

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrate | J Med Chem 46: 1989-96 (2003) Article DOI: 10.1021/jm020515w BindingDB Entry DOI: 10.7270/Q2SQ8ZRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Concentration of the compound required for the neuroprotective effect determined by inhibition of GCP II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392045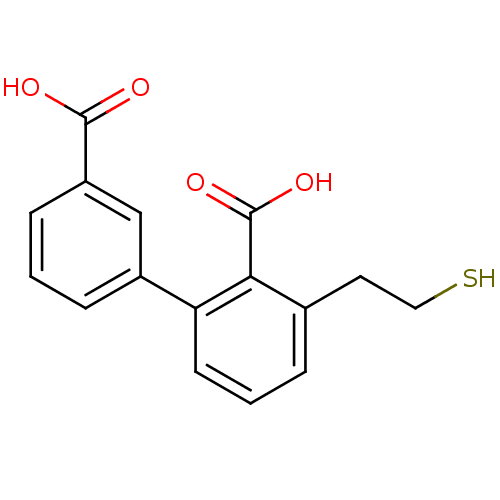 (CHEMBL2152561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins School of Medicine Curated by ChEMBL | Assay Description Inhibition of GCP-2 (unknown origin) | Drug Metab Dispos 40: 2315-23 (2012) Article DOI: 10.1124/dmd.112.046821 BindingDB Entry DOI: 10.7270/Q2W66NG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129200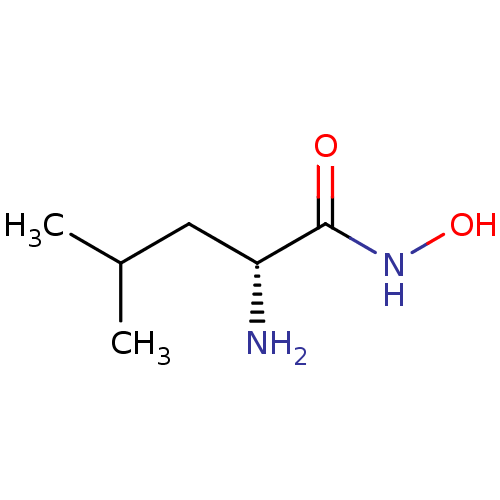 ((R)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801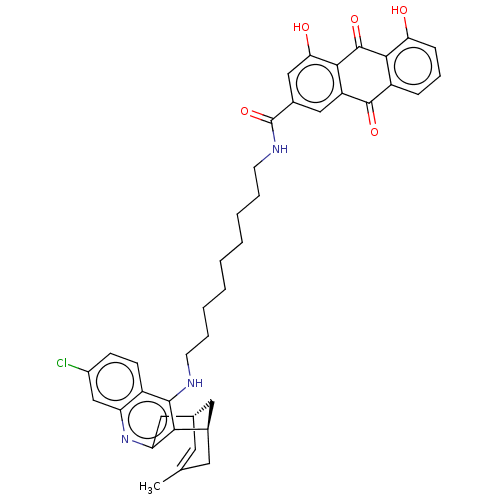 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-inhibitor complex by Li... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-substrate-inhibitor com... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50109593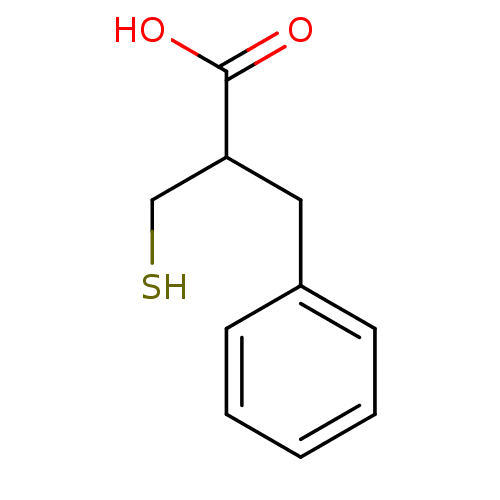 (2-Benzyl-3-mercapto-propionic acid | 2-Mercaptomet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrate | J Med Chem 46: 1989-96 (2003) Article DOI: 10.1021/jm020515w BindingDB