 Found 523 hits with Last Name = 'deng' and Initial = 'c'
Found 523 hits with Last Name = 'deng' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493380
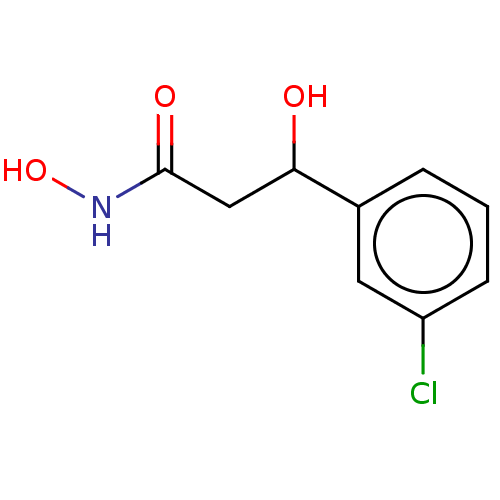
(CHEMBL2425469)Show InChI InChI=1S/C9H10ClNO3/c10-7-3-1-2-6(4-7)8(12)5-9(13)11-14/h1-4,8,12,14H,5H2,(H,11,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis |
Eur J Med Chem 68: 212-21 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.047
BindingDB Entry DOI: 10.7270/Q28G8PNR |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493373
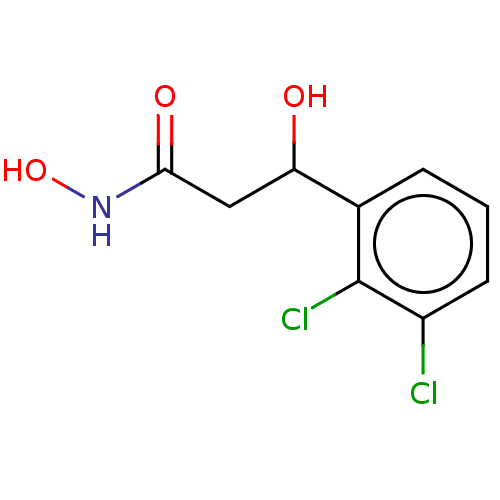
(CHEMBL2425478)Show InChI InChI=1S/C9H9Cl2NO3/c10-6-3-1-2-5(9(6)11)7(13)4-8(14)12-15/h1-3,7,13,15H,4H2,(H,12,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Mixed-type competitive inhibition of Helicobacter pylori ATCC 43504 urease using urea as substrate by Lineweaver-burk plot analysis |
Eur J Med Chem 68: 212-21 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.047
BindingDB Entry DOI: 10.7270/Q28G8PNR |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504
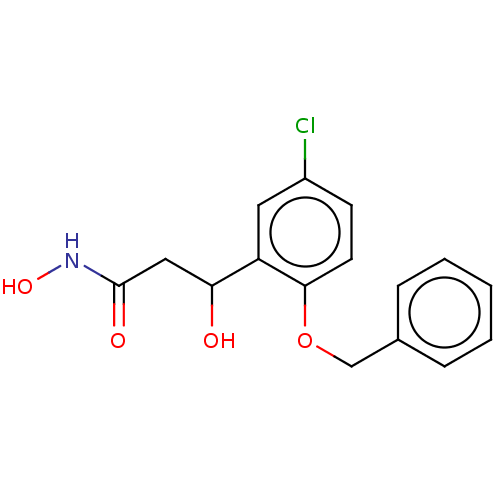
(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534504

(CHEMBL4590676)Show InChI InChI=1S/C16H16ClNO4/c17-12-6-7-15(22-10-11-4-2-1-3-5-11)13(8-12)14(19)9-16(20)18-21/h1-8,14,19,21H,9-10H2,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-urea-inhibitor complex by non-linear fitt... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50493364

(CHEMBL2425480)Show InChI InChI=1S/C9H10ClNO4/c10-5-1-2-7(12)6(3-5)8(13)4-9(14)11-15/h1-3,8,12-13,15H,4H2,(H,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori urease using urea as substrate assessed as inhibition constant for enzyme-inhibitor complex by non-linear fitting a... |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Urease subunit beta
(Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM24961
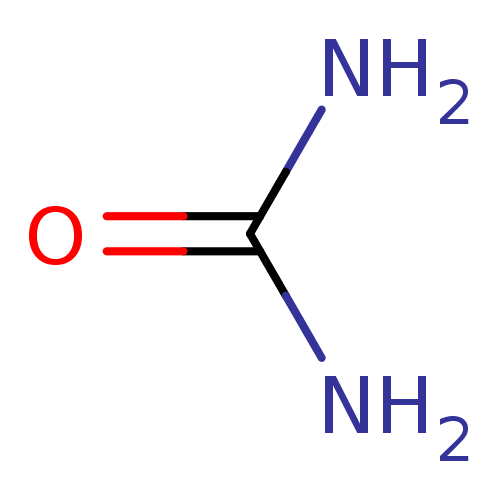
(Urea) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 6.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University
Curated by ChEMBL
| Assay Description
Substrate inhibition of Helicobacter pylori urease in presence of >4 mM urea by indophenol method |
Bioorg Med Chem 24: 4519-4527 (2016)
Article DOI: 10.1016/j.bmc.2016.07.052
BindingDB Entry DOI: 10.7270/Q24F1V76 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456700

