 Found 351 hits with Last Name = 'engel' and Initial = 'c'
Found 351 hits with Last Name = 'engel' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cruzipain
(Trypanosoma cruzi) | BDBM50306596
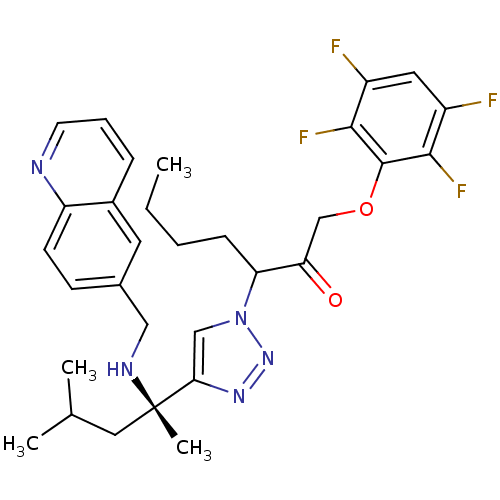
(3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@](C)(CC(C)C)NCc1ccc2ncccc2c1 |r| Show InChI InChI=1S/C31H35F4N5O2/c1-5-6-9-25(26(41)18-42-30-28(34)22(32)14-23(33)29(30)35)40-17-27(38-39-40)31(4,15-19(2)3)37-16-20-10-11-24-21(13-20)8-7-12-36-24/h7-8,10-14,17,19,25,37H,5-6,9,15-16,18H2,1-4H3/t25?,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306600
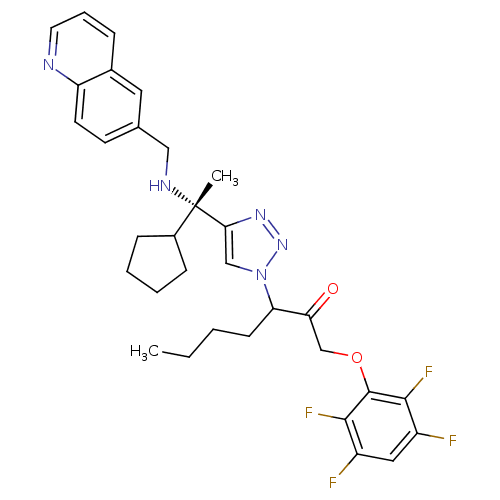
(3-(4-((S)-1-cyclopentyl-1-(quinolin-6-ylmethylamin...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCCC1 |r| Show InChI InChI=1S/C32H35F4N5O2/c1-3-4-11-26(27(42)19-43-31-29(35)23(33)16-24(34)30(31)36)41-18-28(39-40-41)32(2,22-9-5-6-10-22)38-17-20-12-13-25-21(15-20)8-7-14-37-25/h7-8,12-16,18,22,26,38H,3-6,9-11,17,19H2,1-2H3/t26?,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306598
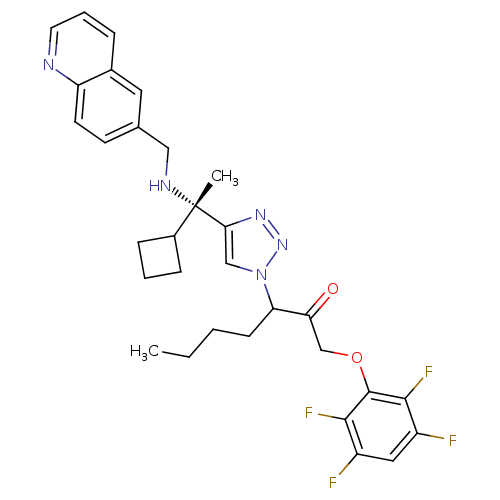
(3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCC1 |r| Show InChI InChI=1S/C31H33F4N5O2/c1-3-4-10-25(26(41)18-42-30-28(34)22(32)15-23(33)29(30)35)40-17-27(38-39-40)31(2,21-8-5-9-21)37-16-19-11-12-24-20(14-19)7-6-13-36-24/h6-7,11-15,17,21,25,37H,3-5,8-10,16,18H2,1-2H3/t25?,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306597
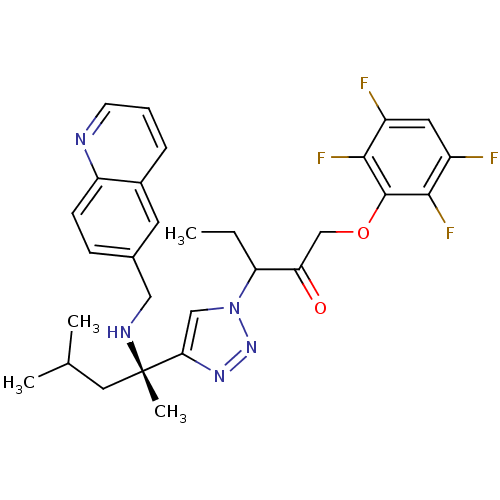
(3-(4-((S)-4-methyl-2-(quinolin-6-ylmethylamino)pen...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@](C)(CC(C)C)NCc1ccc2ncccc2c1 |r| Show InChI InChI=1S/C29H31F4N5O2/c1-5-23(24(39)16-40-28-26(32)20(30)12-21(31)27(28)33)38-15-25(36-37-38)29(4,13-17(2)3)35-14-18-8-9-22-19(11-18)7-6-10-34-22/h6-12,15,17,23,35H,5,13-14,16H2,1-4H3/t23?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306599
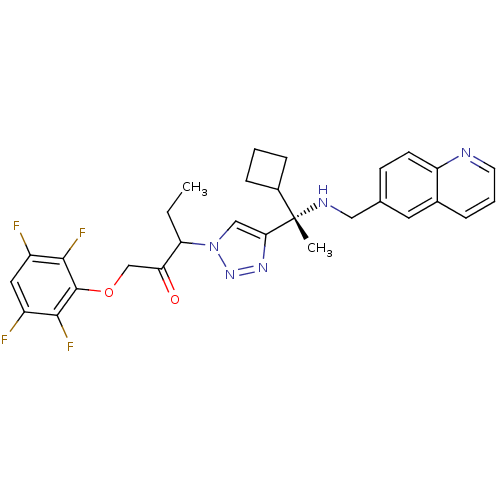
(3-(4-((S)-1-cyclobutyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCC1 |r| Show InChI InChI=1S/C29H29F4N5O2/c1-3-23(24(39)16-40-28-26(32)20(30)13-21(31)27(28)33)38-15-25(36-37-38)29(2,19-7-4-8-19)35-14-17-9-10-22-18(12-17)6-5-11-34-22/h5-6,9-13,15,19,23,35H,3-4,7-8,14,16H2,1-2H3/t23?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306592
