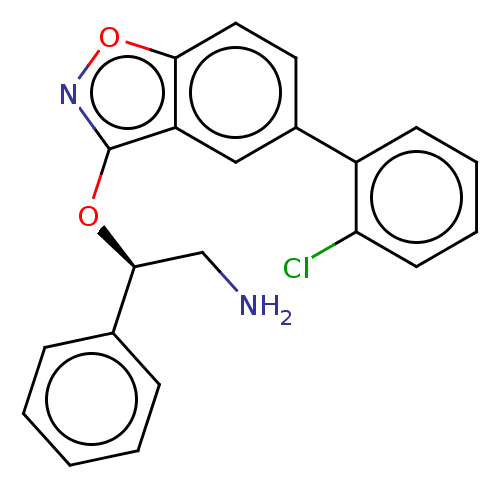
(CHEMBL4445556)Show SMILES NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C21H17ClN2O2/c22-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(24-26-19)25-20(13-23)14-6-2-1-3-7-14/h1-12,20H,13,23H2/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510294
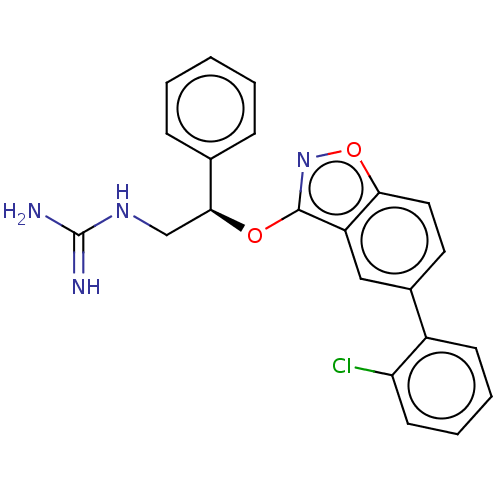
(CHEMBL4592805)Show SMILES NC(=N)NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C22H19ClN4O2/c23-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(27-29-19)28-20(13-26-22(24)25)14-6-2-1-3-7-14/h1-12,20H,13H2,(H4,24,25,26)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50178832

(CHEMBL203535 | N-(2'-(phenylamino)-4,4'-bipyridin-...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)C1CCOC1 Show InChI InChI=1S/C21H20N4O2/c26-21(17-8-11-27-14-17)25-20-13-16(7-10-23-20)15-6-9-22-19(12-15)24-18-4-2-1-3-5-18/h1-7,9-10,12-13,17H,8,11,14H2,(H,22,24)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334936
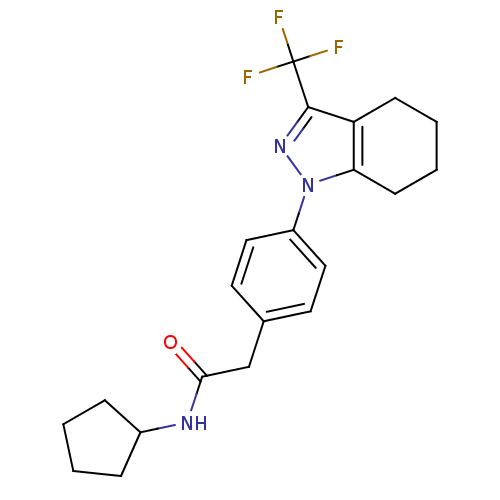
(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415620