Entry DOI: 10.7270/Q2SQ8ZRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM50117763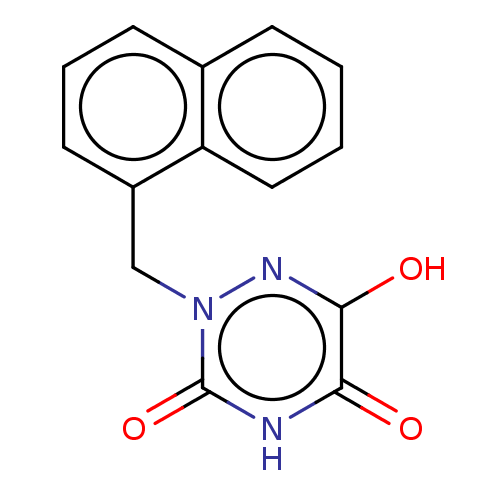 (CHEMBL3613921 | US9505753, 5u) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human DAAO expressed in HEK cells by double reciprocal plot analysis in presence of D-serine | J Med Chem 58: 7258-72 (2015) Article DOI: 10.1021/acs.jmedchem.5b00482 BindingDB Entry DOI: 10.7270/Q2SF2XZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM50129202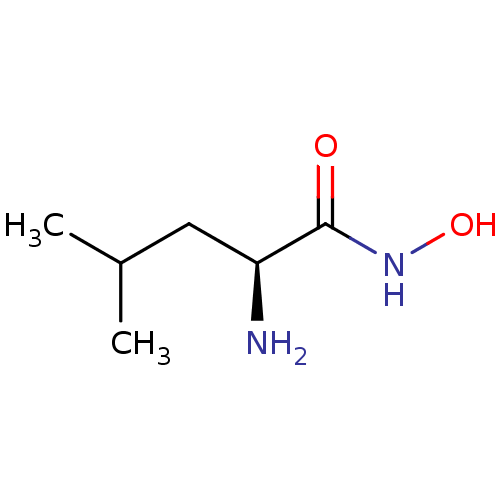 ((S)-2-Amino-4-methyl-pentanoic acid hydroxyamide |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of metalloprotease from family M28, Aeromonas proteolytica aminopeptidase | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM108460 (CHEMBL2178393 | US11191732, Example 1 | US8604016,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis | J Med Chem 55: 10551-63 (2012) Article DOI: 10.1021/jm301191p BindingDB Entry DOI: 10.7270/Q2VD70M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-1 (Human) | BDBM50266275 (CHEMBL456494 | Tajixanthone hydrate) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México Curated by ChEMBL | Assay Description Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v... | Bioorg Med Chem 17: 2167-74 (2009) Article DOI: 10.1016/j.bmc.2008.10.079 BindingDB Entry DOI: 