 Found 872 hits with Last Name = 'thomas' and Initial = 'bf'
Found 872 hits with Last Name = 'thomas' and Initial = 'bf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176988
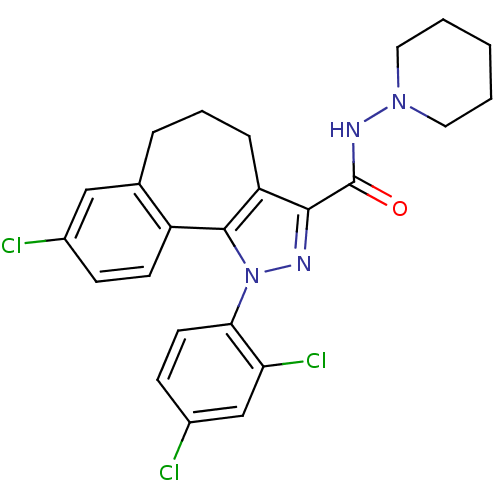
(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity to CB1 receptor (unknown origin) |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor expressed in CHO cells |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534464

(CHEMBL4483714)Show SMILES CCc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:8| Show InChI InChI=1S/C28H29NO/c1-2-21-11-12-22(26-8-4-3-7-25(21)26)19-24-20-23(27-9-5-6-10-28(24)27)13-14-29-15-17-30-18-16-29/h3-12,19-20H,2,13-18H2,1H3/b24-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534464

(CHEMBL4483714)Show SMILES CCc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:8| Show InChI InChI=1S/C28H29NO/c1-2-21-11-12-22(26-8-4-3-7-25(21)26)19-24-20-23(27-9-5-6-10-28(24)27)13-14-29-15-17-30-18-16-29/h3-12,19-20H,2,13-18H2,1H3/b24-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.859 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534476
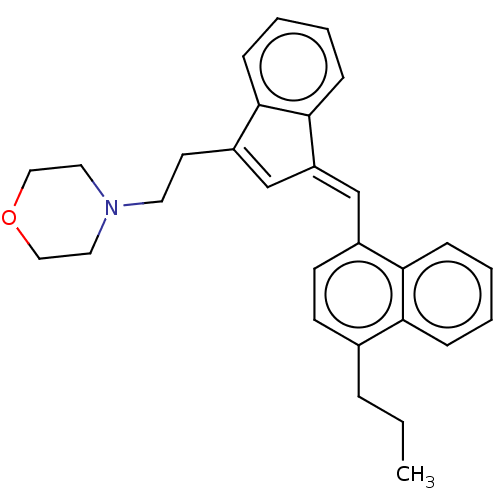
(CHEMBL4443660)Show SMILES CCCc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H31NO/c1-2-7-22-12-13-23(27-9-4-3-8-26(22)27)20-25-21-24(28-10-5-6-11-29(25)28)14-15-30-16-18-31-19-17-30/h3-6,8-13,20-21H,2,7,14-19H2,1H3/b25-20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50114673
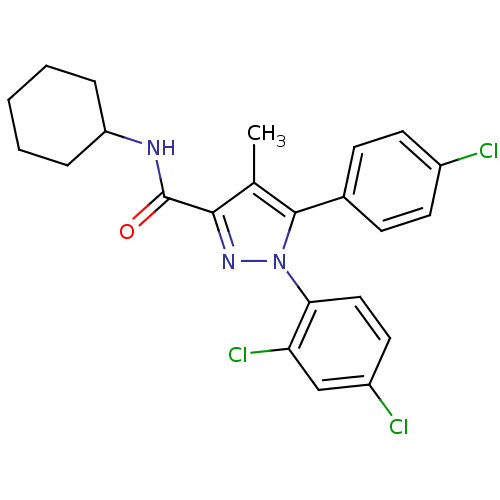
(5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C23H22Cl3N3O/c1-14-21(23(30)27-18-5-3-2-4-6-18)28-29(20-12-11-17(25)13-19(20)26)22(14)15-7-9-16(24)10-8-15/h7-13,18H,2-6H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation |
J Med Chem 45: 2708-19 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3DQW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation |
J Med Chem 45: 2708-19 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3DQW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534476

