 Found 620 hits with Last Name = 'metcalf' and Initial = 'bw'
Found 620 hits with Last Name = 'metcalf' and Initial = 'bw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403349
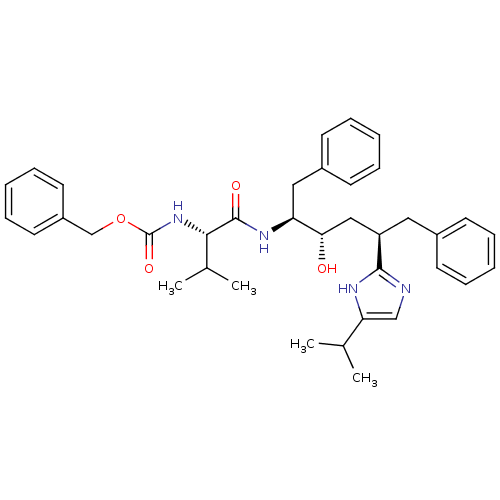
(CHEMBL407551)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C37H46N4O4/c1-25(2)32-23-38-35(39-32)30(20-27-14-8-5-9-15-27)22-33(42)31(21-28-16-10-6-11-17-28)40-36(43)34(26(3)4)41-37(44)45-24-29-18-12-7-13-19-29/h5-19,23,25-26,30-31,33-34,42H,20-22,24H2,1-4H3,(H,38,39)(H,40,43)(H,41,44)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403357