(CHEMBL1088633)Show InChI InChI=1S/C16H13Cl2NO/c1-20-15-8-10-5-6-19-16(13(10)9-14(15)18)11-3-2-4-12(17)7-11/h2-4,7-9H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334935
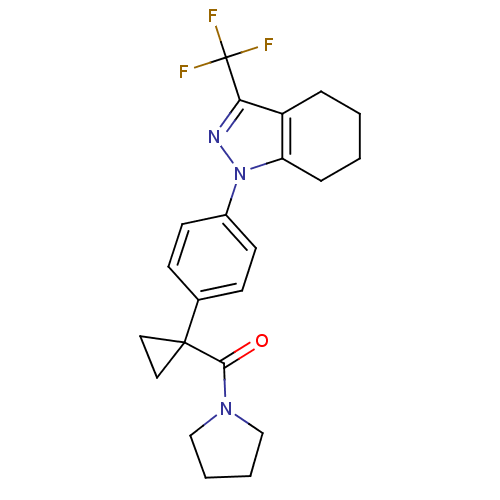
(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50279345
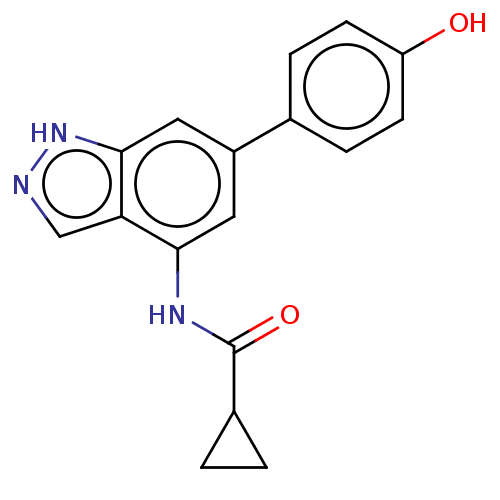
(CHEMBL4172211)Show InChI InChI=1S/C17H15N3O2/c21-13-5-3-10(4-6-13)12-7-15(19-17(22)11-1-2-11)14-9-18-20-16(14)8-12/h3-9,11,21H,1-2H2,(H,18,20)(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415616

(CHEMBL1087421)Show SMILES COc1cc2CCN=C(c3ccc(Cl)c(Cl)c3)c2cc1Cl |t:7| Show InChI InChI=1S/C16H12Cl3NO/c1-21-15-7-9-4-5-20-16(11(9)8-14(15)19)10-2-3-12(17)13(18)6-10/h2-3,6-8H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50178832

(CHEMBL203535 | N-(2'-(phenylamino)-4,4'-bipyridin-...)Show SMILES O=C(Nc1cc(ccn1)-c1ccnc(Nc2ccccc2)c1)C1CCOC1 Show InChI InChI=1S/C21H20N4O2/c26-21(17-8-11-27-14-17)25-20-13-16(7-10-23-20)15-6-9-22-19(12-15)24-18-4-2-1-3-5-18/h1-7,9-10,12-13,17H,8,11,14H2,(H,22,24)(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 by high throughput screening |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415612
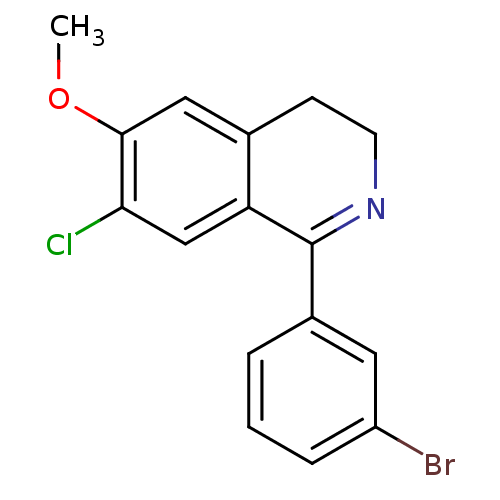
(CHEMBL1083185)Show InChI InChI=1S/C16H13BrClNO/c1-20-15-8-10-5-6-19-16(13(10)9-14(15)18)11-3-2-4-12(17)7-11/h2-4,7-9H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510286
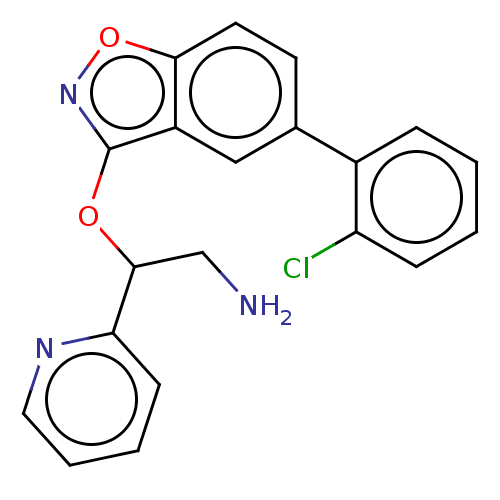
(CHEMBL4467227)Show InChI InChI=1S/C20H16ClN3O2/c21-16-6-2-1-5-14(16)13-8-9-18-15(11-13)20(24-26-18)25-19(12-22)17-7-3-4-10-23-17/h1-11,19H,12,22H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510292
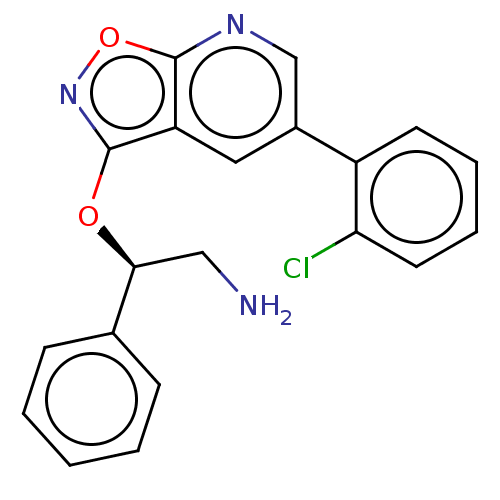
(CHEMBL4471339)Show SMILES NC[C@H](Oc1noc2ncc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN3O2/c21-17-9-5-4-8-15(17)14-10-16-19(23-12-14)26-24-20(16)25-18(11-22)13-6-2-1-3-7-13/h1-10,12,18H,11,22H2/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50279329

