

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498230 (CHEMBL3577576) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498229 (CHEMBL3577575) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50489369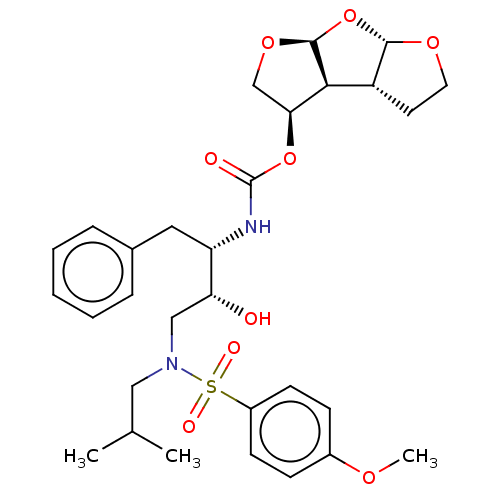 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 wildtype protease using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition mea... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50489369 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease R8Q mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition m... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (MOUSE) | BDBM50050531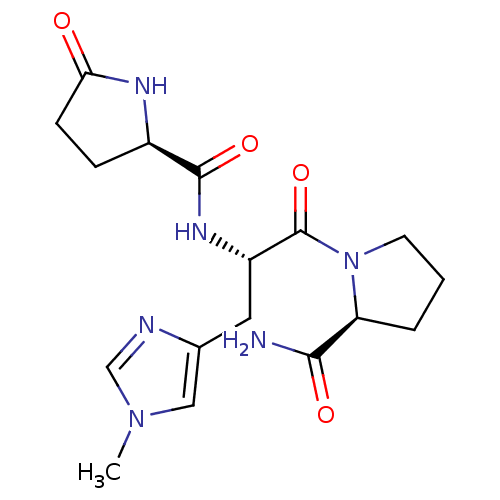 (1-[3-(1-methyl-4-imidazolyl)-2-[2-oxo-(5S)-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Binding affinity against TRH-R receptor in AtT-20 mouse pituitary tumor cells | J Med Chem 39: 1571-4 (1996) Article DOI: 10.1021/jm960053k BindingDB Entry DOI: 10.7270/Q2H70GGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984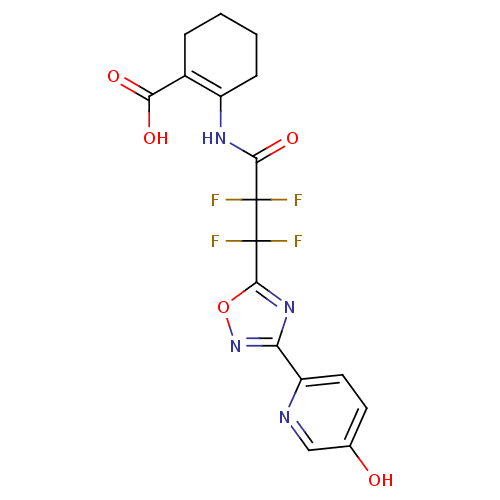 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50089636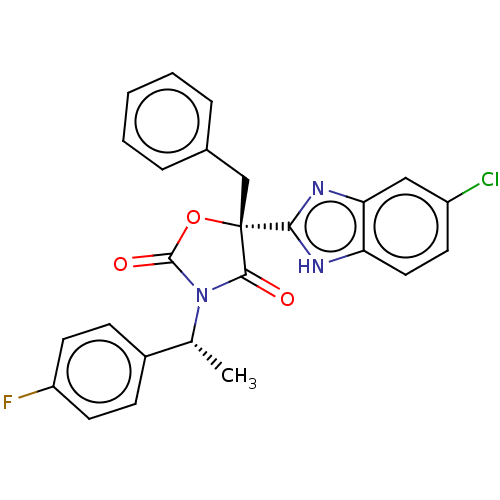 (CHEMBL3578271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to mineralocorticoid receptor (unknown origin) | ACS Med Chem Lett 6: 461-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00010 BindingDB Entry DOI: 10.7270/Q21J9CH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977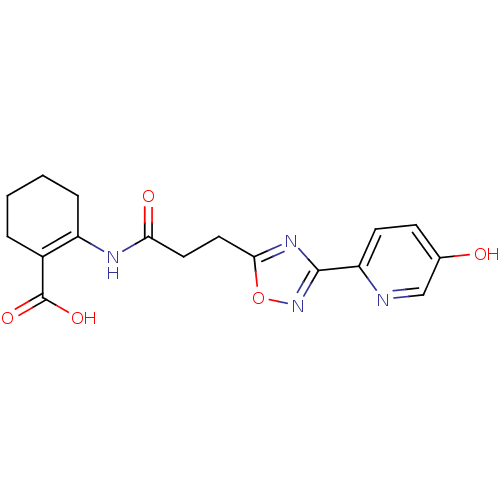 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196446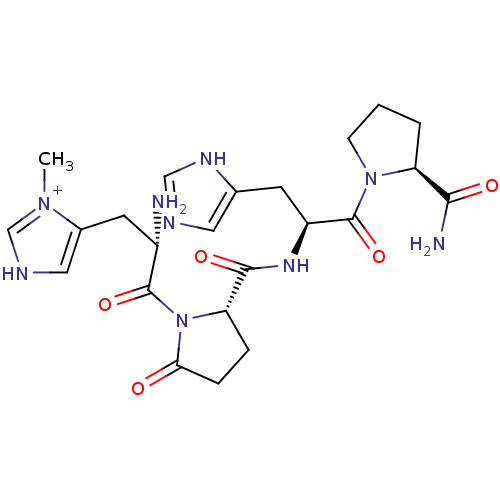 (CHEMBL224730 | [N tau(1)-Me-His]-TRH) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50173826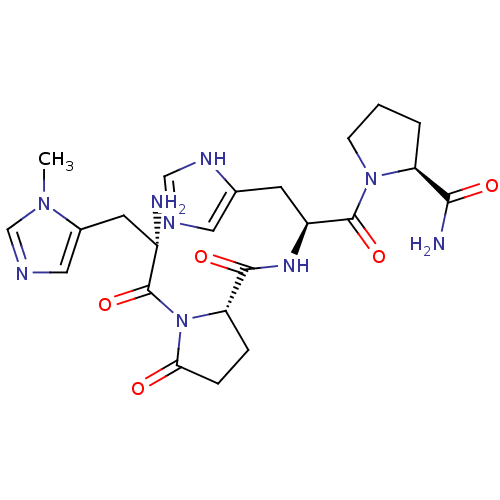 (1-[(S)-2-Amino-3-(3-methyl-3H-imidazol-4-yl)-propi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 1 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50489369 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease V82A mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition ... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196451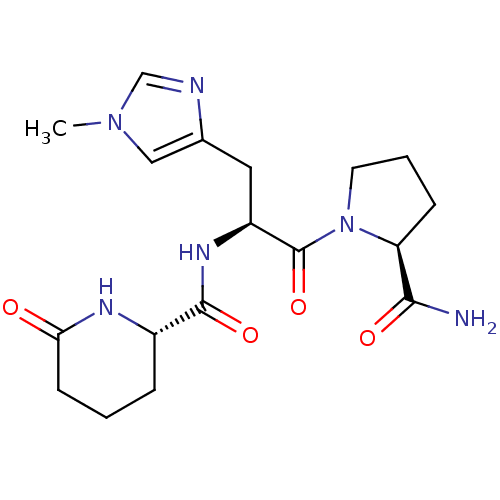 ((2S)-1-{(2S)-3-(1-methyl-1H-4-imidazolyl)-2-[(2S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50489369 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease I54M mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition ... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533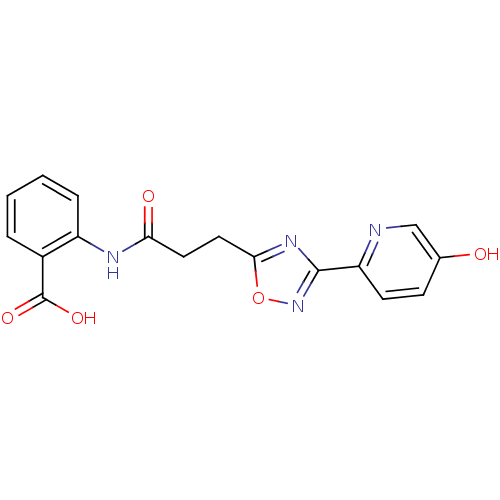 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976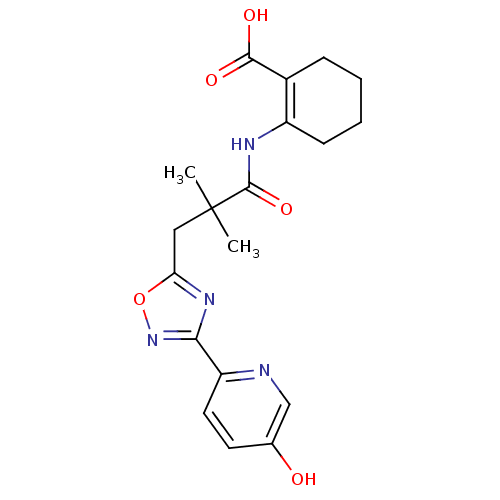 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50300121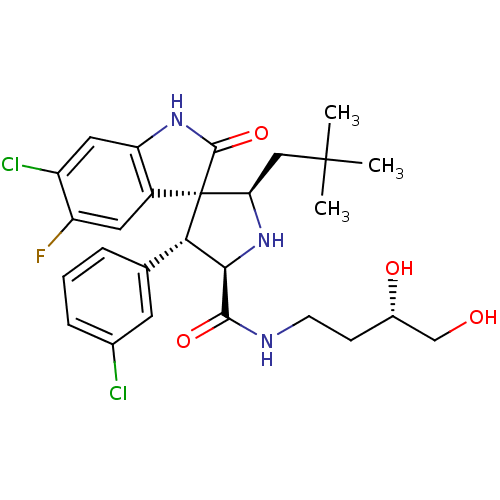 ((2'R,3S,4'R,5'R)-6-chloro-4'-(3-chlorophenyl)-N-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to MDM2 | Eur J Med Chem 56: 10-16 (2012) Article DOI: 10.1016/j.ejmech.2012.08.003 BindingDB Entry DOI: 10.7270/Q2K938SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50249989 (CHEMBL1387422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583530 (CHEMBL5092661) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50489369 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease D30N mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition ... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583533 (CHEMBL5084153) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 2 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (MOUSE) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Binding affinity against TRH-R receptor in AtT-20 mouse pituitary tumor cells | J Med Chem 39: 1571-4 (1996) Article DOI: 10.1021/jm960053k BindingDB Entry DOI: 10.7270/Q2H70GGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50196457 ((2S)-1-{(2S)-3-(1-ethyl-1H-4-imidazolyl)-2-[(2S)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979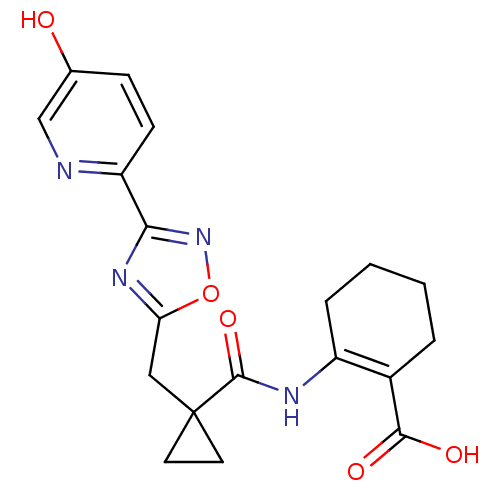 