(CHEMBL4443660)Show SMILES CCCc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C29H31NO/c1-2-7-22-12-13-23(27-9-4-3-8-26(22)27)20-25-21-24(28-10-5-6-11-29(25)28)14-15-30-16-18-31-19-17-30/h3-6,8-13,20-21H,2,7,14-19H2,1H3/b25-20+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471438
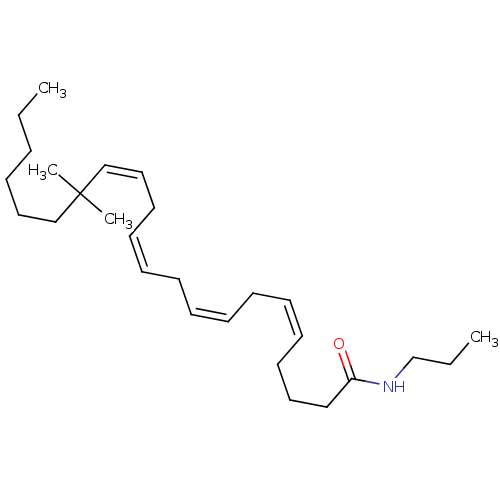
(CHEMBL122884)Show SMILES CCCCCCC(C)(C)\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCC Show InChI InChI=1S/C27H47NO/c1-5-7-8-20-23-27(3,4)24-21-18-16-14-12-10-9-11-13-15-17-19-22-26(29)28-25-6-2/h9-10,13-16,21,24H,5-8,11-12,17-20,22-23,25H2,1-4H3,(H,28,29)/b10-9-,15-13-,16-14-,24-21- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471436
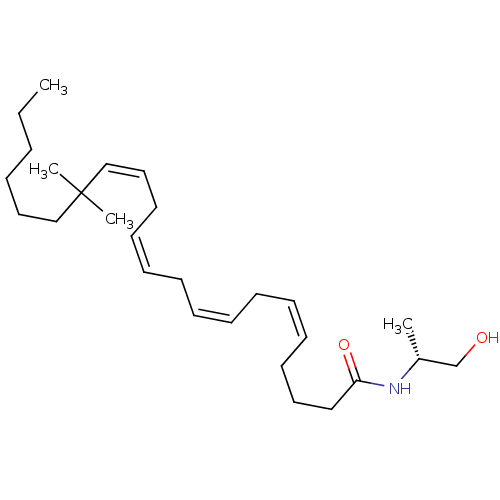
(CHEMBL123120)Show SMILES CCCCCCC(C)(C)\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N[C@H](C)CO Show InChI InChI=1S/C27H47NO2/c1-5-6-7-19-22-27(3,4)23-20-17-15-13-11-9-8-10-12-14-16-18-21-26(30)28-25(2)24-29/h8-9,12-15,20,23,25,29H,5-7,10-11,16-19,21-22,24H2,1-4H3,(H,28,30)/b9-8-,14-12-,15-13-,23-20-/t25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50060605

((5Z,8Z,11Z,14Z)-16,16-Dimethyl-docosa-5,8,11,14-te...)Show SMILES CCCCCCC(C)(C)\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCO Show InChI InChI=1S/C26H45NO2/c1-4-5-6-18-21-26(2,3)22-19-16-14-12-10-8-7-9-11-13-15-17-20-25(29)27-23-24-28/h7-8,11-14,19,22,28H,4-6,9-10,15-18,20-21,23-24H2,1-3H3,(H,27,29)/b8-7-,13-11-,14-12-,22-19- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068669
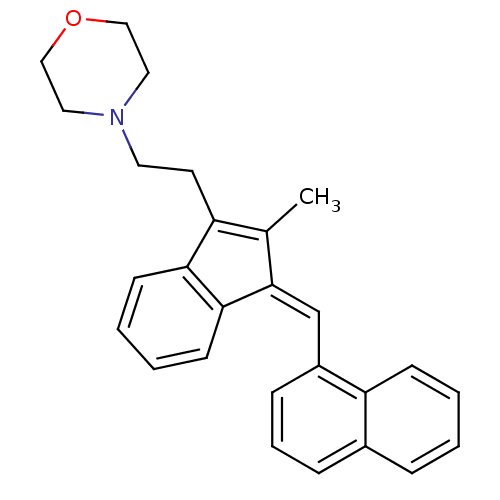
(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50200170