10.7270/Q2V124NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-1 (Human) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México Curated by ChEMBL | Assay Description Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v... | Bioorg Med Chem 17: 2167-74 (2009) Article DOI: 10.1016/j.bmc.2008.10.079 BindingDB Entry DOI: 10.7270/Q2V124NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Sus scrofa (pig)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Competitive inhibition of pig kidney DAAO using D-Alanine as substrate by Michaelis-Menten plot analysis | Bioorg Med Chem Lett 23: 3910-3 (2013) Article DOI: 10.1016/j.bmcl.2013.04.062 BindingDB Entry DOI: 10.7270/Q2K35W2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calmodulin-1 (Human) | BDBM50266274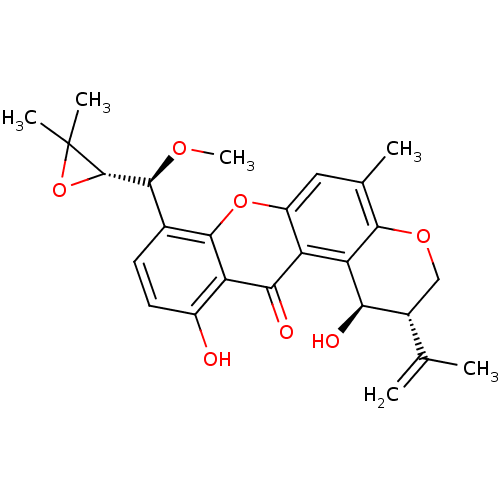 (14-methoxytajixanthone | CHEMBL515370) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional Autónoma de México Curated by ChEMBL | Assay Description Inhibition of recombinant calmodulin (unknown origin) mediated bovine brain PDE1 activation assessed as effect on inorganic phosphate release using v... | Bioorg Med Chem 17: 2167-74 (2009) Article DOI: 10.1016/j.bmc.2008.10.079 BindingDB Entry DOI: 10.7270/Q2V124NN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50007037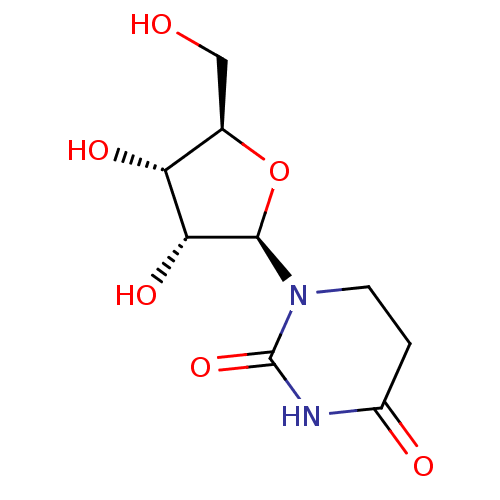 (CHEBI:23774 | CHEMBL3237555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human cytidine deaminase by spectrophotometrically | J Med Chem 57: 2582-8 (2014) Article DOI: 10.1021/jm401856k BindingDB Entry DOI: 10.7270/Q2NK3GJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099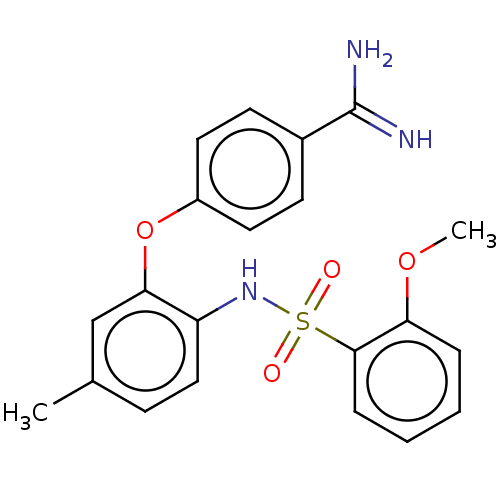 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytidine deaminase (Homo sapiens (Human)) | BDBM50007025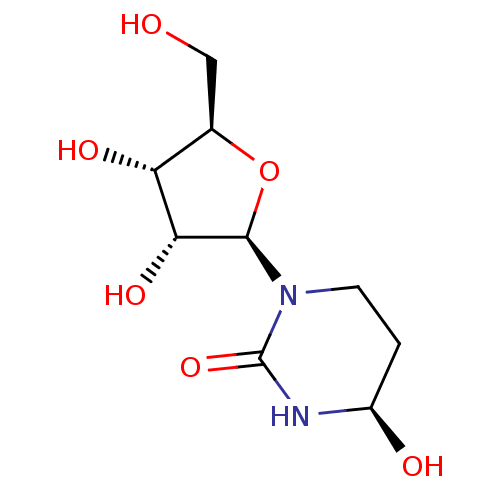 (TETRAHYDROURIDINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human cytidine deaminase by spectrophotometrically | J Med Chem 57: 2582-8 (2014) Article DOI: 10.1021/jm401856k BindingDB Entry DOI: 10.7270/Q2NK3GJG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101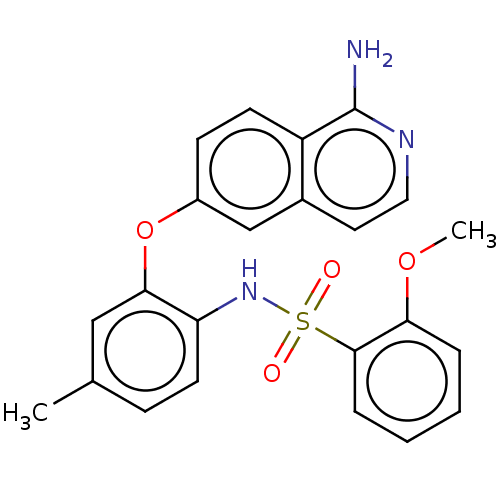 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 2.47E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3.73E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509101 (CHEMBL4460098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 1.21E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 BindingDB Entry DOI: 10.7270/Q2125WZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Glutamate carboxypeptidase II | Bioorg Med Chem Lett 13: 2097-100 (2003) BindingDB Entry DOI: 10.7270/Q2Z60NF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of