(CHEMBL419286)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403360
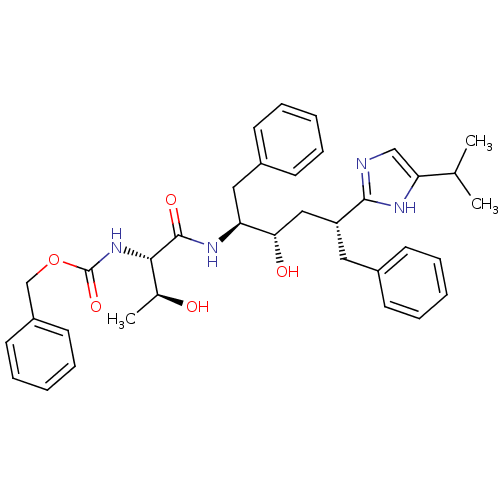
(CHEMBL1790592)Show SMILES CC(C)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)[C@H](C)O)Cc1ccccc1 Show InChI InChI=1S/C36H44N4O5/c1-24(2)31-22-37-34(38-31)29(19-26-13-7-4-8-14-26)21-32(42)30(20-27-15-9-5-10-16-27)39-35(43)33(25(3)41)40-36(44)45-23-28-17-11-6-12-18-28/h4-18,22,24-25,29-30,32-33,41-42H,19-21,23H2,1-3H3,(H,37,38)(H,39,43)(H,40,44)/t25-,29+,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403351
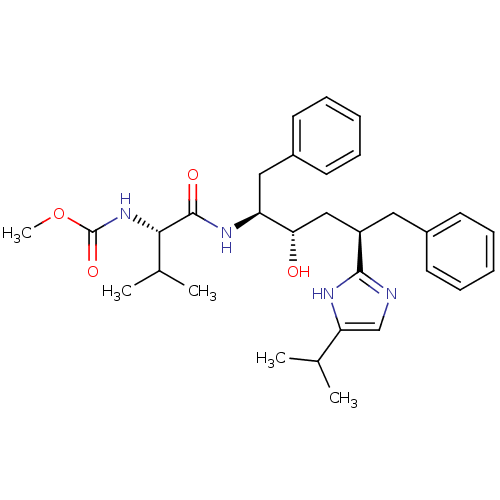
(CHEMBL79698)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O4/c1-20(2)26-19-32-29(33-26)24(16-22-12-8-6-9-13-22)18-27(36)25(17-23-14-10-7-11-15-23)34-30(37)28(21(3)4)35-31(38)39-5/h6-15,19-21,24-25,27-28,36H,16-18H2,1-5H3,(H,32,33)(H,34,37)(H,35,38)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403358
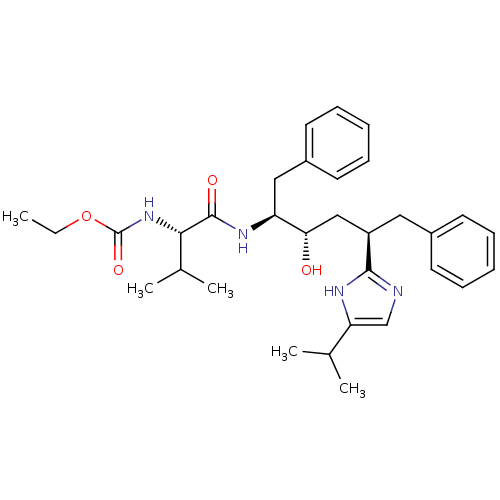
(CHEMBL78531)Show SMILES CCOC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C32H44N4O4/c1-6-40-32(39)36-29(22(4)5)31(38)35-26(18-24-15-11-8-12-16-24)28(37)19-25(17-23-13-9-7-10-14-23)30-33-20-27(34-30)21(2)3/h7-16,20-22,25-26,28-29,37H,6,17-19H2,1-5H3,(H,33,34)(H,35,38)(H,36,39)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279858
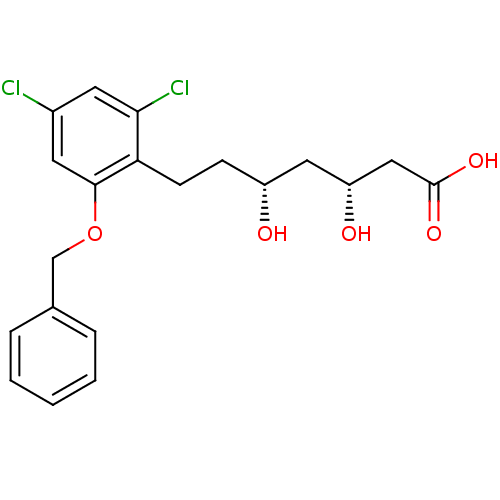
((3R,5R)-7-(2-Benzyloxy-4,6-dichloro-phenyl)-3,5-di...)Show SMILES O[C@H](CCc1c(Cl)cc(Cl)cc1OCc1ccccc1)C[C@@H](O)CC(O)=O Show InChI InChI=1S/C20H22Cl2O5/c21-14-8-18(22)17(7-6-15(23)10-16(24)11-20(25)26)19(9-14)27-12-13-4-2-1-3-5-13/h1-5,8-9,15-16,23-24H,6-7,10-12H2,(H,25,26)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403352

(CHEMBL81517)Show SMILES CC(C)[C@H](NC=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C30H40N4O3/c1-20(2)26-18-31-29(33-26)24(15-22-11-7-5-8-12-22)17-27(36)25(16-23-13-9-6-10-14-23)34-30(37)28(21(3)4)32-19-35/h5-14,18-21,24-25,27-28,36H,15-17H2,1-4H3,(H,31,33)(H,32,35)(H,34,37)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406356

(CHEMBL426217)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3C=C[C@]12C)C(O)=O |c:17,26,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,12,14-17,20-23H,8-11,13H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057468

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3C=CC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:14,18,t:16| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7-8,11,15-17,20-23H,9-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403348
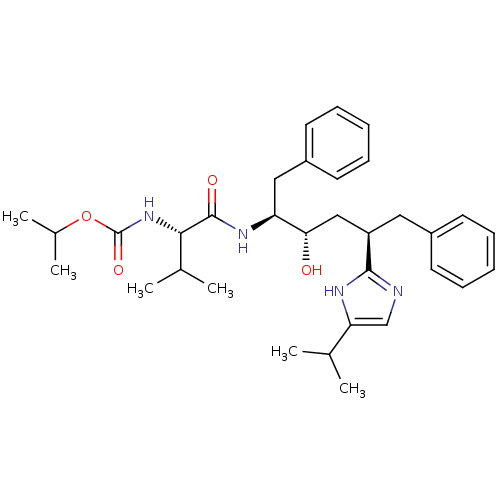
(CHEMBL421709)Show SMILES CC(C)OC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C33H46N4O4/c1-21(2)28-20-34-31(35-28)26(17-24-13-9-7-10-14-24)19-29(38)27(18-25-15-11-8-12-16-25)36-32(39)30(22(3)4)37-33(40)41-23(5)6/h7-16,20-23,26-27,29-30,38H,17-19H2,1-6H3,(H,34,35)(H,36,39)(H,37,40)/t26-,27+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50452771