(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)N[C@H]2[C@@]3(C)CC[C@H](C3)C2(C)C)cc1 |r| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay |
J Med Chem 56: 6593-612 (2013)
Article DOI: 10.1021/jm400070u
BindingDB Entry DOI: 10.7270/Q2BG2RXH |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328956

(CHEMBL1269774 | N-{11-[(11-Aminoundecyl)amino]unde...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCCCCCNCCCCCCCCCCCN Show InChI InChI=1S/C39H58Cl3N5O/c1-31-37(46-47(36-25-24-34(41)30-35(36)42)38(31)32-20-22-33(40)23-21-32)39(48)45-29-19-15-11-7-3-6-10-14-18-28-44-27-17-13-9-5-2-4-8-12-16-26-43/h20-25,30,44H,2-19,26-29,43H2,1H3,(H,45,48) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534485

(CHEMBL4435655)Show InChI InChI=1S/C24H22FNO/c1-2-3-6-14-26-16-22(20-13-12-18(25)15-23(20)26)24(27)21-11-7-9-17-8-4-5-10-19(17)21/h4-5,7-13,15-16H,2-3,6,14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50212395

(CHEMBL123515)Show SMILES COc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:8| Show InChI InChI=1S/C27H27NO2/c1-29-27-11-10-20(24-7-4-5-9-26(24)27)18-22-19-21(23-6-2-3-8-25(22)23)12-13-28-14-16-30-17-15-28/h2-11,18-19H,12-17H2,1H3/b22-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50114673

(5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NC1CCCCC1 Show InChI InChI=1S/C23H22Cl3N3O/c1-14-21(23(30)27-18-5-3-2-4-6-18)28-29(20-12-11-17(25)13-19(20)26)22(14)15-7-9-16(24)10-8-15/h7-13,18H,2-6H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation |
J Med Chem 45: 2708-19 (2002)
BindingDB Entry DOI: 10.7270/Q2TX3DQW |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50221714
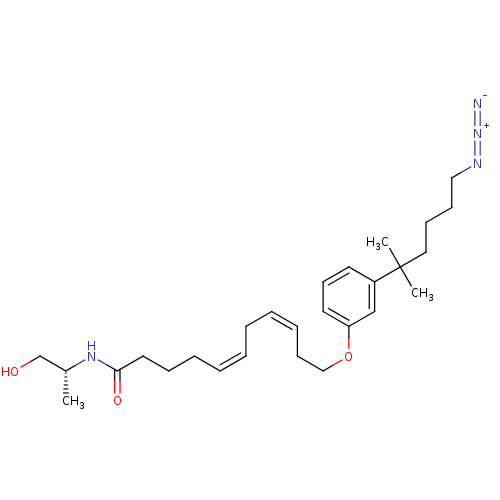
(11-[3-(5-azido-1,1-dimethylpentyl)phenoxy]undeca-5...)Show SMILES C[C@H](CO)NC(=O)CCC\C=C/C\C=C/CCOc1cccc(c1)C(C)(C)CCCCN=[N+]=[N-] Show InChI InChI=1S/C27H42N4O3/c1-23(22-32)30-26(33)17-10-8-6-4-5-7-9-13-20-34-25-16-14-15-24(21-25)27(2,3)18-11-12-19-29-31-28/h4,6-7,9,14-16,21,23,32H,5,8,10-13,17-20,22H2,1-3H3,(H,30,33)/b6-4-,9-7-/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem 15: 7850-64 (2007)
Article DOI: 10.1016/j.bmc.2007.08.039
BindingDB Entry DOI: 10.7270/Q2348K3B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534457