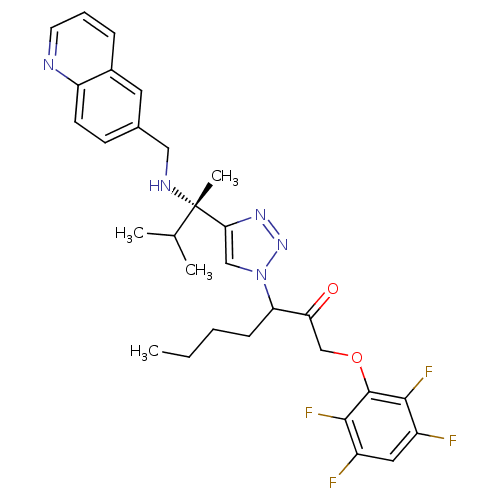
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C(C)C |r| Show InChI InChI=1S/C30H33F4N5O2/c1-5-6-9-24(25(40)17-41-29-27(33)21(31)14-22(32)28(29)34)39-16-26(37-38-39)30(4,18(2)3)36-15-19-10-11-23-20(13-19)8-7-12-35-23/h7-8,10-14,16,18,24,36H,5-6,9,15,17H2,1-4H3/t24?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869
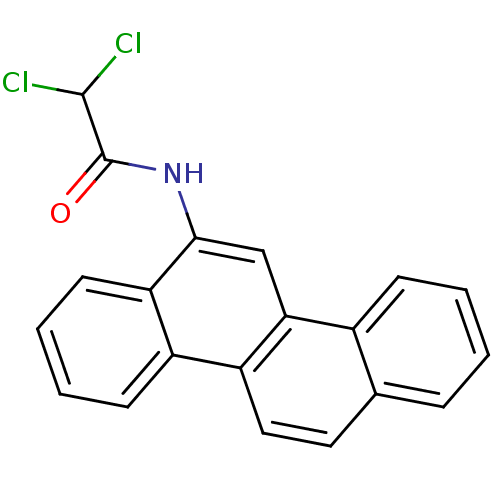
(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128871
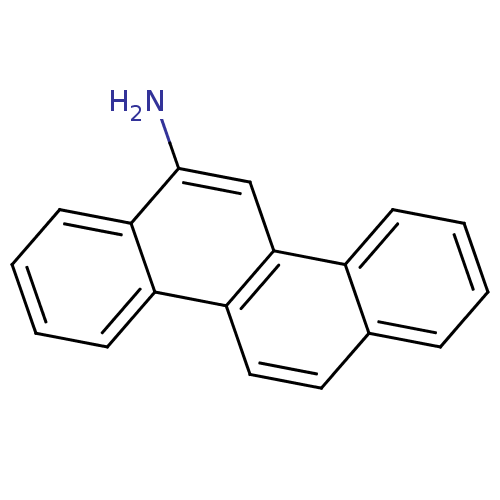
(CHEMBL313154 | Chrysen-6-ylamine)Show InChI InChI=1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306595
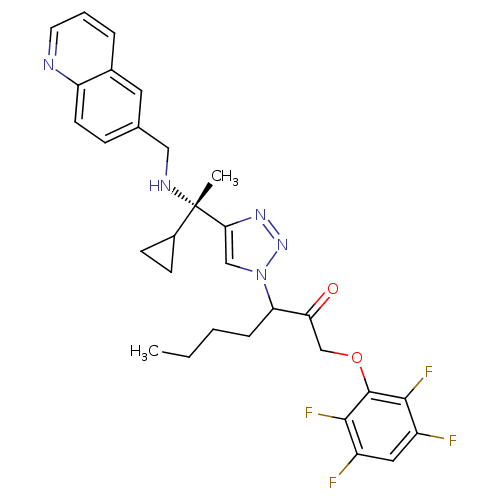
(3-(4-((S)-1-cyclopropyl-1-(quinolin-6-ylmethylamin...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CC1 |r| Show InChI InChI=1S/C30H31F4N5O2/c1-3-4-7-24(25(40)17-41-29-27(33)21(31)14-22(32)28(29)34)39-16-26(37-38-39)30(2,20-9-10-20)36-15-18-8-11-23-19(13-18)6-5-12-35-23/h5-6,8,11-14,16,20,24,36H,3-4,7,9-10,15,17H2,1-2H3/t24?,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306593
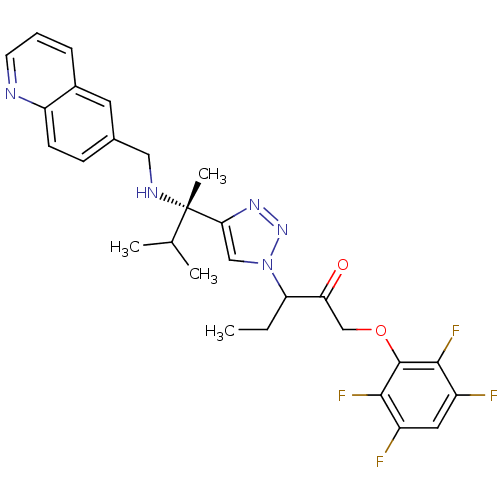
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C(C)C |r| Show InChI InChI=1S/C28H29F4N5O2/c1-5-22(23(38)15-39-27-25(31)19(29)12-20(30)26(27)32)37-14-24(35-36-37)28(4,16(2)3)34-13-17-8-9-21-18(11-17)7-6-10-33-21/h6-12,14,16,22,34H,5,13,15H2,1-4H3/t22?,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306601
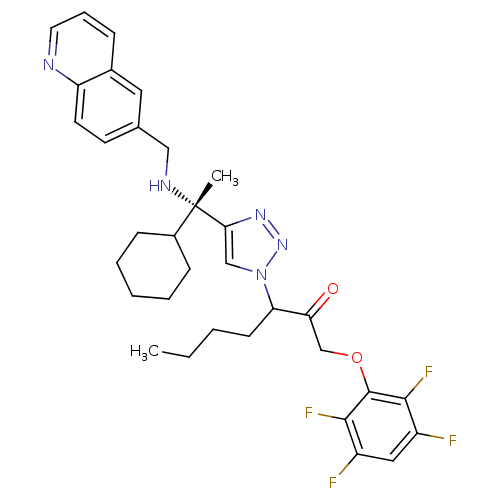
(3-(4-((S)-1-cyclohexyl-1-(quinolin-6-ylmethylamino...)Show SMILES CCCCC(C(=O)COc1c(F)c(F)cc(F)c1F)n1cc(nn1)[C@@](C)(NCc1ccc2ncccc2c1)C1CCCCC1 |r| Show InChI InChI=1S/C33H37F4N5O2/c1-3-4-12-27(28(43)20-44-32-30(36)24(34)17-25(35)31(32)37)42-19-29(40-41-42)33(2,23-10-6-5-7-11-23)39-18-21-13-14-26-22(16-21)9-8-15-38-26/h8-9,13-17,19,23,27,39H,3-7,10-12,18,20H2,1-2H3/t27?,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867
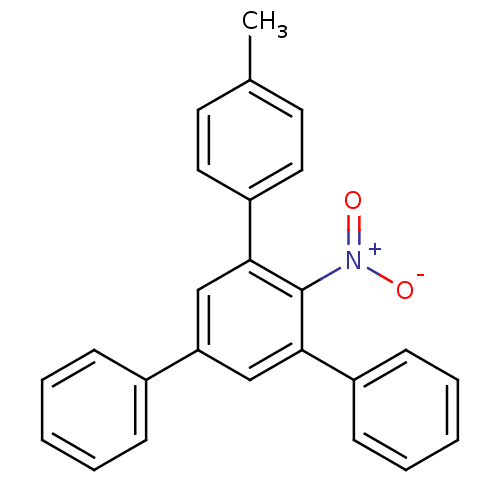
(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128866