N-acetyl-L-aspartyl-[3H]-L-glutamate binding to glutamate carboxypeptidase II (GCP II) | J Med Chem 46: 1989-96 (2003) Article DOI: 10.1021/jm020515w BindingDB Entry DOI: 10.7270/Q2SQ8ZRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins School of Medicine Curated by ChEMBL | Assay Description Inhibition of GCP-2 (unknown origin) | Drug Metab Dispos 40: 2315-23 (2012) Article DOI: 10.1124/dmd.112.046821 BindingDB Entry DOI: 10.7270/Q2W66NG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50304738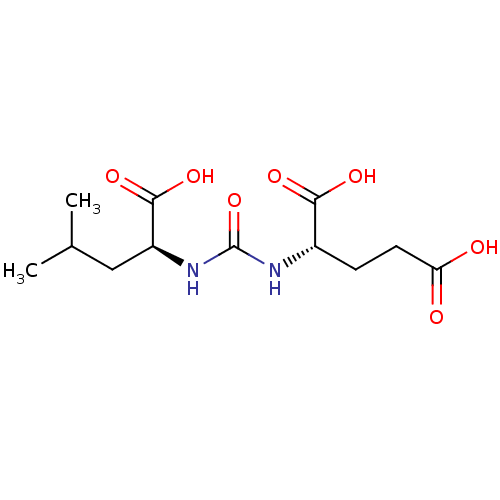 (2-(3-((S)-1-carboxy-3-methylbutyl)ureido)pentanedi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503760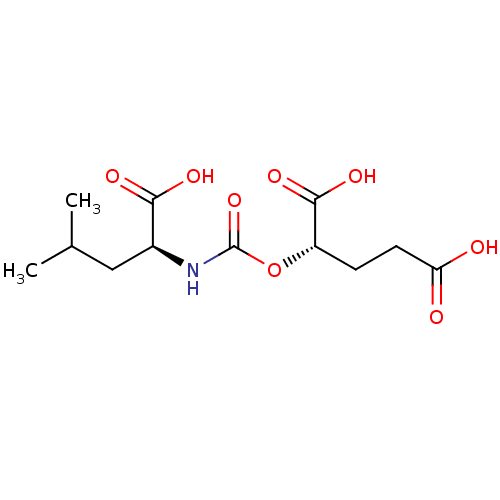 (CHEMBL4442450) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007795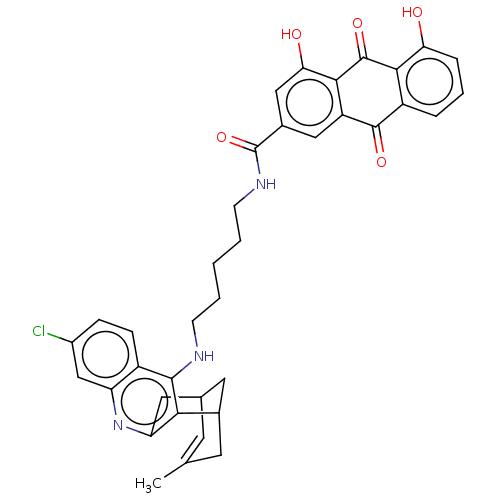 (CHEMBL3233826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007796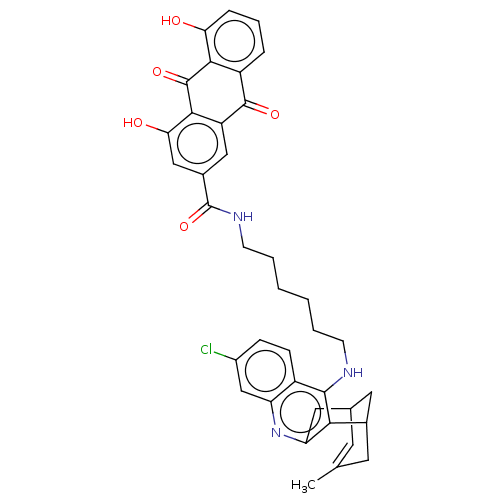 (CHEMBL3233827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392045 (CHEMBL2152561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392040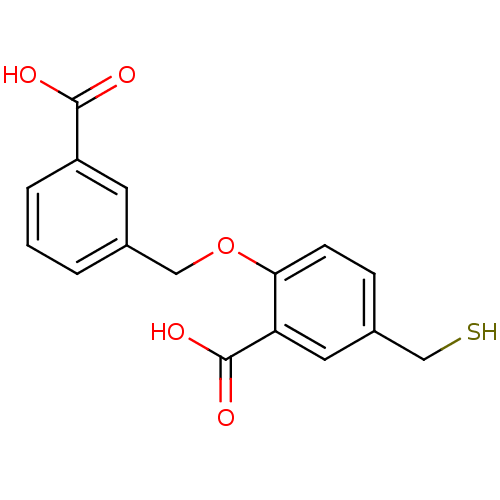 (CHEMBL2152556) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116251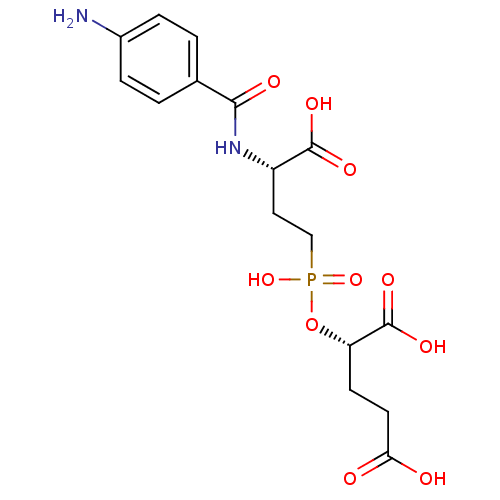 (2-{[3-(4-Amino-benzoylamino)-3-carboxy-propyl]-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007797 (CHEMBL3233828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007799 (CHEMBL3233830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116250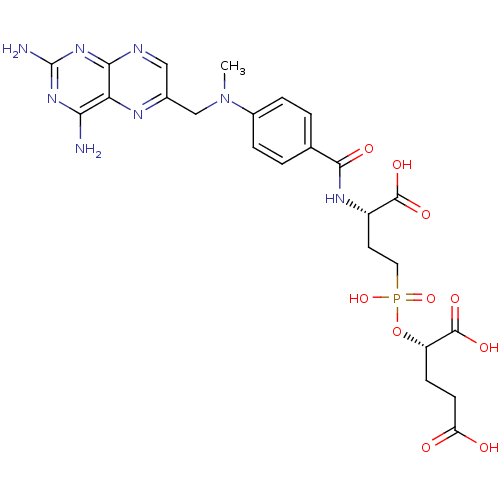 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116253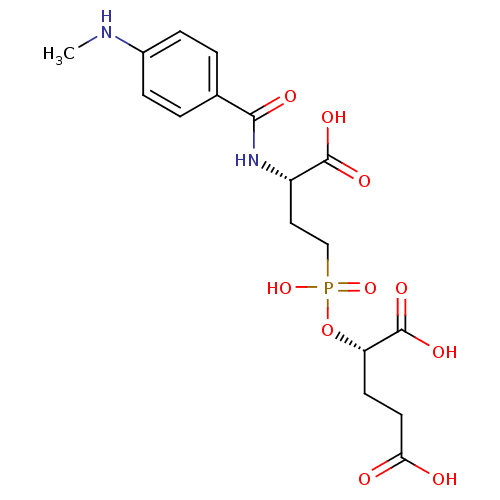 (2-{[3-Carboxy-3-(4-methylamino-benzoylamino)-propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007798 (CHEMBL3233829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Rattus norvegicus) | BDBM50116252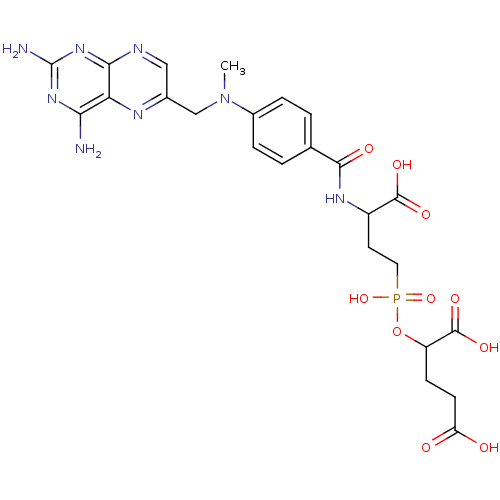 (2-[(3-Carboxy-3-{4-[(2,4-diamino-pteridin-6-ylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit Glutamate carboxypeptidase-II using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate | Bioorg Med Chem Lett 12: 2189-92 (2002) BindingDB Entry DOI: 10.7270/Q21835TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392046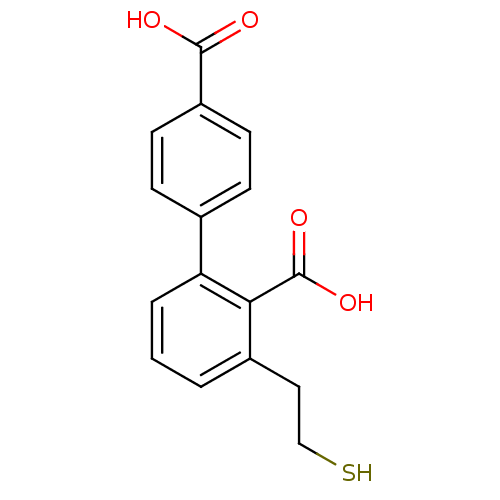 (CHEMBL2152562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007802 (CHEMBL3234038) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503756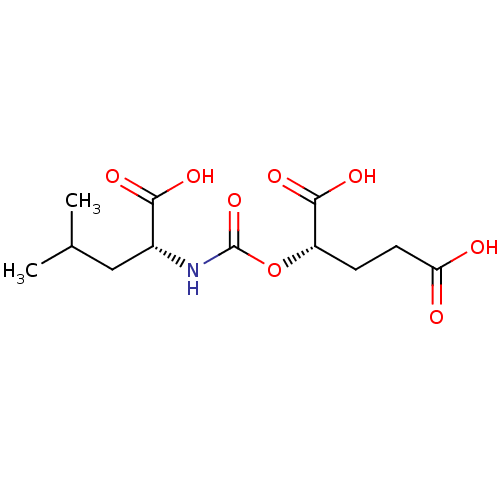 (CHEMBL4473741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521402 (CHEMBL4471834) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Inhibition of human recombinant nSMase expressed in HEK293 cells using sphingomyelin