(CHEMBL4444832)Show SMILES CCCc1ccc(C(=O)c2cn(CCCCCF)c3cccc(F)c23)c2ccccc12 Show InChI InChI=1S/C27H27F2NO/c1-2-9-19-14-15-22(21-11-5-4-10-20(19)21)27(31)23-18-30(17-7-3-6-16-28)25-13-8-12-24(29)26(23)25/h4-5,8,10-15,18H,2-3,6-7,9,16-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50056468
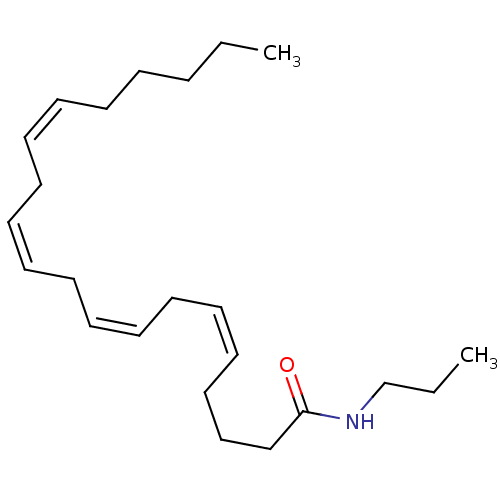
((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid pr...)Show InChI InChI=1S/C23H39NO/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-23(25)24-22-4-2/h8-9,11-12,14-15,17-18H,3-7,10,13,16,19-22H2,1-2H3,(H,24,25)/b9-8-,12-11-,15-14-,18-17- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534481

(CHEMBL4516360)Show InChI InChI=1S/C24H22FNO/c1-2-3-6-15-26-16-21(19-12-8-14-22(25)23(19)26)24(27)20-13-7-10-17-9-4-5-11-18(17)20/h4-5,7-14,16H,2-3,6,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50221714

(11-[3-(5-azido-1,1-dimethylpentyl)phenoxy]undeca-5...)Show SMILES C[C@H](CO)NC(=O)CCC\C=C/C\C=C/CCOc1cccc(c1)C(C)(C)CCCCN=[N+]=[N-] Show InChI InChI=1S/C27H42N4O3/c1-23(22-32)30-26(33)17-10-8-6-4-5-7-9-13-20-34-25-16-14-15-24(21-25)27(2,3)18-11-12-19-29-31-28/h4,6-7,9,14-16,21,23,32H,5,8,10-13,17-20,22H2,1-3H3,(H,30,33)/b6-4-,9-7-/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 7850-64 (2007)
Article DOI: 10.1016/j.bmc.2007.08.039
BindingDB Entry DOI: 10.7270/Q2348K3B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068669