(CHEMBL2311126)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2(C)C=C(CC[C@]12C)C(O)=O |c:26| Show InChI InChI=1S/C26H41NO3/c1-23(2,3)27-21(28)20-8-7-18-17-10-12-24(4)15-16(22(29)30)9-14-26(24,6)19(17)11-13-25(18,20)5/h15,17-20H,7-14H2,1-6H3,(H,27,28)(H,29,30)/t17-,18-,19-,20+,24-,25-,26+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50279859
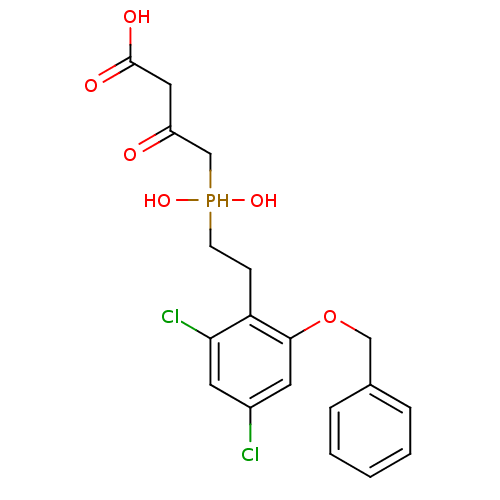
((S)-4-{[2-(2-Benzyloxy-4,6-dichloro-phenyl)-ethyl]...)Show SMILES OC(=O)CC(=O)CP(O)(O)CCc1c(Cl)cc(Cl)cc1OCc1ccccc1 Show InChI InChI=1S/C19H21Cl2O6P/c20-14-8-17(21)16(6-7-28(25,26)12-15(22)10-19(23)24)18(9-14)27-11-13-4-2-1-3-5-13/h1-5,8-9,25-26,28H,6-7,10-12H2,(H,23,24) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The inhibitory activity of the compound against purified recombinant human HMG-CoA reductase was evaluated |
Bioorg Med Chem Lett 1: 151-154 (1991)
Article DOI: 10.1016/S0960-894X(01)80788-5
BindingDB Entry DOI: 10.7270/Q21836DP |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50043604
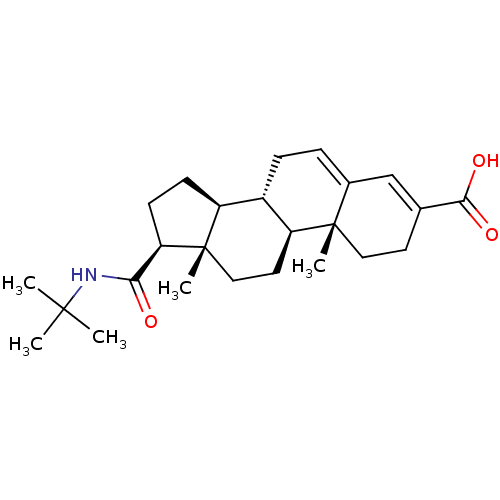
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in rat ventral prostates expressed as apparent inhibition constant; Ran... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50366682
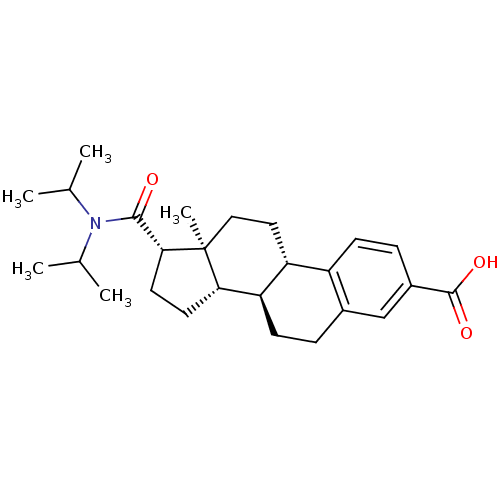
(CHEMBL1627395)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C26H37NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h7-8,14-16,20-23H,6,9-13H2,1-5H3,(H,29,30)/t20-,21-,22+,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50452770

