

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515598 (CHEMBL4526049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515600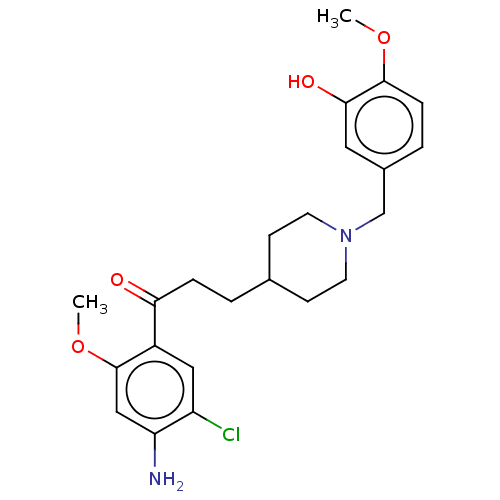 (CHEMBL4580044) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278990 (CHEMBL4164524) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278986 (CHEMBL4161860) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515599 (CHEMBL4457426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278968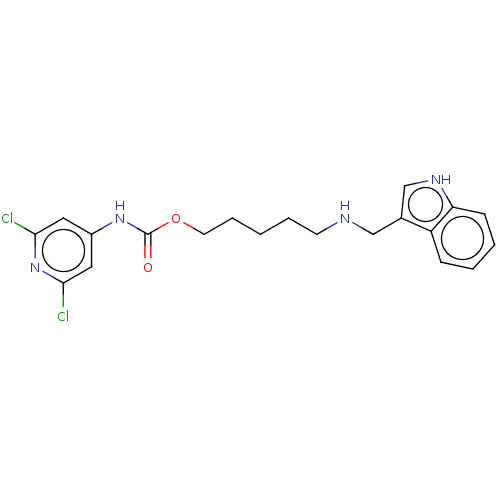 (CHEMBL4164186) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278987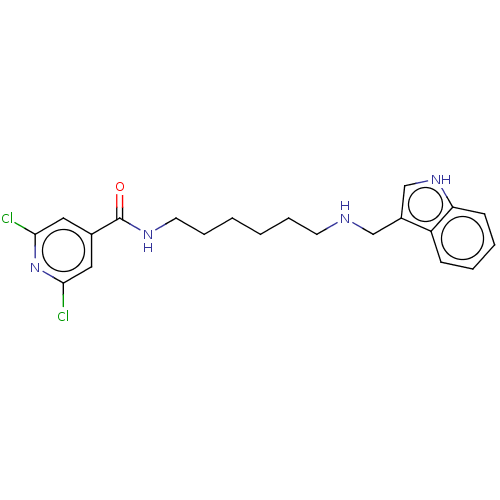 (CHEMBL4165299) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278969 (CHEMBL4160790) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278984 (CHEMBL4168710) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278970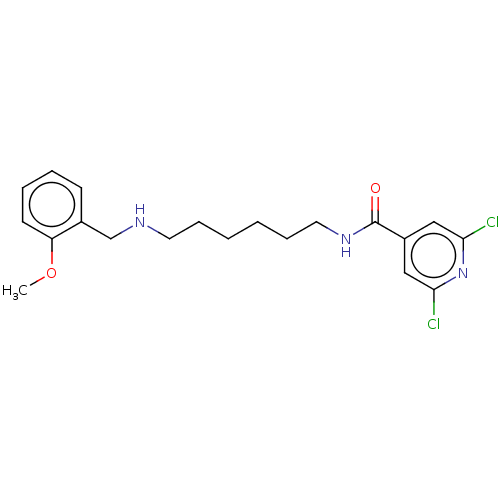 (CHEMBL4175914) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278983 (CHEMBL4172510) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278487 (CHEMBL3585376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Displacement of 125-I echistatin from Vitronectin receptor (alpha v beta3) | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485931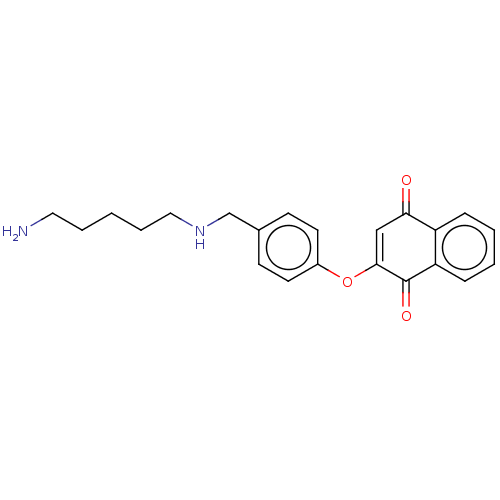 (CHEMBL2178999) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-inhibitor c... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-inhibitor c... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Noncompetitive inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide by Lineweaver-Burk plot | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-substrate-i... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278488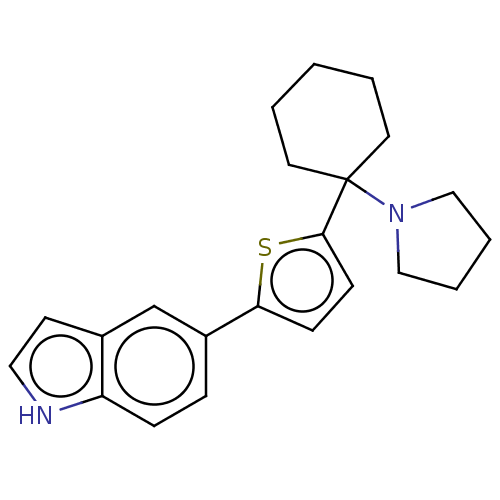 (CHEMBL4159241) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485931 (CHEMBL2178999) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-substrate-i... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278486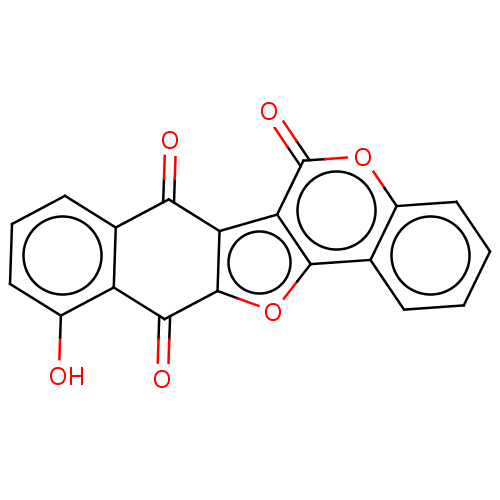 (CHEMBL4160100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed-type inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione d... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM77970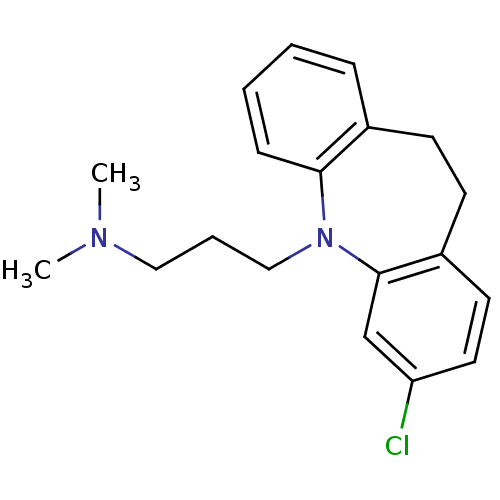 (3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50278489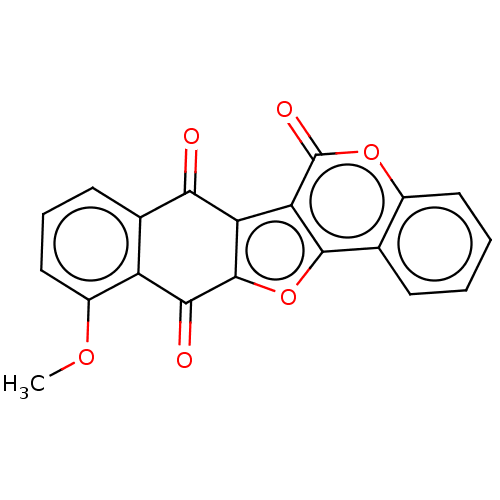 (CHEMBL4170316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Non-competitive inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothi... | Eur J Med Chem 141: 138-148 (2017) Article DOI: 