(CHEMBL4161596)Show SMILES Oc1ccc(cc1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-6-4-12(5-7-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-3-1-2-8-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM8370

(N-[6-(4-hydroxyphenyl)-1H-indazol-3-yl]cyclopropan...)Show InChI InChI=1S/C17H15N3O2/c21-13-6-3-10(4-7-13)12-5-8-14-15(9-12)19-20-16(14)18-17(22)11-1-2-11/h3-9,11,21H,1-2H2,(H2,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50323798
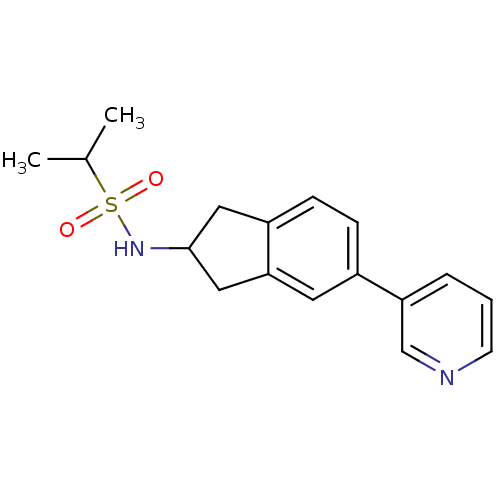
(CHEMBL1214336 | N-[5-(3-pyridinyl)-2,3-dihydro-1H-...)Show InChI InChI=1S/C17H20N2O2S/c1-12(2)22(20,21)19-17-9-14-6-5-13(8-16(14)10-17)15-4-3-7-18-11-15/h3-8,11-12,17,19H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 after 10 mins |
J Med Chem 53: 5801-12 (2010)
Article DOI: 10.1021/jm1005429
BindingDB Entry DOI: 10.7270/Q2FT8N0T |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415624

(CHEMBL1077129)Show InChI InChI=1S/C16H12Cl2FNO/c1-21-14-7-9-5-6-20-16(11(9)8-13(14)18)10-3-2-4-12(17)15(10)19/h2-4,7-8H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50279345

(CHEMBL4172211)Show InChI InChI=1S/C17H15N3O2/c21-13-5-3-10(4-6-13)12-7-15(19-17(22)11-1-2-11)14-9-18-20-16(14)8-12/h3-9,11,21H,1-2H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) after 1 hr by fluorescence polarization assay |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334936