(CHEMBL2311127)Show SMILES [H][C@@]12CC[C@H](C(=O)N(C(C)C)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(F)=C(CC[C@]12C)C(O)=O |c:29| Show InChI InChI=1S/C27H42FNO3/c1-15(2)29(16(3)4)24(30)22-10-9-19-17-7-8-21-23(28)18(25(31)32)11-13-26(21,5)20(17)12-14-27(19,22)6/h15-17,19-22H,7-14H2,1-6H3,(H,31,32)/t17-,19-,20-,21-,22+,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against 5-alpha reductase was determined in human prostatic tissue expressed as apparent inhibition constant |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in human prostatic tissue expressed as apparent inhibition constant; Ra... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406357

(CHEMBL172595)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC(F)=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H40FNO3/c1-15(2)29(16(3)4)24(30)21-8-7-19-18-14-23(28)22-13-17(25(31)32)9-11-27(22,6)20(18)10-12-26(19,21)5/h13,15-16,18-21H,7-12,14H2,1-6H3,(H,31,32)/t18?,19?,20?,21-,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406357

(CHEMBL172595)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC(F)=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H40FNO3/c1-15(2)29(16(3)4)24(30)21-8-7-19-18-14-23(28)22-13-17(25(31)32)9-11-27(22,6)20(18)10-12-26(19,21)5/h13,15-16,18-21H,7-12,14H2,1-6H3,(H,31,32)/t18?,19?,20?,21-,26+,27-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50452770

(CHEMBL2311127)Show SMILES [H][C@@]12CC[C@H](C(=O)N(C(C)C)C(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C(F)=C(CC[C@]12C)C(O)=O |c:29| Show InChI InChI=1S/C27H42FNO3/c1-15(2)29(16(3)4)24(30)22-10-9-19-17-7-8-21-23(28)18(25(31)32)11-13-26(21,5)20(17)12-14-27(19,22)6/h15-17,19-22H,7-14H2,1-6H3,(H,31,32)/t17-,19-,20-,21-,22+,26+,27-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057469

((10R,13S,17S)-17-Diisopropylcarbamoyl-4,10,13-trim...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C(C)=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:15| Show InChI InChI=1S/C28H43NO3/c1-16(2)29(17(3)4)25(30)24-11-10-22-20-8-9-21-18(5)19(26(31)32)12-14-27(21,6)23(20)13-15-28(22,24)7/h9,16-17,20,22-24H,8,10-15H2,1-7H3,(H,31,32)/t20?,22?,23?,24-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50057448

((10R,13S,17S)-17-Diisopropylcarbamoyl-10,13-dimeth...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,15-17,20-23H,8-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Steroid 5-alpha-reductase was determined in rat ventral prostates expressed as apparent inhibition constant; Ran... |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403345
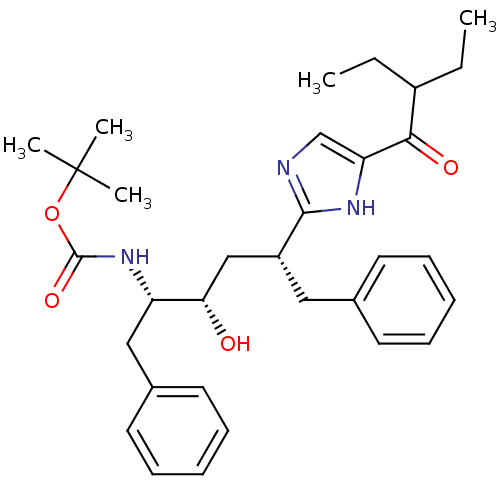
(CHEMBL83739)Show SMILES CCC(CC)C(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C32H43N3O4/c1-6-24(7-2)29(37)27-21-33-30(34-27)25(18-22-14-10-8-11-15-22)20-28(36)26(19-23-16-12-9-13-17-23)35-31(38)39-32(3,4)5/h8-17,21,24-26,28,36H,6-7,18-20H2,1-5H3,(H,33,34)(H,35,38)/t25-,26+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406356