10.1016/j.ejmech.2017.10.005 BindingDB Entry DOI: 10.7270/Q2JD50B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587056 (CHEMBL5080685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0352 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587059 (CHEMBL5092094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.177 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10512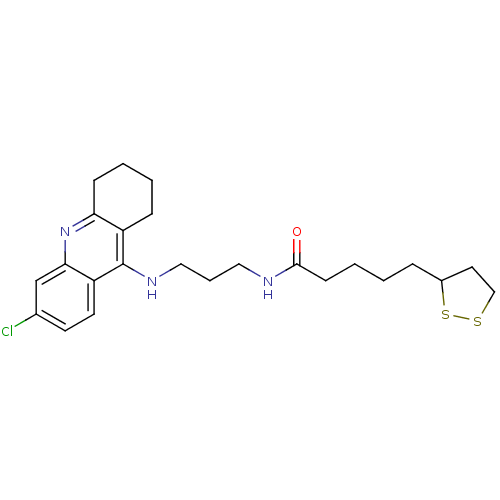 (CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.253 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 52: 7883-6 (2009) Article DOI: 10.1021/jm901123n BindingDB Entry DOI: 10.7270/Q2571C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587057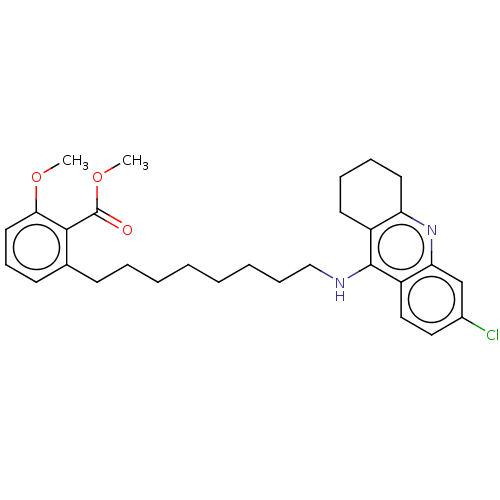 (CHEMBL5078555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.265 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231951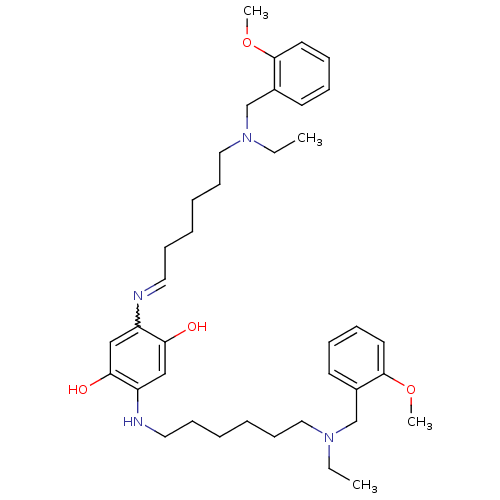 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE by Ellman's method | J Med Chem 52: 7883-6 (2009) Article DOI: 10.1021/jm901123n BindingDB Entry DOI: 10.7270/Q2571C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50231951 (2,5-bis(6-((2-methoxybenzyl)(ethyl)amino)hexylamin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028681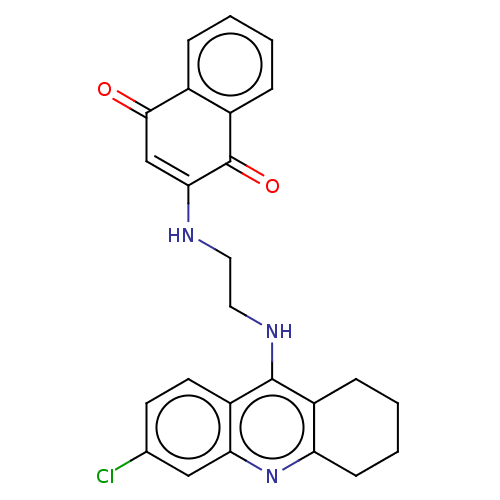 (CHEMBL3356532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221914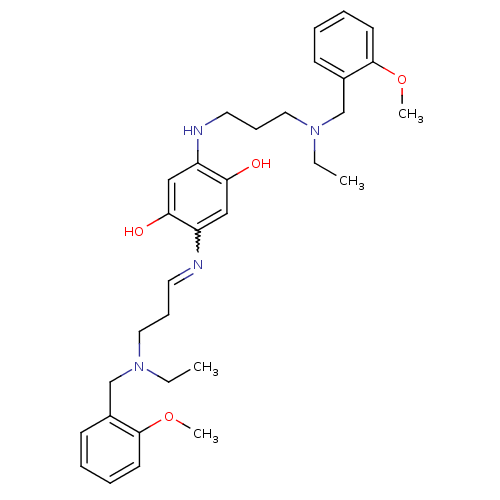 (2,5-bis{3-[ethyl(2-methoxybenzyl)amino]propylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221909 (2,5-bis{5-[ethyl(2-methoxybenzyl)amino]pentylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50587057 (CHEMBL5078555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221918 (2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50587064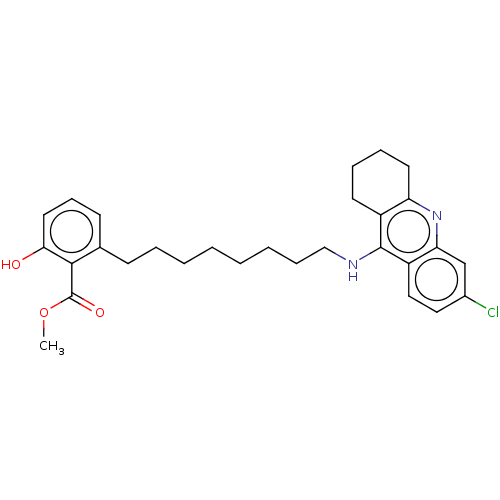 (CHEMBL5082773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221908 (2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028691 (CHEMBL3356951) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587062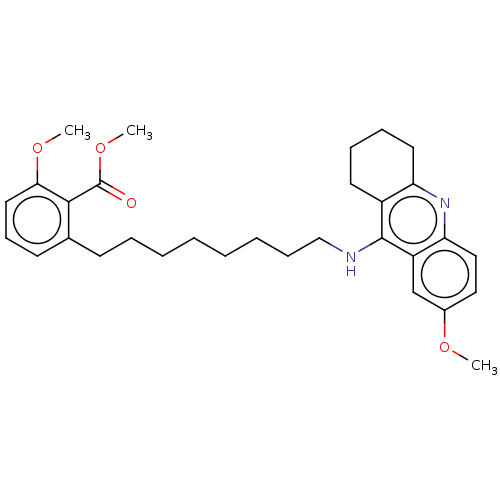 (CHEMBL5084379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587066 (CHEMBL5073819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50587061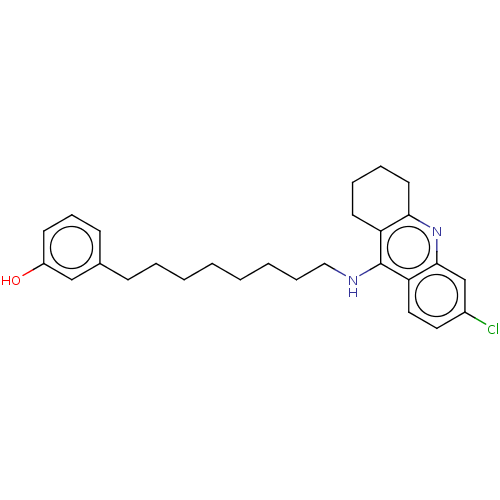 (CHEMBL5072428) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587064 (CHEMBL5082773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50587063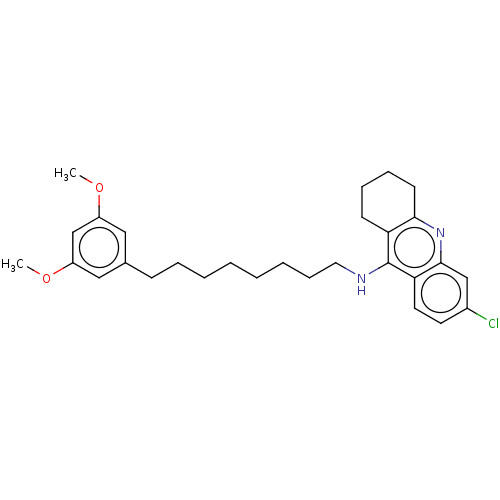 (CHEMBL5077234) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221926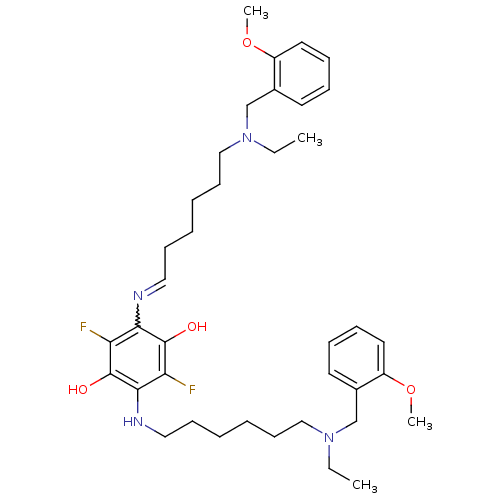 (2,5-bis{6-[ethyl(2-methoxybenzyl)amino]hexylamino}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028682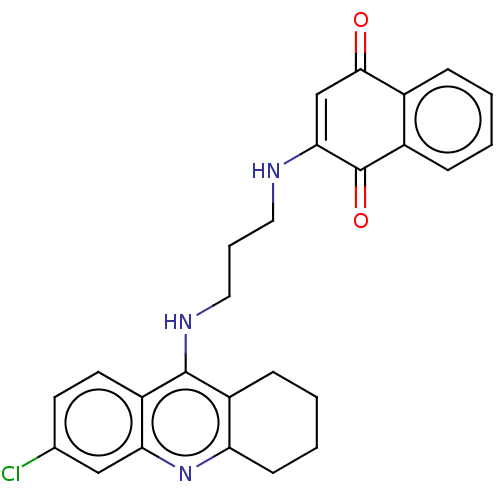 (CHEMBL3356533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028679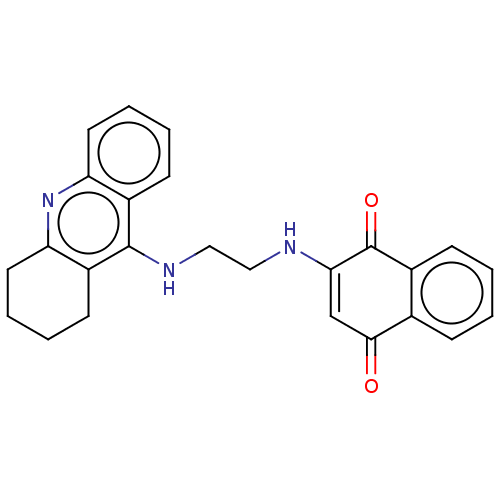 (CHEMBL3356530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50587058 (CHEMBL5088557) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE after 5 mins by Ellman method | J Med Chem 57: 8576-89 (2014) Article DOI: 10.1021/jm5010804 BindingDB Entry DOI: 10.7270/Q25H7HVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515598 (CHEMBL4526049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR 113808 from recombinant human brain 5HT4 receptor measured after 60 mins by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50587055 (CHEMBL5091660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00048 BindingDB Entry DOI: 10.7270/Q24F1VNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50221929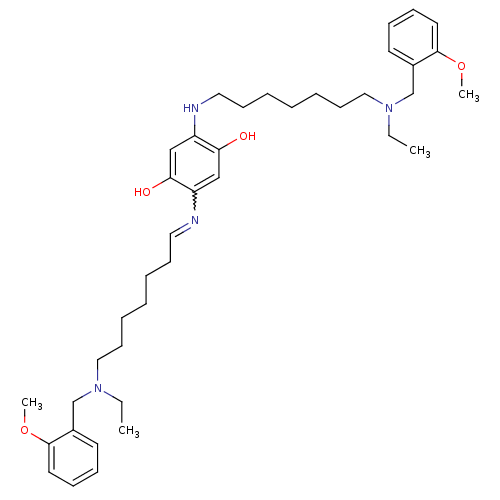 (2,5-bis{7-[ethyl(2-methoxybenzyl)amino]heptylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant acetylcholinesterase | J Med Chem 50: 4882-97 (2007) Article DOI: 10.1021/jm070559a BindingDB Entry DOI: 10.7270/Q218379R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 253 total ) | Next | Last >> |