(CHEMBL4205425)Show SMILES COc1ccccc1C(=O)N1Cc2cc(NC(=O)c3cccc(Cl)c3)ccc2CC1=O Show InChI InChI=1S/C24H19ClN2O4/c1-31-21-8-3-2-7-20(21)24(30)27-14-17-12-19(10-9-15(17)13-22(27)28)26-23(29)16-5-4-6-18(25)11-16/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456689
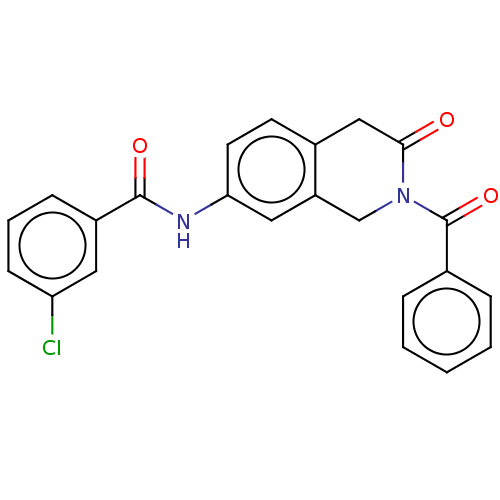
(CHEMBL4205954)Show SMILES Clc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C23H17ClN2O3/c24-19-8-4-7-17(11-19)22(28)25-20-10-9-16-13-21(27)26(14-18(16)12-20)23(29)15-5-2-1-3-6-15/h1-12H,13-14H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456691
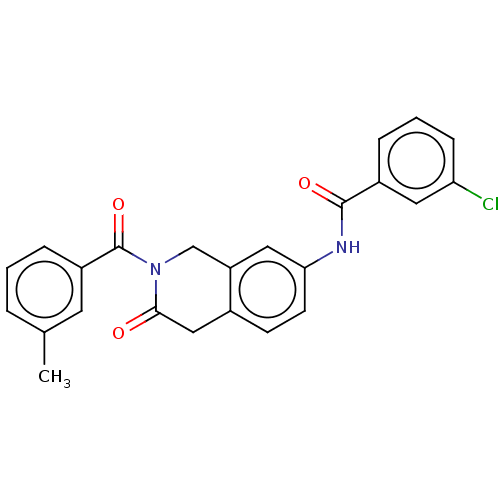
(CHEMBL4210316)Show SMILES Cc1cccc(c1)C(=O)N1Cc2cc(NC(=O)c3cccc(Cl)c3)ccc2CC1=O Show InChI InChI=1S/C24H19ClN2O3/c1-15-4-2-6-18(10-15)24(30)27-14-19-12-21(9-8-16(19)13-22(27)28)26-23(29)17-5-3-7-20(25)11-17/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456692

(CHEMBL4209803)Show SMILES Cc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(C)c1 Show InChI InChI=1S/C25H22N2O3/c1-16-5-3-7-19(11-16)24(29)26-22-10-9-18-14-23(28)27(15-21(18)13-22)25(30)20-8-4-6-17(2)12-20/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456690