(CHEMBL426217)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@]4(C)C3C=C[C@]12C)C(O)=O |c:17,26,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,12,14-17,20-23H,8-11,13H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406358

(CHEMBL366660)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@@H]4C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C26H39NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h6,14-16,19-23H,7-13H2,1-5H3,(H,29,30)/t19-,20?,21?,22?,23+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50391268

(CHEMBL110001)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)[N+]([O-])=O |c:17| Show InChI InChI=1S/C26H42N2O3/c1-16(2)27(17(3)4)24(29)23-10-9-21-20-8-7-18-15-19(28(30)31)11-13-25(18,5)22(20)12-14-26(21,23)6/h15-18,20-23H,7-14H2,1-6H3/t18-,20?,21?,22?,23+,25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against human prostatic Steroid 5-alpha-reductase |
Bioorg Med Chem Lett 1: 27-32 (1991)
Article DOI: 10.1016/S0960-894X(01)81084-2
BindingDB Entry DOI: 10.7270/Q29P31J1 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406353

(CHEMBL172416)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CCC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,15-17,20-23H,7-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403350
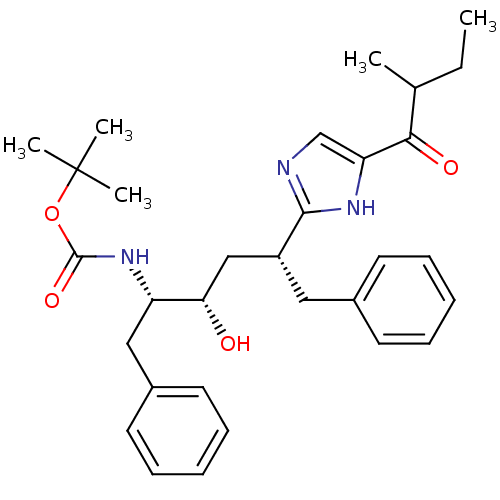
(CHEMBL81190)Show SMILES CCC(C)C(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C31H41N3O4/c1-6-21(2)28(36)26-20-32-29(33-26)24(17-22-13-9-7-10-14-22)19-27(35)25(18-23-15-11-8-12-16-23)34-30(37)38-31(3,4)5/h7-16,20-21,24-25,27,35H,6,17-19H2,1-5H3,(H,32,33)(H,34,37)/t21?,24-,25+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406351
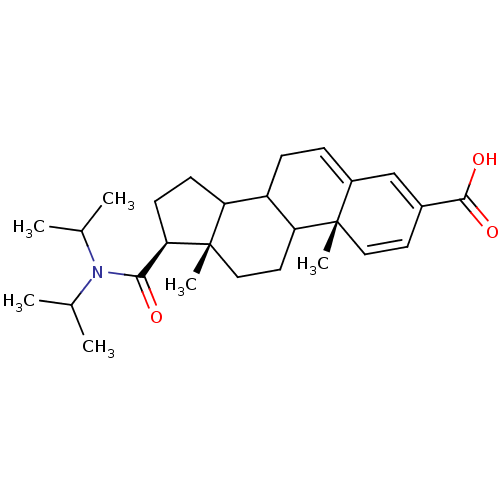
(CHEMBL427113)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(C=C[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,19,t:15| Show InChI InChI=1S/C27H39NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h7,11,13,15-17,20-23H,8-10,12,14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406359