(2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...)Show SMILES [#8-]-[#7+](=O)-c1ccc-2c(c1)\[#6](=[#6](/C#N)C#N)-c1cc(cc(c-21)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O Show InChI InChI=1S/C16H5N5O6/c17-6-8(7-18)15-12-3-9(19(22)23)1-2-11(12)16-13(15)4-10(20(24)25)5-14(16)21(26)27/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128872
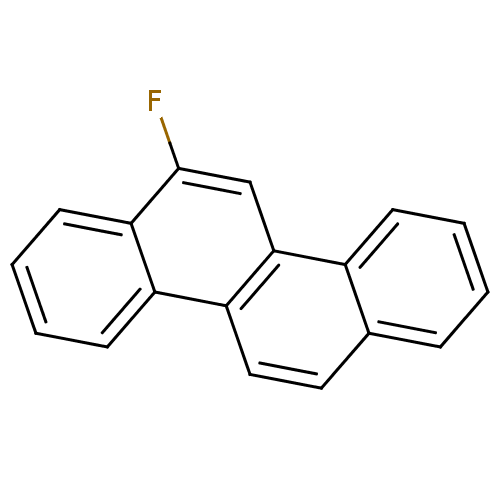
(6-Fluoro-chrysene | CHEMBL83242)Show InChI InChI=1S/C18H11F/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128866