(CHEMBL4217663)Show SMILES Clc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C23H16Cl2N2O3/c24-18-5-1-3-15(9-18)22(29)26-20-8-7-14-12-21(28)27(13-17(14)11-20)23(30)16-4-2-6-19(25)10-16/h1-11H,12-13H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456693
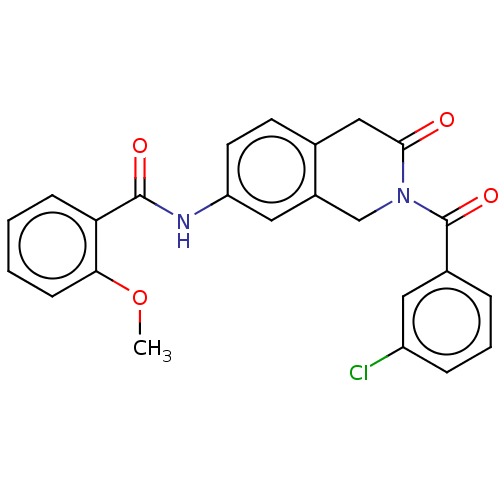
(CHEMBL4214430)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C24H19ClN2O4/c1-31-21-8-3-2-7-20(21)23(29)26-19-10-9-15-13-22(28)27(14-17(15)12-19)24(30)16-5-4-6-18(25)11-16/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456699
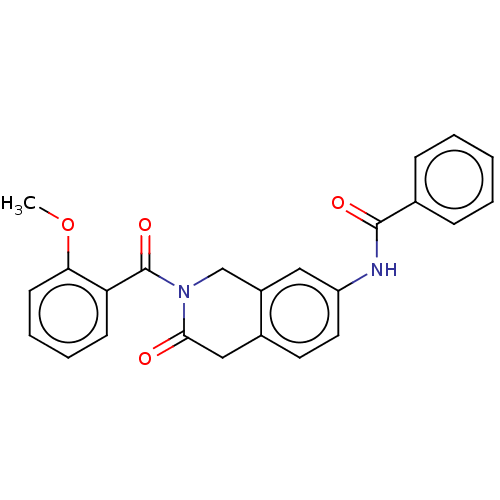
(CHEMBL4213253)Show SMILES COc1ccccc1C(=O)N1Cc2cc(NC(=O)c3ccccc3)ccc2CC1=O Show InChI InChI=1S/C24H20N2O4/c1-30-21-10-6-5-9-20(21)24(29)26-15-18-13-19(12-11-17(18)14-22(26)27)25-23(28)16-7-3-2-4-8-16/h2-13H,14-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456696
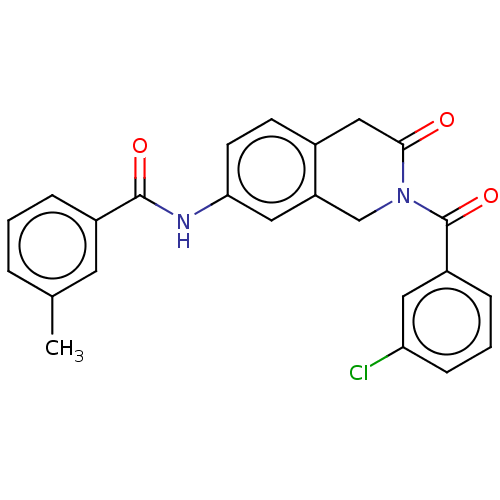
(CHEMBL4214707)Show SMILES Cc1cccc(c1)C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C24H19ClN2O3/c1-15-4-2-5-17(10-15)23(29)26-21-9-8-16-13-22(28)27(14-19(16)12-21)24(30)18-6-3-7-20(25)11-18/h2-12H,13-14H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456682
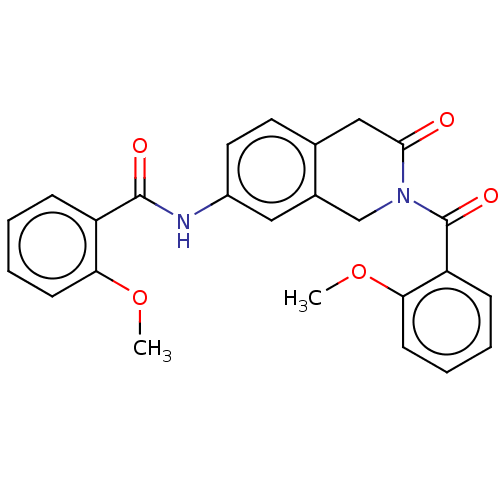
(CHEMBL4210041)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1OC Show InChI InChI=1S/C25H22N2O5/c1-31-21-9-5-3-7-19(21)24(29)26-18-12-11-16-14-23(28)27(15-17(16)13-18)25(30)20-8-4-6-10-22(20)32-2/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456694
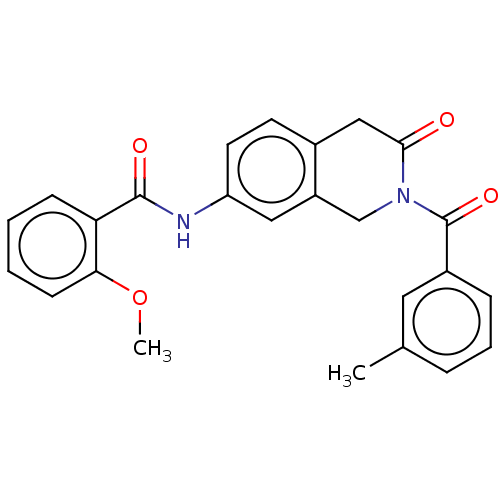
(CHEMBL4205144)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1cccc(C)c1 Show InChI InChI=1S/C25H22N2O4/c1-16-6-5-7-18(12-16)25(30)27-15-19-13-20(11-10-17(19)14-23(27)28)26-24(29)21-8-3-4-9-22(21)31-2/h3-13H,14-15H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456688
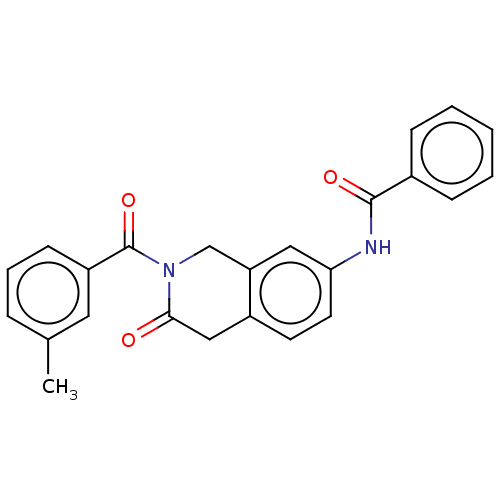
(CHEMBL4218191)Show SMILES Cc1cccc(c1)C(=O)N1Cc2cc(NC(=O)c3ccccc3)ccc2CC1=O Show InChI InChI=1S/C24H20N2O3/c1-16-6-5-9-19(12-16)24(29)26-15-20-13-21(11-10-18(20)14-22(26)27)25-23(28)17-7-3-2-4-8-17/h2-13H,14-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456687
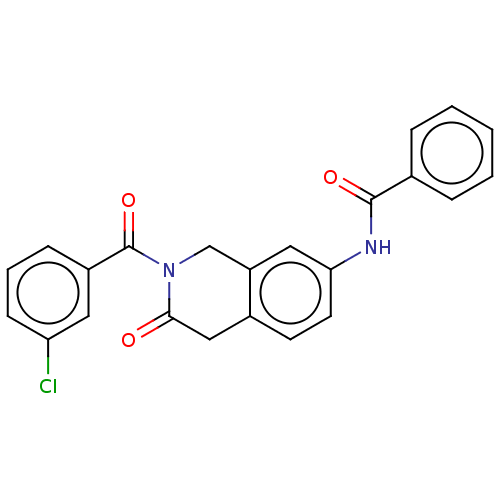
(CHEMBL4208866)Show SMILES Clc1cccc(c1)C(=O)N1Cc2cc(NC(=O)c3ccccc3)ccc2CC1=O Show InChI InChI=1S/C23H17ClN2O3/c24-19-8-4-7-17(11-19)23(29)26-14-18-12-20(10-9-16(18)13-21(26)27)25-22(28)15-5-2-1-3-6-15/h1-12H,13-14H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456698
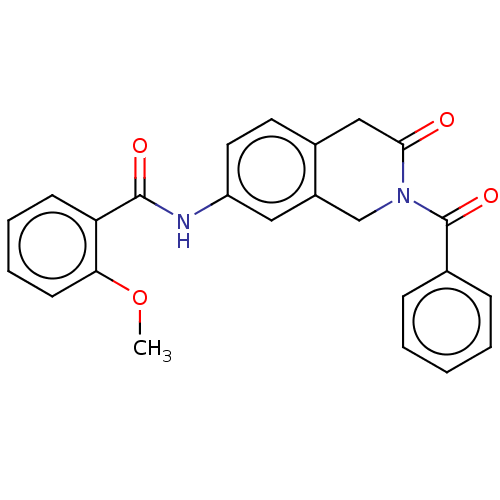
(CHEMBL4209518)Show SMILES COc1ccccc1C(=O)Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C24H20N2O4/c1-30-21-10-6-5-9-20(21)23(28)25-19-12-11-17-14-22(27)26(15-18(17)13-19)24(29)16-7-3-2-4-8-16/h2-13H,14-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325177
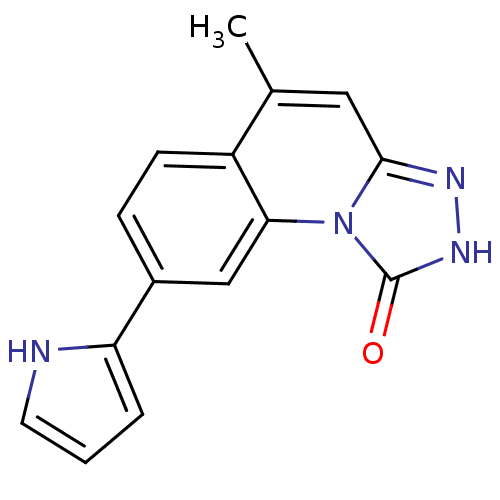
(5-methyl-8-(1H-pyrrol-2-yl)-[1,2,4]triazolo[4,3-a]...)Show InChI InChI=1S/C15H12N4O/c1-9-7-14-17-18-15(20)19(14)13-8-10(4-5-11(9)13)12-3-2-6-16-12/h2-8,16H,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325177