(CHEMBL172025)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(C=C[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,19| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,13,15-17,19-23H,7-10,12,14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406359

(CHEMBL172025)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(C=C[C@]4(C)C3CC[C@]12C)C(O)=O |c:17,19| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,13,15-17,19-23H,7-10,12,14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50057469

((10R,13S,17S)-17-Diisopropylcarbamoyl-4,10,13-trim...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C(C)=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:15| Show InChI InChI=1S/C28H43NO3/c1-16(2)29(17(3)4)25(30)24-11-10-22-20-8-9-21-18(5)19(26(31)32)12-14-27(21,6)23(20)13-15-28(22,24)7/h9,16-17,20,22-24H,8,10-15H2,1-7H3,(H,31,32)/t20?,22?,23?,24-,27+,28+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50391269

(CHEMBL48467)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:17| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h15-17,19-23H,7-14H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against 5-alpha reductase was determined in rat ventral prostates expressed as apparent inhibition constant |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406361

(CHEMBL172951)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,16-17,19-23H,7-10,12-15H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037126
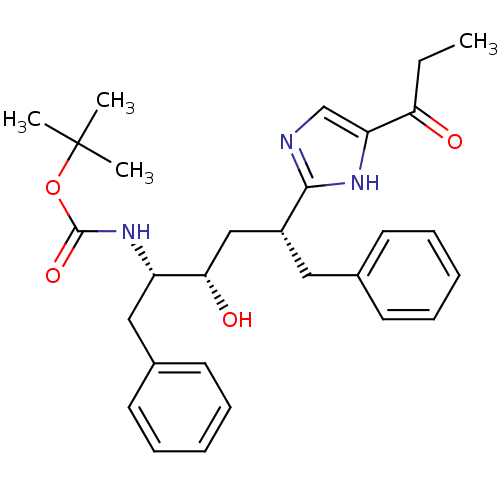
(CHEMBL80098 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-5-phe...)Show SMILES CCC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4/c1-5-25(33)24-19-30-27(31-24)22(16-20-12-8-6-9-13-20)18-26(34)23(17-21-14-10-7-11-15-21)32-28(35)36-29(2,3)4/h6-15,19,22-23,26,34H,5,16-18H2,1-4H3,(H,30,31)(H,32,35)/t22-,23+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406361

(CHEMBL172951)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18| Show InChI InChI=1S/C27H43NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,16-17,19-23H,7-10,12-15H2,1-6H3,(H,30,31)/t19-,20?,21?,22?,23+,26-,27-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50452771

(CHEMBL2311126)Show SMILES [H][C@@]12CC[C@H](C(=O)NC(C)(C)C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2(C)C=C(CC[C@]12C)C(O)=O |c:26| Show InChI InChI=1S/C26H41NO3/c1-23(2,3)27-21(28)20-8-7-18-17-10-12-24(4)15-16(22(29)30)9-14-26(24,6)19(17)11-13-25(18,20)5/h15,17-20H,7-14H2,1-6H3,(H,27,28)(H,29,30)/t17-,18-,19-,20+,24-,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406355

(CHEMBL353340)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CCC4=C(CCC(=C4)C(O)=O)C3CC[C@]12C |c:20,t:16| Show InChI InChI=1S/C26H39NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h14-16,20-23H,6-13H2,1-5H3,(H,29,30)/t20?,21?,22?,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406353

(CHEMBL172416)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CCC4=CC(=CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C27H41NO3/c1-16(2)28(17(3)4)24(29)23-10-9-21-20-8-7-19-15-18(25(30)31)11-13-26(19,5)22(20)12-14-27(21,23)6/h11,15-17,20-23H,7-10,12-14H2,1-6H3,(H,30,31)/t20?,21?,22?,23-,26+,27+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406362

(CHEMBL170473)Show SMILES C[C@]12CCC3C(CC=C4C=C(CC[C@]34C)C(O)=O)C1CC[C@@H]2C#N |c:9,t:7| Show InChI InChI=1S/C21H27NO2/c1-20-9-7-13(19(23)24)11-14(20)3-5-16-17-6-4-15(12-22)21(17,2)10-8-18(16)20/h3,11,15-18H,4-10H2,1-2H3,(H,23,24)/t15-,16?,17?,18?,20+,21-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406358