(2-(2,4,7-Trinitro-fluoren-9-ylidene)-malononitrile...)Show SMILES [#8-]-[#7+](=O)-c1ccc-2c(c1)\[#6](=[#6](/C#N)C#N)-c1cc(cc(c-21)-[#7+](-[#8-])=O)-[#7+](-[#8-])=O Show InChI InChI=1S/C16H5N5O6/c17-6-8(7-18)15-12-3-9(19(22)23)1-2-11(12)16-13(15)4-10(20(24)25)5-14(16)21(26)27/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128865

(6-Nitro-chrysene | CHEMBL82858)Show InChI InChI=1S/C18H11NO2/c20-19(21)18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50306594
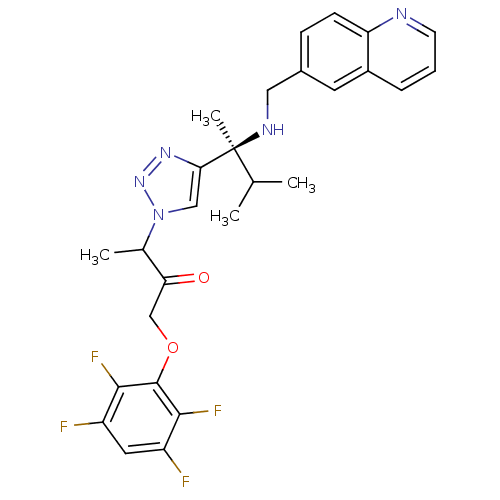
(3-(4-((S)-3-methyl-2-(quinolin-6-ylmethylamino)but...)Show SMILES CC(C)[C@](C)(NCc1ccc2ncccc2c1)c1cn(nn1)C(C)C(=O)COc1c(F)c(F)cc(F)c1F |r| Show InChI InChI=1S/C27H27F4N5O2/c1-15(2)27(4,33-12-17-7-8-21-18(10-17)6-5-9-32-21)23-13-36(35-34-23)16(3)22(37)14-38-26-24(30)19(28)11-20(29)25(26)31/h5-11,13,15-16,33H,12,14H2,1-4H3/t16?,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi cruzain |
J Med Chem 53: 1763-73 (2010)
Article DOI: 10.1021/jm901633v
BindingDB Entry DOI: 10.7270/Q21N8172 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128870

(CHEMBL85685 | chrysene)Show InChI InChI=1S/C18H12/c1-3-7-15-13(5-1)9-11-18-16-8-4-2-6-14(16)10-12-17(15)18/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867

(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128867

(3,5-diphenyl-4'-methyl-2-nitrobiphenyl | CHEMBL875...)Show SMILES Cc1ccc(cc1)-c1cc(cc(-c2ccccc2)c1[N+]([O-])=O)-c1ccccc1 Show InChI InChI=1S/C25H19NO2/c1-18-12-14-21(15-13-18)24-17-22(19-8-4-2-5-9-19)16-23(25(24)26(27)28)20-10-6-3-7-11-20/h2-17H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869

(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82204

(Substrate analogue, 28)Show InChI InChI=1S/C9H14N2O6/c1-17-9(14)3-2-8(13)10-6-7(12)4-5-11(15)16/h2-6H2,1H3,(H,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82199

(Substrate analogue, 18)Show InChI InChI=1S/C4H6FNO3/c5-3-4(7)1-2-6(8)9/h1-3H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128869

(2,2-Dichloro-N-chrysen-6-yl-acetamide | CHEMBL8805...)Show InChI InChI=1S/C20H13Cl2NO/c21-19(22)20(24)23-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11,19H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82198