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068669

(4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...)Show SMILES CC1=C(CCN2CCOCC2)c2ccccc2\C1=C\c1cccc2ccccc12 |c:1| Show InChI InChI=1S/C27H27NO/c1-20-23(13-14-28-15-17-29-18-16-28)25-11-4-5-12-26(25)27(20)19-22-9-6-8-21-7-2-3-10-24(21)22/h2-12,19H,13-18H2,1H3/b27-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand |
J Med Chem 41: 5177-87 (1999)
Article DOI: 10.1021/jm9801197
BindingDB Entry DOI: 10.7270/Q2ST7QJJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50221715
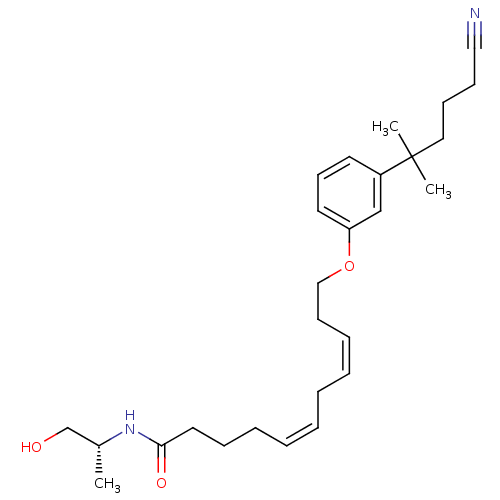
(11-[3-(5-cyano-1,1-dimethylpentyl)phenoxy]-undeca-...)Show SMILES C[C@H](CO)NC(=O)CCC\C=C/C\C=C/CCOc1cccc(c1)C(C)(C)CCCC#N Show InChI InChI=1S/C27H40N2O3/c1-23(22-30)29-26(31)17-10-8-6-4-5-7-9-13-20-32-25-16-14-15-24(21-25)27(2,3)18-11-12-19-28/h4,6-7,9,14-16,21,23,30H,5,8,10-13,17-18,20,22H2,1-3H3,(H,29,31)/b6-4-,9-7-/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB2 receptor expressed in CHO cells |
Bioorg Med Chem 15: 7850-64 (2007)
Article DOI: 10.1016/j.bmc.2007.08.039
BindingDB Entry DOI: 10.7270/Q2348K3B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534481

(CHEMBL4516360)Show InChI InChI=1S/C24H22FNO/c1-2-3-6-15-26-16-21(19-12-8-14-22(25)23(19)26)24(27)20-13-7-10-17-9-4-5-11-18(17)20/h4-5,7-14,16H,2-3,6,15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471437

(CHEMBL121384)Show SMILES CCCCCCC(C)(C)\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCOC Show InChI InChI=1S/C27H47NO2/c1-5-6-7-19-22-27(2,3)23-20-17-15-13-11-9-8-10-12-14-16-18-21-26(29)28-24-25-30-4/h8-9,12-15,20,23H,5-7,10-11,16-19,21-22,24-25H2,1-4H3,(H,28,29)/b9-8-,14-12-,15-13-,23-20- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50212395

(CHEMBL123515)Show SMILES COc1ccc(\C=C2/C=C(CCN3CCOCC3)c3ccccc23)c2ccccc12 |t:8| Show InChI InChI=1S/C27H27NO2/c1-29-27-11-10-20(24-7-4-5-9-26(24)27)18-22-19-21(23-6-2-3-8-25(22)23)12-13-28-14-16-30-17-15-28/h2-11,18-19H,12-17H2,1H3/b22-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50534463

(CHEMBL4550629)Show SMILES C(CC1=C\C(=C/c2ccc3CCc4cccc2c34)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C28H27NO/c1-2-6-26-24(19-23(25(26)5-1)12-13-29-14-16-30-17-15-29)18-22-11-10-21-9-8-20-4-3-7-27(22)28(20)21/h1-7,10-11,18-19H,8-9,12-17H2/b24-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50534498