as substrate by alkaline phosphatase, choline oxidase and horser... | Eur J Med Chem 170: 276-289 (2019) Article DOI: 10.1016/j.ejmech.2019.03.015 BindingDB Entry DOI: 10.7270/Q27H1NZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17762 (3-[2-carboxy-2-(3-sulfanylpropyl)ethyl]benzoic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503754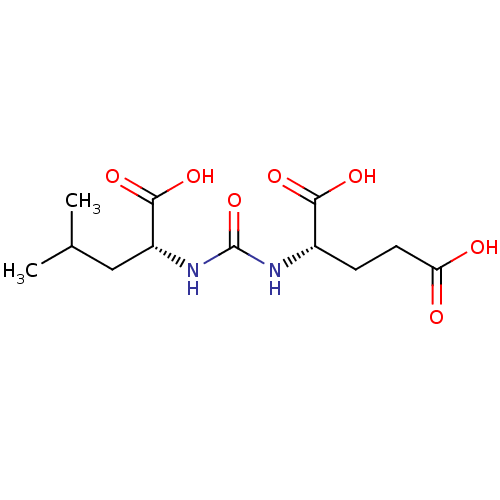 (CHEMBL4458733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50392041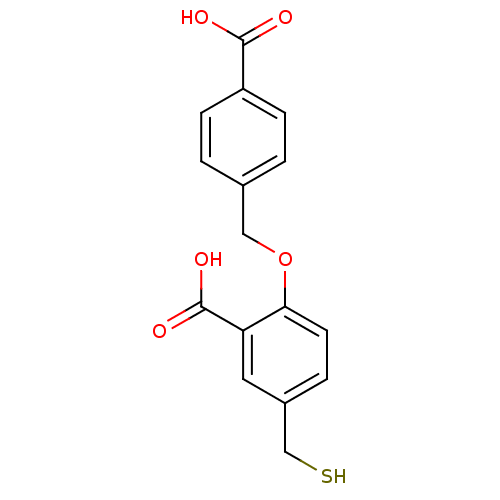 (CHEMBL2152557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant GCP2 using N-acetyl-L-aspartyl-[3H]-L-glutamate as substrate by microplate assay | J Med Chem 55: 5922-32 (2012) Article DOI: 10.1021/jm300488m BindingDB Entry DOI: 10.7270/Q21J9BWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50503757 (CHEMBL4541841) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of N-terminally tagged human recombinant GCP2 (44 to 750 residues) extracellular domain expressed in Drosophila melanogaster S2 cells prei... | Bioorg Med Chem 27: 255-264 (2019) Article DOI: 10.1016/j.bmc.2018.11.022 BindingDB Entry DOI: 10.7270/Q2F47SC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007803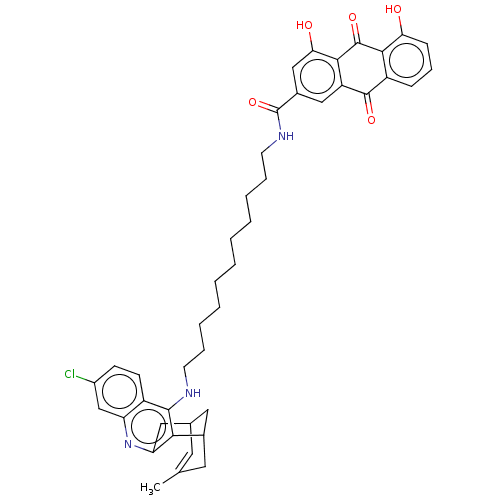 (CHEMBL3234039) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 783 total ) | Next | Last >> |