(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50415616

(CHEMBL1087421)Show SMILES COc1cc2CCN=C(c3ccc(Cl)c(Cl)c3)c2cc1Cl |t:7| Show InChI InChI=1S/C16H12Cl3NO/c1-21-15-7-9-4-5-20-16(11(9)8-14(15)19)10-2-3-12(17)13(18)6-10/h2-3,6-8H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal histidine-tagged human full length JNK2-alpha-2 by radiometric filter binding assay |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415614
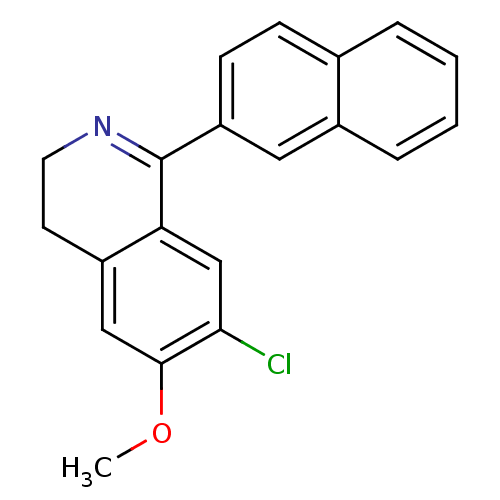
(CHEMBL1088053)Show InChI InChI=1S/C20H16ClNO/c1-23-19-11-15-8-9-22-20(17(15)12-18(19)21)16-7-6-13-4-2-3-5-14(13)10-16/h2-7,10-12H,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50279347
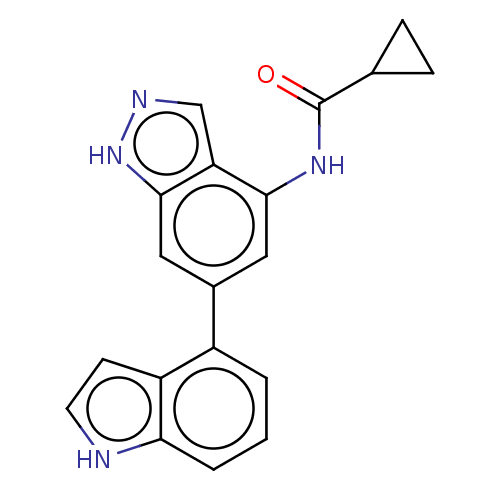
(CHEMBL4163479)Show SMILES O=C(Nc1cc(cc2[nH]ncc12)-c1cccc2[nH]ccc12)C1CC1 Show InChI InChI=1S/C19H16N4O/c24-19(11-4-5-11)22-17-8-12(9-18-15(17)10-21-23-18)13-2-1-3-16-14(13)6-7-20-16/h1-3,6-11,20H,4-5H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334939

(1-({4-[3-(Trifluoromethyl)-4,5,6,7-tetrahydro-1H-i...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)18-15-4-1-2-5-16(15)25(23-18)14-9-7-13(8-10-14)12-24-11-3-6-17(24)26/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334948

(CHEMBL1649654 | N-methyl-N-(2-phenylethyl)-4-[3-(t...)Show SMILES CN(CCc1ccccc1)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-29(16-15-17-7-3-2-4-8-17)23(31)18-11-13-19(14-12-18)30-21-10-6-5-9-20(21)22(28-30)24(25,26)27/h2-4,7-8,11-14H,5-6,9-10,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334940
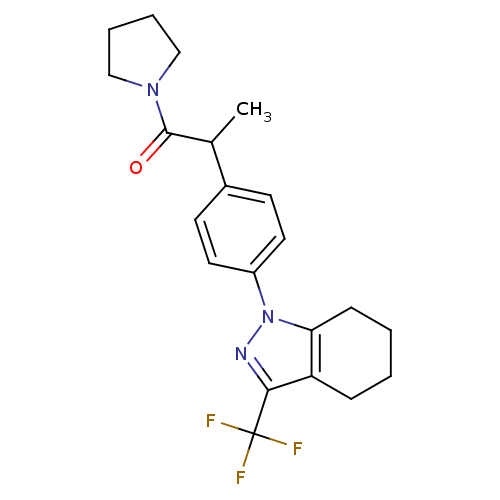
(1-{4-[1-methyl-2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES CC(C(=O)N1CCCC1)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N3O/c1-14(20(28)26-12-4-5-13-26)15-8-10-16(11-9-15)27-18-7-3-2-6-17(18)19(25-27)21(22,23)24/h8-11,14H,2-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50415620