(CHEMBL4474739)Show SMILES FCCCCCn1cc(C(=O)c2cccc3ccccc23)c2c(F)cccc12 Show InChI InChI=1S/C24H21F2NO/c25-14-4-1-5-15-27-16-20(23-21(26)12-7-13-22(23)27)24(28)19-11-6-9-17-8-2-3-10-18(17)19/h2-3,6-13,16H,1,4-5,14-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328952
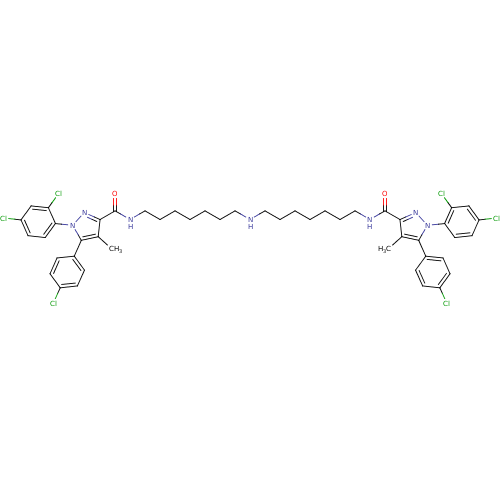
(CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCNCCCCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C48H51Cl6N7O2/c1-31-43(58-60(41-23-21-37(51)29-39(41)53)45(31)33-13-17-35(49)18-14-33)47(62)56-27-11-7-3-5-9-25-55-26-10-6-4-8-12-28-57-48(63)44-32(2)46(34-15-19-36(50)20-16-34)61(59-44)42-24-22-38(52)30-40(42)54/h13-24,29-30,55H,3-12,25-28H2,1-2H3,(H,56,62)(H,57,63) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328956

(CHEMBL1269774 | N-{11-[(11-Aminoundecyl)amino]unde...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCCCCCNCCCCCCCCCCCN Show InChI InChI=1S/C39H58Cl3N5O/c1-31-37(46-47(36-25-24-34(41)30-35(36)42)38(31)32-20-22-33(40)23-21-32)39(48)45-29-19-15-11-7-3-6-10-14-18-28-44-27-17-13-9-5-2-4-8-12-16-26-43/h20-25,30,44H,2-19,26-29,43H2,1H3,(H,45,48) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471435
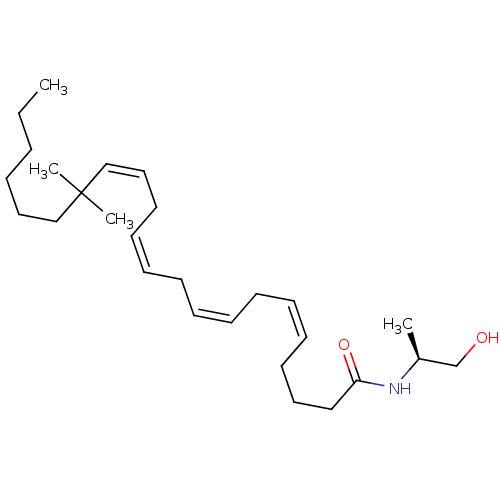
(CHEMBL120428)Show SMILES CCCCCCC(C)(C)\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)N[C@@H](C)CO Show InChI InChI=1S/C27H47NO2/c1-5-6-7-19-22-27(3,4)23-20-17-15-13-11-9-8-10-12-14-16-18-21-26(30)28-25(2)24-29/h8-9,12-15,20,23,25,29H,5-7,10-11,16-19,21-22,24H2,1-4H3,(H,28,30)/b9-8-,14-12-,15-13-,23-20-/t25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane |
J Med Chem 40: 3626-34 (1997)
Article DOI: 10.1021/jm9702950
BindingDB Entry DOI: 10.7270/Q261131W |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328957
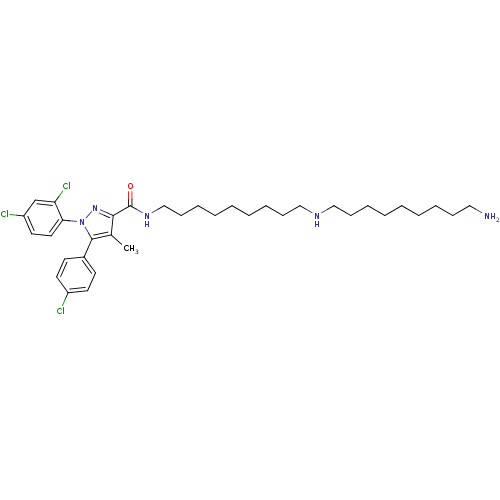
(CHEMBL1269773 | N-{9-[(9-Aminononyl)amino]nonyl}-5...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NCCCCCCCCCNCCCCCCCCCN Show InChI InChI=1S/C35H50Cl3N5O/c1-27-33(42-43(32-21-20-30(37)26-31(32)38)34(27)28-16-18-29(36)19-17-28)35(44)41-25-15-11-7-3-6-10-14-24-40-23-13-9-5-2-4-8-12-22-39/h16-21,26,40H,2-15,22-25,39H2,1H3,(H,41,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50221715