(5-methyl-8-(1H-pyrrol-2-yl)-[1,2,4]triazolo[4,3-a]...)Show InChI InChI=1S/C15H12N4O/c1-9-7-14-17-18-15(20)19(14)13-8-10(4-5-11(9)13)12-3-2-6-16-12/h2-8,16H,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 22: 2330-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.043
BindingDB Entry DOI: 10.7270/Q2GM88B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50456697
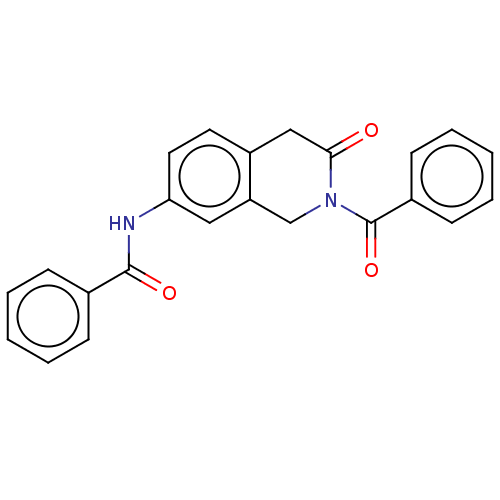
(CHEMBL4213792)Show SMILES O=C(Nc1ccc2CC(=O)N(Cc2c1)C(=O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H18N2O3/c26-21-14-18-11-12-20(24-22(27)16-7-3-1-4-8-16)13-19(18)15-25(21)23(28)17-9-5-2-6-10-17/h1-13H,14-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239258
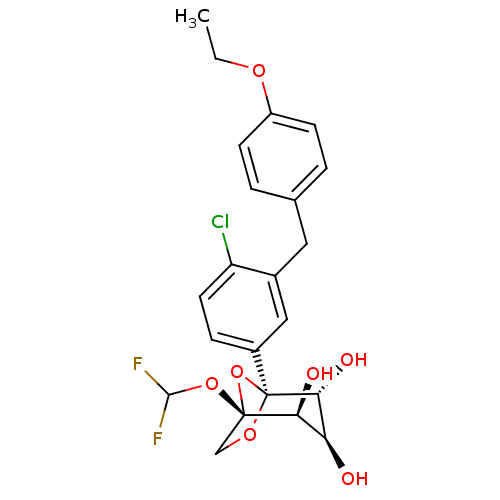
(CHEMBL4070727)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](OC(F)F)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H23ClF2O7/c1-2-29-15-6-3-12(4-7-15)9-13-10-14(5-8-16(13)23)22-19(28)17(26)18(27)21(32-22,11-30-22)31-20(24)25/h3-8,10,17-20,26-28H,2,9,11H2,1H3/t17-,18-,19+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239257