(CHEMBL1088633)Show InChI InChI=1S/C16H13Cl2NO/c1-20-15-8-10-5-6-19-16(13(10)9-14(15)18)11-3-2-4-12(17)7-11/h2-4,7-9H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal histidine-tagged human full length JNK2-alpha-2 by radiometric filter binding assay |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50279328
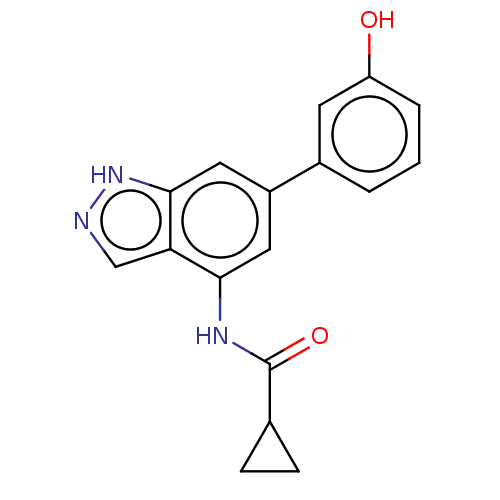
(CHEMBL4176717)Show InChI InChI=1S/C17H15N3O2/c21-13-3-1-2-11(6-13)12-7-15(19-17(22)10-4-5-10)14-9-18-20-16(14)8-12/h1-3,6-10,21H,4-5H2,(H,18,20)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) after 1 hr by fluorescence polarization assay |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50279329

(CHEMBL4161596)Show SMILES Oc1ccc(cc1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-6-4-12(5-7-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-3-1-2-8-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using YRRAAVPPSPSLSRHSSPHQ(pS)EDEEE as substrate in presence of [gamma-33P]-ATP |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50279344
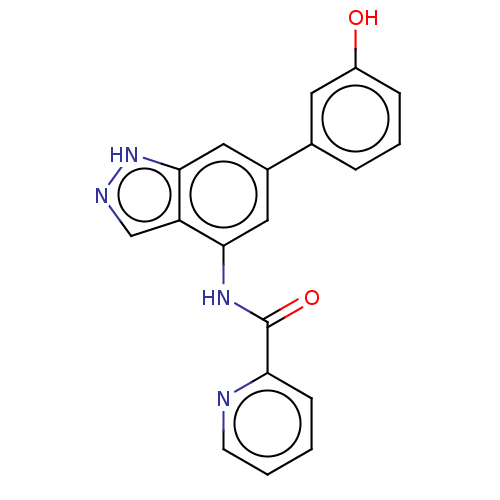
(CHEMBL4174177)Show SMILES Oc1cccc(c1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-5-3-4-12(8-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-6-1-2-7-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) after 1 hr by fluorescence polarization assay |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50279344