(11-[3-(5-cyano-1,1-dimethylpentyl)phenoxy]-undeca-...)Show SMILES C[C@H](CO)NC(=O)CCC\C=C/C\C=C/CCOc1cccc(c1)C(C)(C)CCCC#N Show InChI InChI=1S/C27H40N2O3/c1-23(22-30)29-26(31)17-10-8-6-4-5-7-9-13-20-32-25-16-14-15-24(21-25)27(2,3)18-11-12-19-28/h4,6-7,9,14-16,21,23,30H,5,8,10-13,17-18,20,22H2,1-3H3,(H,29,31)/b6-4-,9-7-/t23-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organix, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem 15: 7850-64 (2007)
Article DOI: 10.1016/j.bmc.2007.08.039
BindingDB Entry DOI: 10.7270/Q2348K3B |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328967
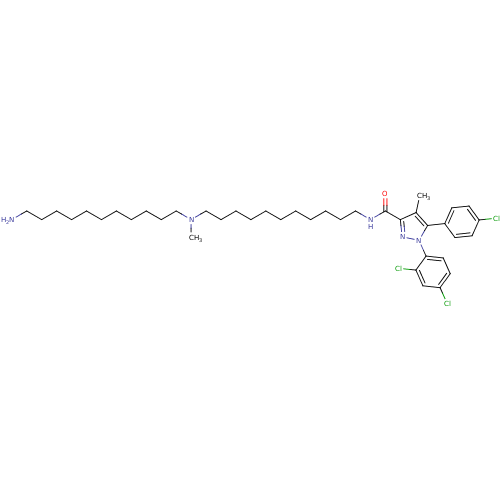
(CHEMBL1269776 | N-{11-[(11-Aminoundecyl)(methyl)am...)Show SMILES CN(CCCCCCCCCCCN)CCCCCCCCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C40H60Cl3N5O/c1-32-38(46-48(37-26-25-35(42)31-36(37)43)39(32)33-21-23-34(41)24-22-33)40(49)45-28-18-14-10-6-4-8-12-16-20-30-47(2)29-19-15-11-7-3-5-9-13-17-27-44/h21-26,31H,3-20,27-30,44H2,1-2H3,(H,45,49) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50176988

(8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...)Show SMILES Clc1ccc(c(Cl)c1)-n1nc(C(=O)NN2CCCCC2)c2CCCc3cc(Cl)ccc3-c12 Show InChI InChI=1S/C24H23Cl3N4O/c25-16-7-9-18-15(13-16)5-4-6-19-22(24(32)29-30-11-2-1-3-12-30)28-31(23(18)19)21-10-8-17(26)14-20(21)27/h7-10,13-14H,1-6,11-12H2,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50068666