(CHEMBL4071777)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](OCC#N)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C23H24ClNO7/c1-2-29-17-6-3-14(4-7-17)11-15-12-16(5-8-18(15)24)23-21(28)19(26)20(27)22(32-23,13-31-23)30-10-9-25/h3-8,12,19-21,26-28H,2,10-11,13H2,1H3/t19-,20-,21+,22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239256
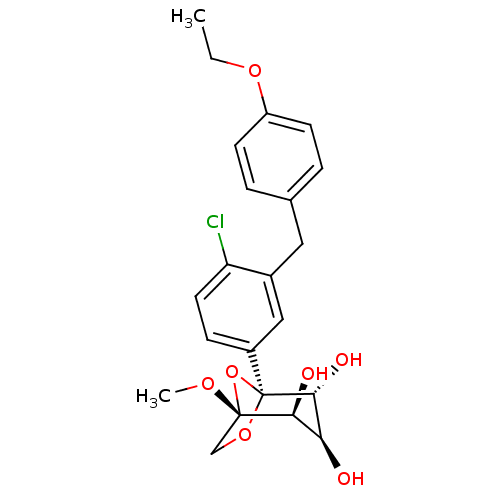
(CHEMBL4082283)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](OC)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-3-28-16-7-4-13(5-8-16)10-14-11-15(6-9-17(14)23)22-20(26)18(24)19(25)21(27-2,30-22)12-29-22/h4-9,11,18-20,24-26H,3,10,12H2,1-2H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50235017
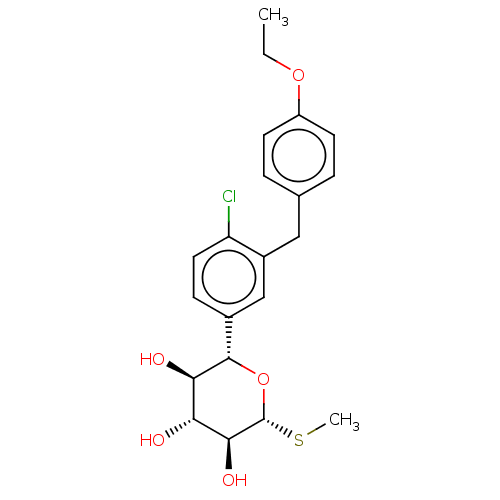
(LP-802034 | LX-4211 | Sotagliflozin)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5S/c1-3-26-15-7-4-12(5-8-15)10-14-11-13(6-9-16(14)22)20-18(24)17(23)19(25)21(27-20)28-2/h4-9,11,17-21,23-25H,3,10H2,1-2H3/t17-,18-,19+,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50235017