(Substrate analogue, 17)Show InChI InChI=1S/C4H7NO3/c1-4(6)2-3-5(7)8/h2-3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.80E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82205

(Substrate analogue, 29)Show InChI InChI=1S/C7H11N3O6/c11-6(1-3-9(13)14)5-8-7(12)2-4-10(15)16/h1-5H2,(H,8,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+4 | -26.9 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50128868

(5-(2,4-Dichloro-phenoxy)-3-(2-fluoro-phenyl)-[1,2,...)Show SMILES Fc1ccccc1-c1nnc2c3ccccc3nc(Oc3ccc(Cl)cc3Cl)n12 Show InChI InChI=1S/C21H11Cl2FN4O/c22-12-9-10-18(15(23)11-12)29-21-25-17-8-4-2-6-14(17)20-27-26-19(28(20)21)13-5-1-3-7-16(13)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT) |
J Med Chem 46: 2548-50 (2003)
Article DOI: 10.1021/jm030061i
BindingDB Entry DOI: 10.7270/Q2CF9PGG |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82192

(Substrate analogue, 9)Show InChI InChI=1S/C5H7FO3/c6-3-4(7)1-2-5(8)9/h1-3H2,(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.50E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82203

(Substrate analogue, 24)Show InChI InChI=1S/C10H15NO6/c1-17-10(16)5-3-8(13)11-6-7(12)2-4-9(14)15/h2-6H2,1H3,(H,11,13)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.43E+5 | -21.5 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82200

(Substrate analogue, 19)Show InChI InChI=1S/C4H7NO4/c1-9-4(6)2-3-5(7)8/h2-3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.88E+6 | -16.2 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82191
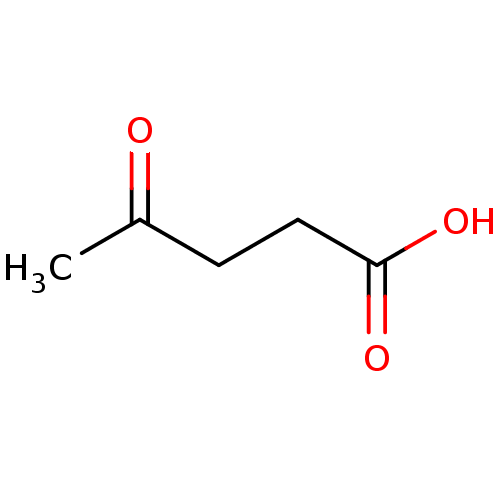
(Substrate analogue, 8)Show InChI InChI=1S/C5H8O3/c1-4(6)2-3-5(7)8/h2-3H2,1H3,(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 2.20E+6 | -15.8 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82206
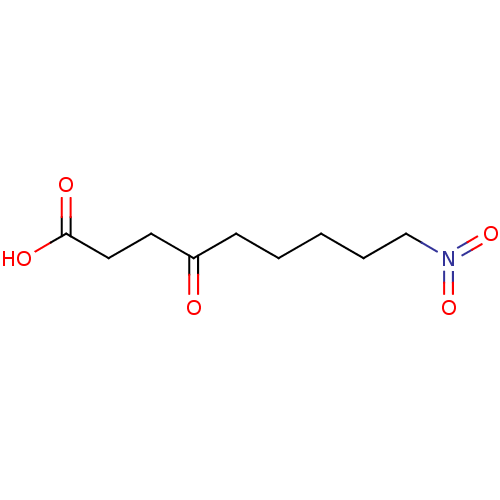
(Substrate analogue, 31)Show InChI InChI=1S/C9H15NO5/c11-8(5-6-9(12)13)4-2-1-3-7-10(14)15/h1-7H2,(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+6 | -13.7 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM26121
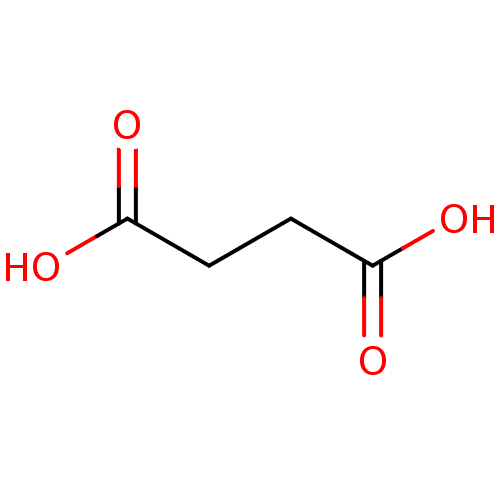
(SUCCINIC ACID | Substrate analogue, 11 | Succinate...)Show InChI InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+7 | -11.3 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82208
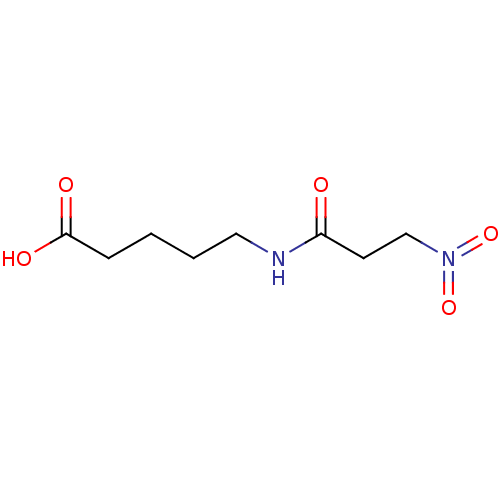
(Substrate analogue, 33)Show InChI InChI=1S/C8H14N2O5/c11-7(4-6-10(14)15)9-5-2-1-3-8(12)13/h1-6H2,(H,9,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.82E+7 | -10.3 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82196