(4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...)Show SMILES C(CC1=C\C(=C/c2cccc3ccccc23)c2ccccc12)N1CCOCC1 |t:2| Show InChI InChI=1S/C26H25NO/c1-2-9-24-20(6-1)7-5-8-21(24)18-23-19-22(25-10-3-4-11-26(23)25)12-13-27-14-16-28-17-15-27/h1-11,18-19H,12-17H2/b23-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB2 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
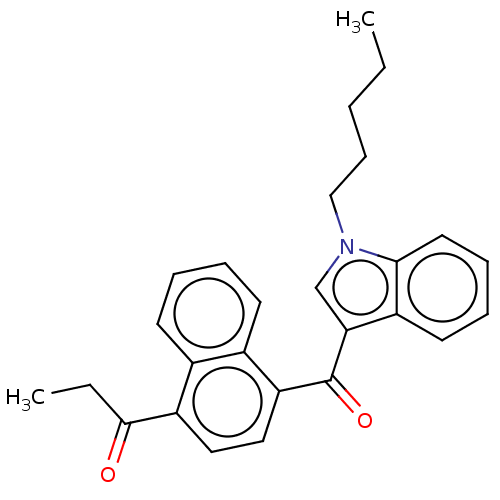
(Homo sapiens (Human)) | BDBM50534467
(CHEMBL4549096)Show SMILES CCCCCn1cc(C(=O)c2ccc(C(=O)CC)c3ccccc23)c2ccccc12 Show InChI InChI=1S/C27H27NO2/c1-3-5-10-17-28-18-24(21-13-8-9-14-25(21)28)27(30)23-16-15-22(26(29)4-2)19-11-6-7-12-20(19)23/h6-9,11-16,18H,3-5,10,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human CB1 receptor |
J Med Chem 59: 7525-43 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00516
BindingDB Entry DOI: 10.7270/Q2862KX2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
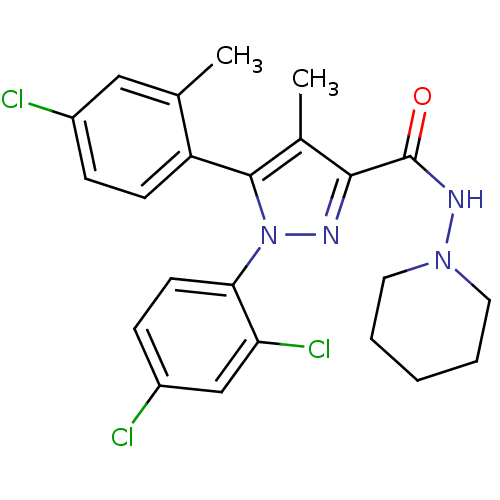
(Homo sapiens (Human)) | BDBM50271287
(5-(4-Chloro-2-methylphenyl)-1-(2,4-dichlorophenyl)...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1C)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 |(23.68,.56,;24.82,-.46,;26.33,-.13,;27.11,-1.46,;26.08,-2.61,;24.67,-1.99,;23.34,-2.76,;23.35,-4.32,;22,-5.09,;20.67,-4.32,;19.34,-5.08,;20.68,-2.77,;22,-2,;21.99,-.46,;26.41,-4.12,;25.27,-5.15,;25.59,-6.65,;27.06,-7.12,;27.39,-8.63,;28.2,-6.08,;27.87,-4.58,;29.01,-3.54,;26.95,1.28,;26.04,2.52,;28.48,1.44,;29.1,2.85,;30.64,3.02,;31.26,4.41,;30.35,5.66,;28.82,5.5,;28.19,4.09,)| Show InChI InChI=1S/C23H23Cl3N4O/c1-14-12-16(24)6-8-18(14)22-15(2)21(23(31)28-29-10-4-3-5-11-29)27-30(22)20-9-7-17(25)13-19(20)26/h6-9,12-13H,3-5,10-11H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50328960

(CHEMBL1269775 | N-{9-[(9-Aminononyl)(methyl)amino]...)Show SMILES CN(CCCCCCCCCN)CCCCCCCCCNC(=O)c1nn(c(c1C)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C36H52Cl3N5O/c1-28-34(42-44(33-22-21-31(38)27-32(33)39)35(28)29-17-19-30(37)20-18-29)36(45)41-24-14-10-6-4-8-12-16-26-43(2)25-15-11-7-3-5-9-13-23-40/h17-22,27H,3-16,23-26,40H2,1-2H3,(H,41,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 53: 7048-60 (2010)
Article DOI: 10.1021/jm1006676
BindingDB Entry DOI: 10.7270/Q27P8ZMC |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 51: 3526-39 (2008)
Article DOI: 10.1021/jm8000778
BindingDB Entry DOI: 10.7270/Q29K4B0X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data