(LP-802034 | LX-4211 | Sotagliflozin)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](SC)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H25ClO5S/c1-3-26-15-7-4-12(5-8-15)10-14-11-13(6-9-16(14)22)20-18(24)17(23)19(25)21(27-20)28-2/h4-9,11,17-21,23-25H,3,10H2,1-2H3/t17-,18-,19+,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20880
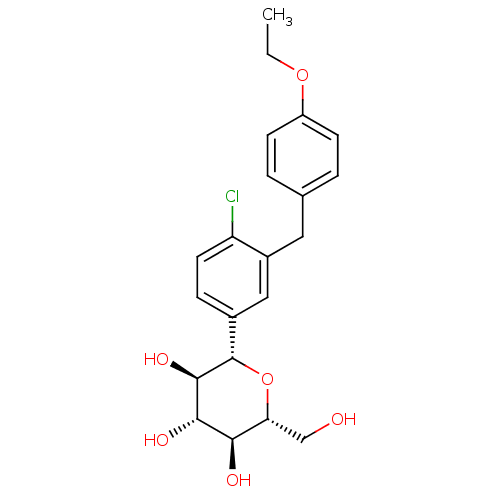
((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-3
(Homo sapiens (Human)) | BDBM10246
((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Fifth Affiliated Hospital of Guangzhou Medical University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human recombinant caspase 3 expressed in Escherichia coli using acetyl-Asp-Glu-Val-Asp-7- amido-4-methylcoumarin (Ac-DEVD-AM... |
Bioorg Med Chem Lett 26: 3195-201 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.031
BindingDB Entry DOI: 10.7270/Q2X350CV |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50386885
(CANAGLIFLOZIN | CANAGLIFLOZIN HYDRATE | US10752604...)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ccc(F)cc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition and measured after... |
Eur J Med Chem 146: 460-470 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.081
BindingDB Entry DOI: 10.7270/Q2NZ8B7G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50457968
(CHEMBL4207750)Show SMILES CCS(=O)(=O)Nc1cc(cnc1OC)-c1ccc2ncnc(Nc3ccc(F)c(Cl)c3)c2c1 Show InChI InChI=1S/C22H19ClFN5O3S/c1-3-33(30,31)29-20-9-14(11-25-22(20)32-2)13-4-7-19-16(8-13)21(27-12-26-19)28-15-5-6-18(24)17(23)10-15/h4-12,29H,3H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition and measured after... |
Eur J Med Chem 146: 460-470 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.081
BindingDB Entry DOI: 10.7270/Q2NZ8B7G |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50342885
((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phe...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239266
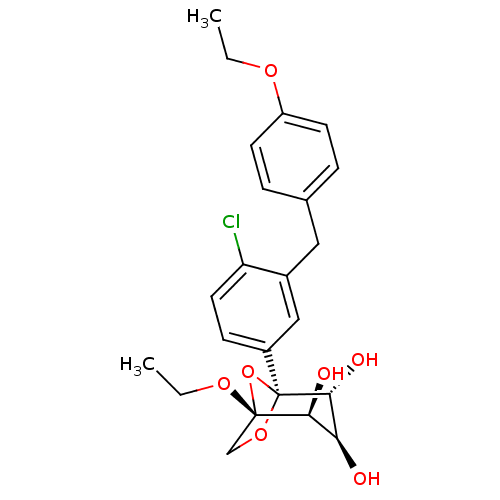
(CHEMBL4092561)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](OCC)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C23H27ClO7/c1-3-28-17-8-5-14(6-9-17)11-15-12-16(7-10-18(15)24)23-21(27)19(25)20(26)22(31-23,13-30-23)29-4-2/h5-10,12,19-21,25-27H,3-4,11,13H2,1-2H3/t19-,20-,21+,22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50243148

(1-(3-((S)-piperidin-3-ylcarbamoyl)-5-(thiophen-2-y...)Show SMILES NC(=O)Nc1sc(cc1C(=O)N[C@H]1CCCNC1)-c1cccs1 |r| Show InChI InChI=1S/C15H18N4O2S2/c16-15(21)19-14-10(7-12(23-14)11-4-2-6-22-11)13(20)18-9-3-1-5-17-8-9/h2,4,6-7,9,17H,1,3,5,8H2,(H,18,20)(H3,16,19,21)/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 18: 4242-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.016
BindingDB Entry DOI: 10.7270/Q2J102Z9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379546

(CHEMBL2012879)Show InChI InChI=1S/C16H13N3O2S/c1-21-8-12-7-15-17-18-16(20)19(15)14-6-10(2-3-13(12)14)11-4-5-22-9-11/h2-7,9H,8H2,1H3,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 22: 2330-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.043
BindingDB Entry DOI: 10.7270/Q2GM88B0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50243624

((R)-1-(3-(azepan-3-ylcarbamoyl)-5-phenylthiophen-2...)Show SMILES NC(=O)Nc1sc(cc1C(=O)N[C@@H]1CCCCNC1)-c1ccccc1 |r| Show InChI InChI=1S/C18H22N4O2S/c19-18(24)22-17-14(10-15(25-17)12-6-2-1-3-7-12)16(23)21-13-8-4-5-9-20-11-13/h1-3,6-7,10,13,20H,4-5,8-9,11H2,(H,21,23)(H3,19,22,24)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 18: 4242-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.016
BindingDB Entry DOI: 10.7270/Q2J102Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239265
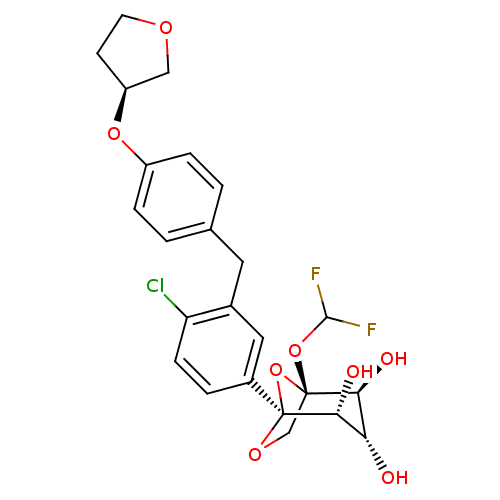
(CHEMBL4073410)Show SMILES O[C@H]1[C@H](O)[C@@]2(CO[C@@](O2)([C@@H]1O)c1ccc(Cl)c(Cc2ccc(O[C@H]3CCOC3)cc2)c1)OC(F)F |r| Show InChI InChI=1S/C24H25ClF2O8/c25-18-6-3-15(24-21(30)19(28)20(29)23(35-24,12-32-24)34-22(26)27)10-14(18)9-13-1-4-16(5-2-13)33-17-7-8-31-11-17/h1-6,10,17,19-22,28-30H,7-9,11-12H2/t17-,19-,20-,21+,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by Ellman's method |
Eur J Med Chem 138: 738-747 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.006
BindingDB Entry DOI: 10.7270/Q2HM5C2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379547