(Substrate analogue, 14)Show InChI InChI=1S/C4H7NO4/c5-1-4(8)9-2-3(6)7/h1-2,5H2,(H,6,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.82E+7 | -10.3 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82197

(Substrate analogue, 15)Show InChI InChI=1S/C4H7NO3/c1-3(6)5-2-4(7)8/h2H2,1H3,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.88E+7 | -10.2 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82207
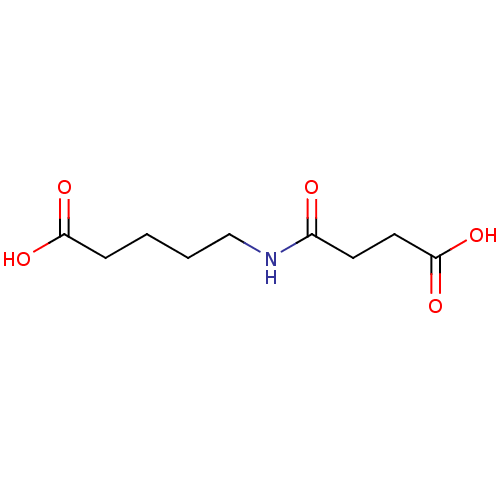
(Substrate analogue, 32)Show InChI InChI=1S/C9H15NO5/c11-7(4-5-9(14)15)10-6-2-1-3-8(12)13/h1-6H2,(H,10,11)(H,12,13)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.29E+7 | -9.74 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82195
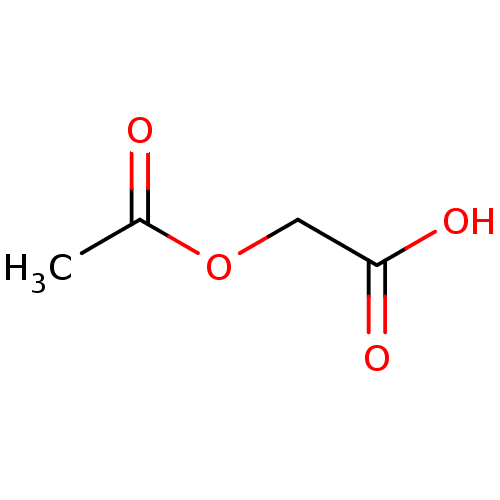
(Substrate analogue, 13)Show InChI InChI=1S/C4H6O4/c1-3(5)8-2-4(6)7/h2H2,1H3,(H,6,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.45E+7 | -9.56 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82194

(Substrate analogue, 12)Show InChI InChI=1S/C5H9NO3/c1-6-4(7)2-3-5(8)9/h2-3H2,1H3,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.64E+7 | -8.54 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82193
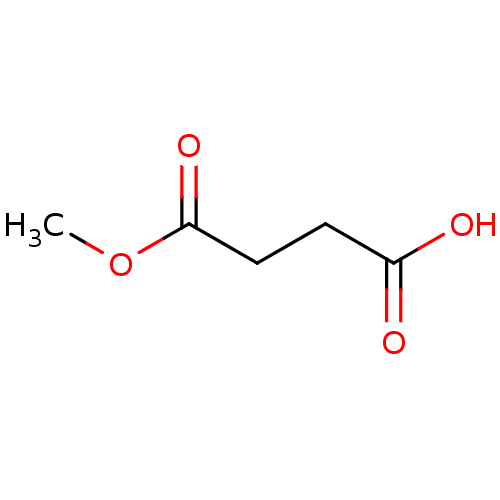
(Substrate analogue, 10)Show InChI InChI=1S/C5H8O4/c1-9-5(8)3-2-4(6)7/h2-3H2,1H3,(H,6,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 3.84E+7 | -8.41 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82201
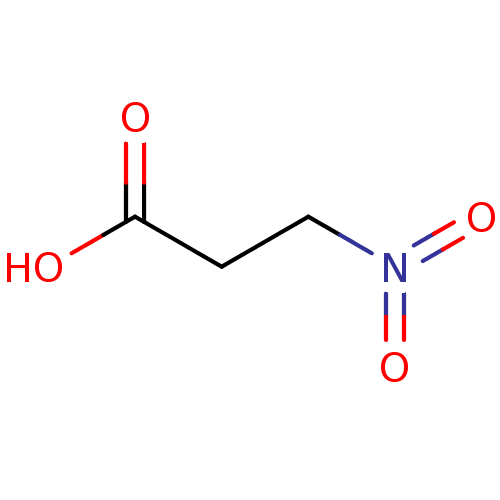
(Substrate analogue, 20)Show InChI InChI=1S/C3H5NO4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.49E+7 | -8.00 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM50137044