(CHEMBL366660)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC=C4C=C(CC[C@@H]4C3CC[C@]12C)C(O)=O |c:17,t:15| Show InChI InChI=1S/C26H39NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h6,14-16,19-23H,7-13H2,1-5H3,(H,29,30)/t19-,20?,21?,22?,23+,26-/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403362

(CHEMBL312709)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C1CCCC1 Show InChI InChI=1S/C32H41N3O4/c1-32(2,3)39-31(38)35-26(19-23-14-8-5-9-15-23)28(36)20-25(18-22-12-6-4-7-13-22)30-33-21-27(34-30)29(37)24-16-10-11-17-24/h4-9,12-15,21,24-26,28,36H,10-11,16-20H2,1-3H3,(H,33,34)(H,35,38)/t25-,26+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403353

(CHEMBL309773)Show SMILES CC(C)[C@@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50403606
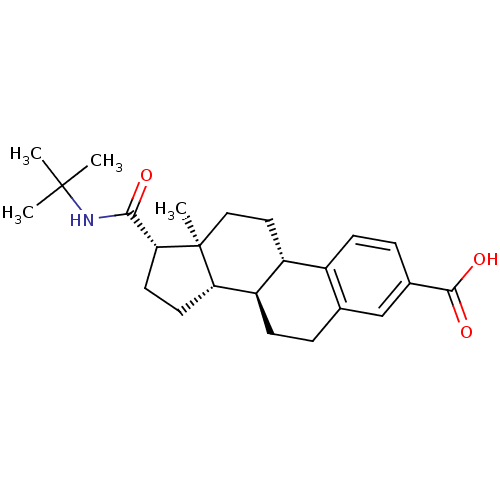
(CHEMBL1627951)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C24H33NO3/c1-23(2,3)25-21(26)20-10-9-19-18-8-5-14-13-15(22(27)28)6-7-16(14)17(18)11-12-24(19,20)4/h6-7,13,17-20H,5,8-12H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-,19+,20-,24+/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase of rat ventral prostate tissue |
J Med Chem 33: 937-42 (1990)
BindingDB Entry DOI: 10.7270/Q2F76DR5 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406352

(CHEMBL174294)Show SMILES CC(CO)[C@@H]1CCC2C3CCC4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:12| Show InChI InChI=1S/C23H36O3/c1-14(13-24)18-6-7-19-17-5-4-16-12-15(21(25)26)8-10-22(16,2)20(17)9-11-23(18,19)3/h12,14,16-20,24H,4-11,13H2,1-3H3,(H,25,26)/t14?,16?,17?,18-,19?,20?,22-,23+/m0/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50406346

(CHEMBL367878)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC(C)=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C28H43NO3/c1-16(2)29(17(3)4)25(30)23-9-8-21-20-14-18(5)24-15-19(26(31)32)10-12-28(24,7)22(20)11-13-27(21,23)6/h15-17,20-23H,8-14H2,1-7H3,(H,31,32)/t20?,21?,22?,23-,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in human prostatic tissue. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1/2
(Rattus norvegicus) | BDBM50406346

(CHEMBL367878)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC(C)=C4C=C(CC[C@]4(C)C3CC[C@]12C)C(O)=O |c:18,t:16| Show InChI InChI=1S/C28H43NO3/c1-16(2)29(17(3)4)25(30)23-9-8-21-20-14-18(5)24-15-19(26(31)32)10-12-28(24,7)22(20)11-13-27(21,23)6/h15-17,20-23H,8-14H2,1-7H3,(H,31,32)/t20?,21?,22?,23-,27+,28-/m1/s1 | MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Steroid 5-alpha-reductase in rat ventral prostates. |
J Med Chem 33: 943-50 (1990)
BindingDB Entry DOI: 10.7270/Q25D8QSB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data