(CHEMBL2012859)Show SMILES CN1CCN(Cc2cc(cs2)-c2ccc3c(N)cc4n[nH]c(=O)n4c3c2)CC1 Show InChI InChI=1S/C20H22N6OS/c1-24-4-6-25(7-5-24)11-15-8-14(12-28-15)13-2-3-16-17(21)10-19-22-23-20(27)26(19)18(16)9-13/h2-3,8-10,12H,4-7,11,21H2,1H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 22: 2330-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.043
BindingDB Entry DOI: 10.7270/Q2GM88B0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50325145
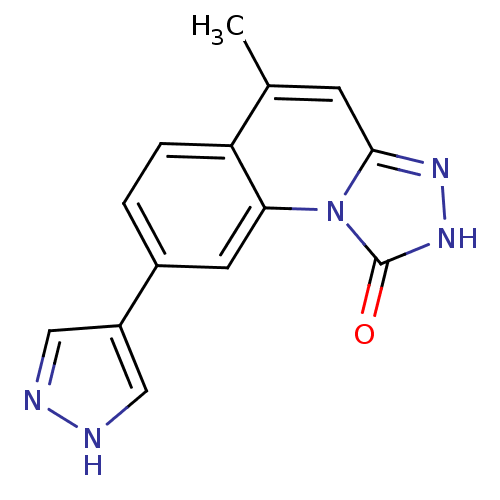
(5-methyl-8-(1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a...)Show InChI InChI=1S/C14H11N5O/c1-8-4-13-17-18-14(20)19(13)12-5-9(2-3-11(8)12)10-6-15-16-7-10/h2-7H,1H3,(H,15,16)(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of His6-CHK1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 20: 5133-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.015
BindingDB Entry DOI: 10.7270/Q2FF3SJZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50243151
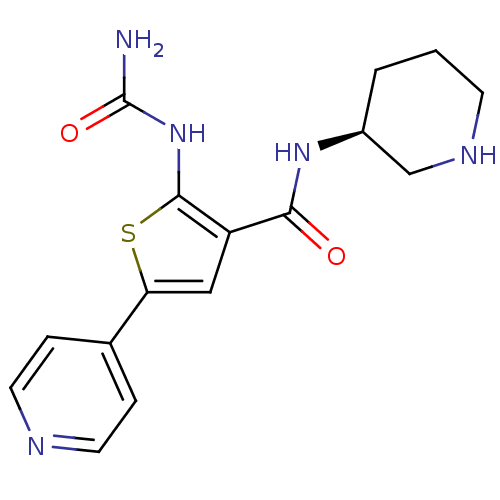
((S)-1-(3-(piperidin-3-ylcarbamoyl)-5-(pyridin-4-yl...)Show SMILES NC(=O)Nc1sc(cc1C(=O)N[C@H]1CCCNC1)-c1ccncc1 |r| Show InChI InChI=1S/C16H19N5O2S/c17-16(23)21-15-12(14(22)20-11-2-1-5-19-9-11)8-13(24-15)10-3-6-18-7-4-10/h3-4,6-8,11,19H,1-2,5,9H2,(H,20,22)(H3,17,21,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 18: 4242-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.016
BindingDB Entry DOI: 10.7270/Q2J102Z9 |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239259
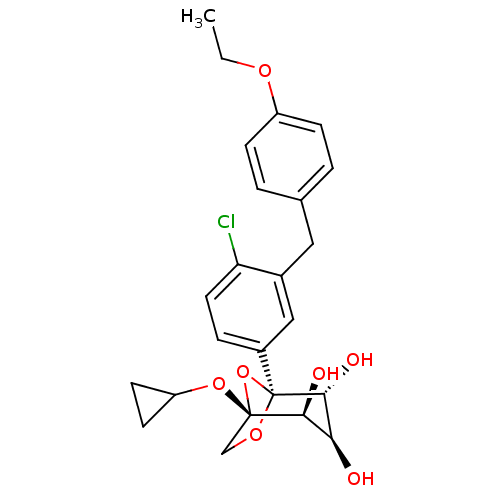
(CHEMBL4097849)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](OC4CC4)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C24H27ClO7/c1-2-29-17-6-3-14(4-7-17)11-15-12-16(5-10-19(15)25)24-22(28)20(26)21(27)23(32-24,13-30-24)31-18-8-9-18/h3-7,10,12,18,20-22,26-28H,2,8-9,11,13H2,1H3/t20-,21-,22+,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50243201