(Substrate analogue, 16 | [(ammonioacetyl)amino]ace...)Show InChI InChI=1S/C4H8N2O3/c5-1-3(7)6-2-4(8)9/h1-2,5H2,(H,6,7)(H,8,9) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+8 | >-4.89 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM82202

(Substrate analogue, 22)Show InChI InChI=1S/C4H8N2O3/c1-5-4(7)2-3-6(8)9/h2-3H2,1H3,(H,5,7) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.23E+8 | -3.87 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433404

(CHEMBL2380641)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cnc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(2.53,-27.78,;3.31,-29.1,;3.3,-30.64,;1.97,-29.87,;4.85,-29.11,;5.62,-30.43,;7.15,-30.43,;7.92,-29.1,;7.15,-27.77,;5.61,-27.78,;9.45,-29.1,;10.22,-30.44,;11.76,-30.44,;12.53,-29.1,;11.75,-27.76,;10.22,-27.77,;14.07,-29.1,;14.84,-30.44,;16.37,-30.44,;17.15,-29.11,;18.69,-29.12,;19.46,-30.45,;19.61,-27.88,;21.15,-27.91,;22.34,-26.63,;22.33,-25.15,;23.68,-24.67,;22.64,-25.9,;22.65,-27.49,;24.05,-28.05,;25.07,-26.78,;26.61,-26.78,;25.08,-25.25,;23.67,-27.12,;16.38,-27.78,;17.16,-26.45,;16.4,-25.1,;14.84,-25.1,;14.07,-26.43,;14.84,-27.77,)| Show InChI InChI=1S/C28H37N7O4S/c1-40(38,39)33-8-6-32(7-9-33)22-17-29-26(30-18-22)34-10-11-35(24-5-3-2-4-23(24)34)27(36)31-25-20-12-19-13-21(25)16-28(37,14-19)15-20/h2-5,17-21,25,37H,6-16H2,1H3,(H,31,36)/t19?,20?,21?,25-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433406
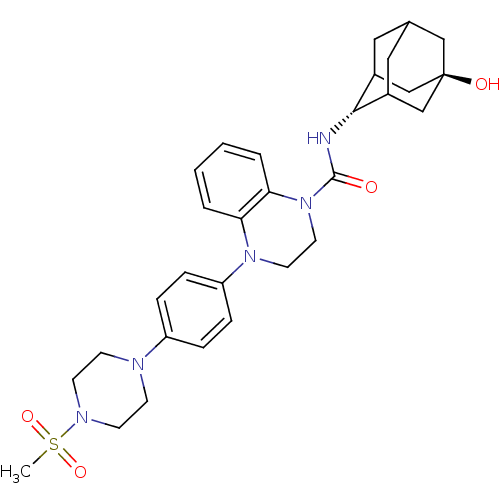
(CHEMBL2380643)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(cc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(1.07,-35.62,;1.84,-36.95,;1.84,-38.49,;.51,-37.71,;3.39,-36.95,;4.15,-38.27,;5.69,-38.27,;6.46,-36.95,;5.69,-35.62,;4.15,-35.62,;7.99,-36.94,;8.76,-38.28,;10.3,-38.28,;11.07,-36.95,;10.29,-35.61,;8.76,-35.61,;12.61,-36.94,;13.38,-38.28,;14.91,-38.29,;15.69,-36.95,;17.23,-36.96,;18,-38.29,;18.15,-35.73,;19.69,-35.75,;20.88,-34.48,;20.87,-32.99,;22.22,-32.51,;21.18,-33.75,;21.19,-35.33,;22.59,-35.9,;23.61,-34.62,;25.15,-34.62,;23.62,-33.09,;22.21,-34.97,;14.92,-35.62,;15.7,-34.29,;14.94,-32.95,;13.38,-32.94,;12.61,-34.28,;13.37,-35.61,)| Show InChI InChI=1S/C30H39N5O4S/c1-40(38,39)33-12-10-32(11-13-33)24-6-8-25(9-7-24)34-14-15-35(27-5-3-2-4-26(27)34)29(36)31-28-22-16-21-17-23(28)20-30(37,18-21)19-22/h2-9,21-23,28,37H,10-20H2,1H3,(H,31,36)/t21?,22?,23?,28-,30- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50433405