(CHEMBL4174177)Show SMILES Oc1cccc(c1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-5-3-4-12(8-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-6-1-2-7-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate pretreated for 15 mins followed by substrate addition measured after 1 hr |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510293
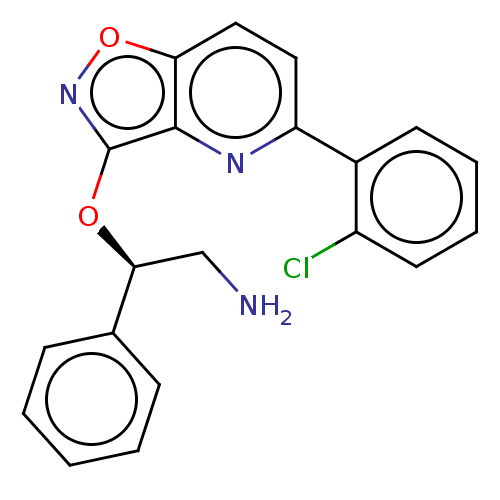
(CHEMBL4528547)Show SMILES NC[C@H](Oc1noc2ccc(nc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C20H16ClN3O2/c21-15-9-5-4-8-14(15)16-10-11-17-19(23-16)20(24-26-17)25-18(12-22)13-6-2-1-3-7-13/h1-11,18H,12,22H2/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510289
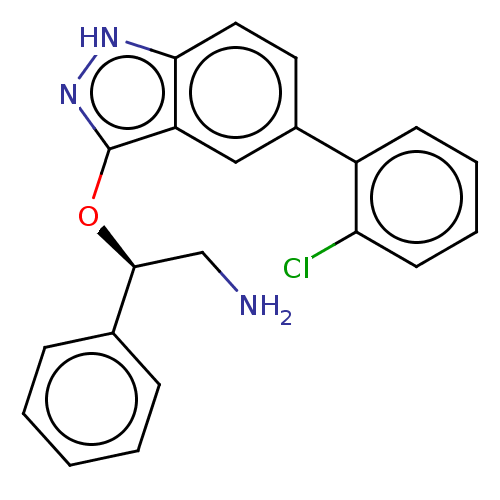
(CHEMBL4588294)Show SMILES NC[C@H](Oc1n[nH]c2ccc(cc12)-c1ccccc1Cl)c1ccccc1 |r| Show InChI InChI=1S/C21H18ClN3O/c22-18-9-5-4-8-16(18)15-10-11-19-17(12-15)21(25-24-19)26-20(13-23)14-6-2-1-3-7-14/h1-12,20H,13,23H2,(H,24,25)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50279344

(CHEMBL4174177)Show SMILES Oc1cccc(c1)-c1cc(NC(=O)c2ccccn2)c2cn[nH]c2c1 Show InChI InChI=1S/C19H14N4O2/c24-14-5-3-4-12(8-14)13-9-17(15-11-21-23-18(15)10-13)22-19(25)16-6-1-2-7-20-16/h1-11,24H,(H,21,23)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50415621