((S)-1-(5-(4-carbamoylphenyl)-3-(piperidin-3-ylcarb...)Show SMILES NC(=O)Nc1sc(cc1C(=O)N[C@H]1CCCNC1)-c1ccc(cc1)C(N)=O |r| Show InChI InChI=1S/C18H21N5O3S/c19-15(24)11-5-3-10(4-6-11)14-8-13(17(27-14)23-18(20)26)16(25)22-12-2-1-7-21-9-12/h3-6,8,12,21H,1-2,7,9H2,(H2,19,24)(H,22,25)(H3,20,23,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 18: 4242-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.016
BindingDB Entry DOI: 10.7270/Q2J102Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50239260
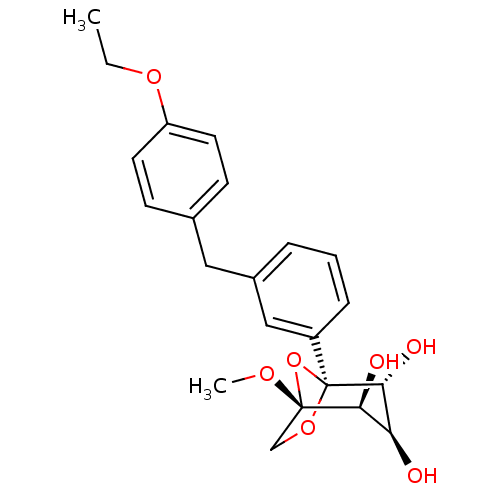
(CHEMBL4090131)Show SMILES CCOc1ccc(Cc2cccc(c2)[C@]23OC[C@](OC)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H26O7/c1-3-27-17-9-7-14(8-10-17)11-15-5-4-6-16(12-15)22-20(25)18(23)19(24)21(26-2,29-22)13-28-22/h4-10,12,18-20,23-25H,3,11,13H2,1-2H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Haisco Pharmaceuticals Group Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount method |
J Med Chem 60: 4173-4184 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01818
BindingDB Entry DOI: 10.7270/Q2W37ZFS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50243047

(1-(3-((S)-piperidin-3-ylcarbamoyl)-5-(3-carbamoylp...)Show SMILES NC(=O)Nc1sc(cc1C(=O)N[C@H]1CCCNC1)-c1cccc(c1)C(N)=O |r| Show InChI InChI=1S/C18H21N5O3S/c19-15(24)11-4-1-3-10(7-11)14-8-13(17(27-14)23-18(20)26)16(25)22-12-5-2-6-21-9-12/h1,3-4,7-8,12,21H,2,5-6,9H2,(H2,19,24)(H,22,25)(H3,20,23,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 18: 4242-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.016
BindingDB Entry DOI: 10.7270/Q2J102Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50389803
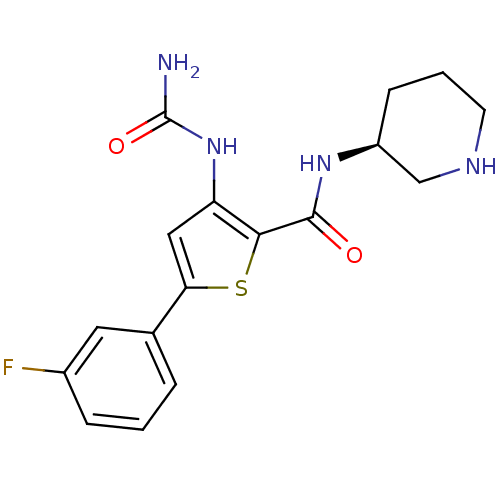
(AZD7762 | CHEMBL2041933)Show SMILES NC(=O)Nc1cc(sc1C(=O)N[C@H]1CCCNC1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C17H19FN4O2S/c18-11-4-1-3-10(7-11)14-8-13(22-17(19)24)15(25-14)16(23)21-12-5-2-6-20-9-12/h1,3-4,7-8,12,20H,2,5-6,9H2,(H,21,23)(H3,19,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Oncology Chemistry, IMED Biotech Unit, AstraZeneca , 35 Gatehouse Drive, Waltham, Massachusetts 02451, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 (unknown origin) |
J Med Chem 61: 1061-1073 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01490
BindingDB Entry DOI: 10.7270/Q2PC34T5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50379548

(CHEMBL2012858)Show InChI InChI=1S/C16H15N5OS/c1-18-7-11-4-10(8-23-11)9-2-3-12-13(17)6-15-19-20-16(22)21(15)14(12)5-9/h2-6,8,18H,7,17H2,1H3,(H,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 22: 2330-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.043
BindingDB Entry DOI: 10.7270/Q2GM88B0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50389803

(AZD7762 | CHEMBL2041933)Show SMILES NC(=O)Nc1cc(sc1C(=O)N[C@H]1CCCNC1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C17H19FN4O2S/c18-11-4-1-3-10(7-11)14-8-13(22-17(19)24)15(25-14)16(23)21-12-5-2-6-20-9-12/h1,3-4,7-8,12,20H,2,5-6,9H2,(H,21,23)(H3,19,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CHK1 expressed in insect cells using biotinylaminohexanoyl-KKVSRSGLYRSPMPENLNRPR as substrate after 2 hrs by scintill... |
J Med Chem 55: 5130-42 (2012)
Article DOI: 10.1021/jm300025r
BindingDB Entry DOI: 10.7270/Q2RV0PR6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data