(CHEMBL2380642)Show SMILES CS(=O)(=O)N1CCN(CC1)c1ccc(nc1)N1CCN(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c2ccccc12 |r,wU:23.24,wD:30.33,TLB:31:30:23.28.27:25,22:23:30.33.29:27.26.25,22:23:25:30.33.32,THB:29:30:23.28.27:25,29:28:25:30.33.32,32:30:23:27.26.25,32:26:23:30.33.29,(25.74,-29.77,;26.51,-31.1,;26.5,-32.64,;25.17,-31.86,;28.05,-31.1,;28.82,-32.42,;30.36,-32.43,;31.12,-31.1,;30.35,-29.77,;28.81,-29.77,;32.66,-31.09,;33.42,-32.43,;34.96,-32.43,;35.73,-31.1,;34.96,-29.76,;33.42,-29.76,;37.28,-31.1,;38.05,-32.43,;39.58,-32.44,;40.35,-31.11,;41.89,-31.11,;42.66,-32.45,;42.82,-29.88,;44.35,-29.9,;45.55,-28.63,;45.54,-27.14,;46.89,-26.66,;45.85,-27.9,;45.85,-29.48,;47.26,-30.05,;48.27,-28.77,;49.81,-28.77,;48.28,-27.24,;46.88,-29.12,;39.59,-29.77,;40.37,-28.44,;39.6,-27.1,;38.05,-27.09,;37.28,-28.43,;38.04,-29.76,)| Show InChI InChI=1S/C29H38N6O4S/c1-40(38,39)33-10-8-32(9-11-33)23-6-7-26(30-19-23)34-12-13-35(25-5-3-2-4-24(25)34)28(36)31-27-21-14-20-15-22(27)18-29(37,16-20)17-21/h2-7,19-22,27,37H,8-18H2,1H3,(H,31,36)/t20?,21?,22?,27-,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant 11beta-HSD1 by scintillation proximity assay |
Bioorg Med Chem Lett 23: 2414-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.018
BindingDB Entry DOI: 10.7270/Q2NV9KMN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438080
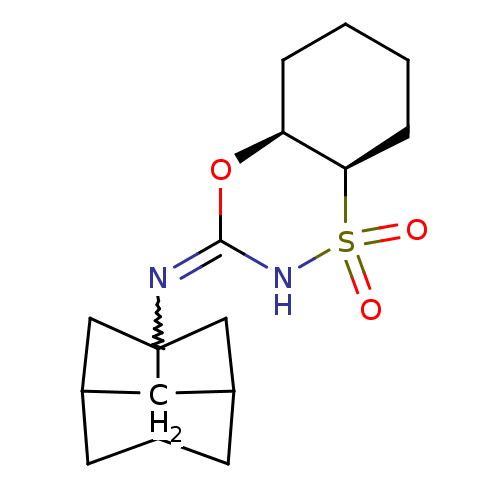
(CHEMBL2409607)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CC3CC1CC(C2)C3 |r,w:12.14,TLB:20:19:16:13.14,THB:20:13:16:19.18.21,TEB:12:13:16:19.18.21,12:13:18:16.15.21| Show InChI InChI=1S/C16H24N2O3S/c19-22(20)14-4-2-1-3-13(14)21-15(18-22)17-16-8-10-5-11(9-16)7-12(16)6-10/h10-14H,1-9H2,(H,17,18)/t10?,11?,12?,13-,14+,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50438083
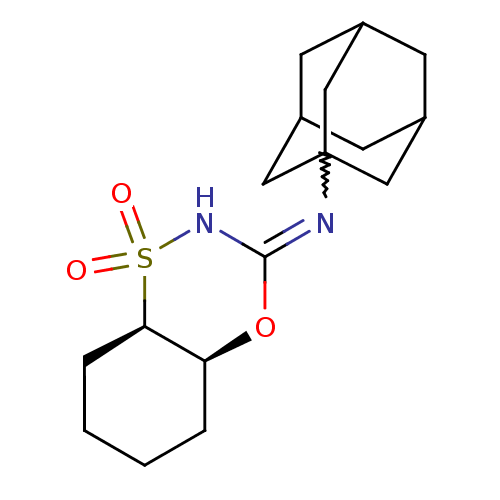
(CHEMBL2409755)Show SMILES O=S1(=O)NC(O[C@H]2CCCC[C@@H]12)=NC12CC3CC(CC(C3)C1)C2 |r,w:12.14,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,TEB:12:13:16:20.19.18,12:13:16.15.20:18| Show InChI InChI=1S/C17H26N2O3S/c20-23(21)15-4-2-1-3-14(15)22-16(19-23)18-17-8-11-5-12(9-17)7-13(6-11)10-17/h11-15H,1-10H2,(H,18,19)/t11?,12?,13?,14-,15+,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Deutschland GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 |
Bioorg Med Chem Lett 23: 4685-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.102
BindingDB Entry DOI: 10.7270/Q2BV7J2H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data