(CHEMBL1088634)Show InChI InChI=1S/C17H16ClNO/c1-11-4-3-5-13(8-11)17-14-10-15(18)16(20-2)9-12(14)6-7-19-17/h3-5,8-10H,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His(6)-tagged truncated human JNK3 transfected in baculovirus expression system by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 2230-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.098
BindingDB Entry DOI: 10.7270/Q2Z320WR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334937
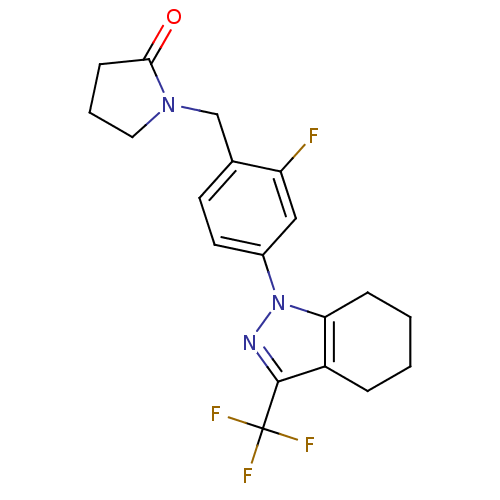
(1-({2-Fluoro-4-[3-(trifluoromethyl)-4,5,6,7-tetrah...)Show SMILES Fc1cc(ccc1CN1CCCC1=O)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C19H19F4N3O/c20-15-10-13(8-7-12(15)11-25-9-3-6-17(25)27)26-16-5-2-1-4-14(16)18(24-26)19(21,22)23/h7-8,10H,1-6,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334949
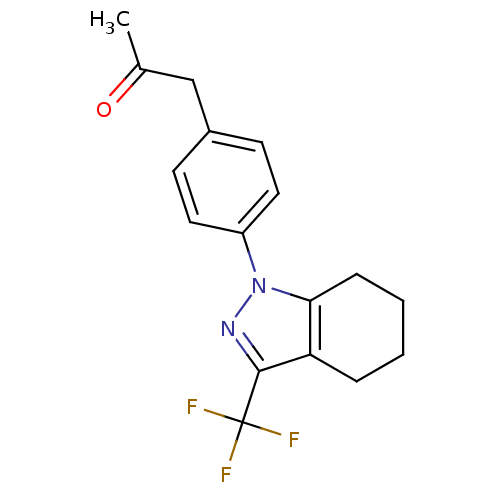
(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C17H17F3N2O/c1-11(23)10-12-6-8-13(9-7-12)22-15-5-3-2-4-14(15)16(21-22)17(18,19)20/h6-9H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334947
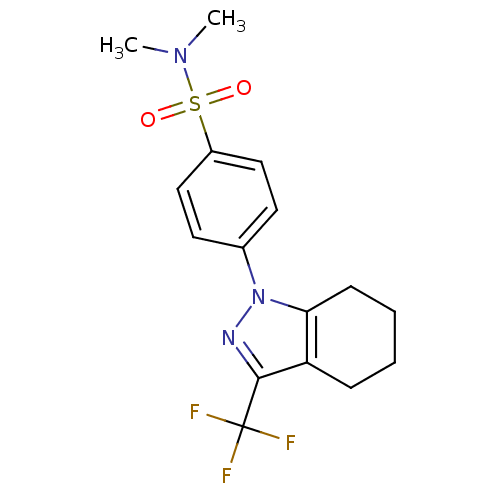
(CHEMBL1649655 | N,N-dimethyl-4-[3-(trifluoromethyl...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C16H18F3N3O2S/c1-21(2)25(23,24)12-9-7-11(8-10-12)22-14-6-4-3-5-13(14)15(20-22)16(17,18)19/h7-10H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Escherichia coli (strain K12)) | BDBM50510287
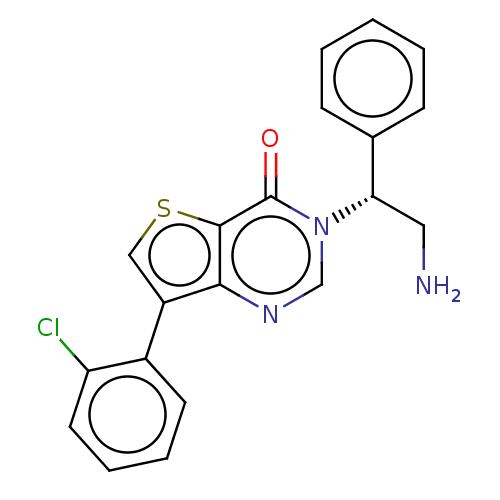
(CHEMBL4468900)Show SMILES NC[C@@H](c1ccccc1)n1cnc2c(csc2c1=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C20H16ClN3OS/c21-16-9-5-4-8-14(16)15-11-26-19-18(15)23-12-24(20(19)25)17(10-22)13-6-2-1-3-7-13/h1-9,11-12,17H,10,22H2/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... |
Bioorg Med Chem Lett 29: 1407-1412 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.029
BindingDB Entry DOI: 10.7270/Q2NC64HZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50279327

(CHEMBL4165288)Show SMILES O=C(Nc1cc(cc2[nH]ncc12)-c1cccc2[nH]ccc12)c1ccccn1 Show InChI InChI=1S/C21H15N5O/c27-21(18-5-1-2-8-22-18)25-19-10-13(11-20-16(19)12-24-26-20)14-4-3-6-17-15(14)7-9-23-17/h1-12,23H,(H,24,26)(H,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50279328

(CHEMBL4176717)Show InChI InChI=1S/C17H15N3O2/c21-13-3-1-2-11(6-13)12-7-15(19-17(22)10-4-5-10)14-9-18-20-16(14)8-12/h1-3,6-10,21H,4-5H2,(H,18,20)(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using TAMRA-ERMRPRKRQGSVRRRV-NH2 as substrate pretreated for 30 mins followed by substrate addition measured afte... |
ACS Med Chem Lett 8: 1093-1098 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00296
BindingDB Entry DOI: 10.7270/Q28S4SDF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data