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50196451 ((2S)-1-{(2S)-3-(1-methyl-1H-4-imidazolyl)-2-[(2S)-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583534 (CHEMBL5089219) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (MOUSE) | BDBM86214 (CAS_32281 | NSC_32281 | Phe2TRH | TRH) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Endocrinology 144: 1842-6 (2003) Article DOI: 10.1210/en.2002-221074 BindingDB Entry DOI: 10.7270/Q2736PGN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Xenopus) | BDBM86214 (CAS_32281 | NSC_32281 | Phe2TRH | TRH) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Endocrinology 144: 1842-6 (2003) Article DOI: 10.1210/en.2002-221074 BindingDB Entry DOI: 10.7270/Q2736PGN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR1 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory constant against thyrotropin releasing hormone receptor 1 expressed in HEK 293EM cells upon incubation at 37 degee C for 1 hr at pH 7.4 us... | J Med Chem 48: 6162-5 (2005) Article DOI: 10.1021/jm0505462 BindingDB Entry DOI: 10.7270/Q2PK0FQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981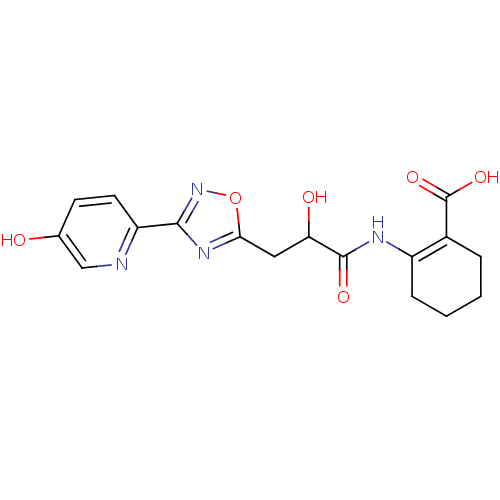 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (MOUSE) | BDBM50072394 (1-[3-(1H-4-imidazolyl)-2-(5-oxotetrahydro-1H-2-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Curated by ChEMBL | Assay Description Ability to bind thyroliberin endocrine receptor (TRH-R) using AtT-20 mouse pituitary tumor cells. | Bioorg Med Chem Lett 8: 3093-6 (1999) Article DOI: 10.1016/S0960-894X(98)00567-8 BindingDB Entry DOI: 10.7270/Q21R6PP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583525 (CHEMBL5094121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583524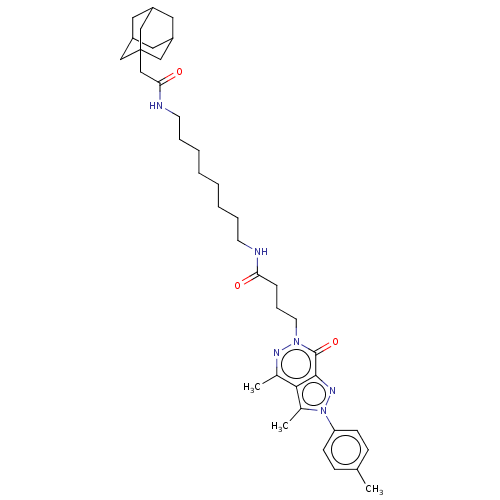 (CHEMBL5077941) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982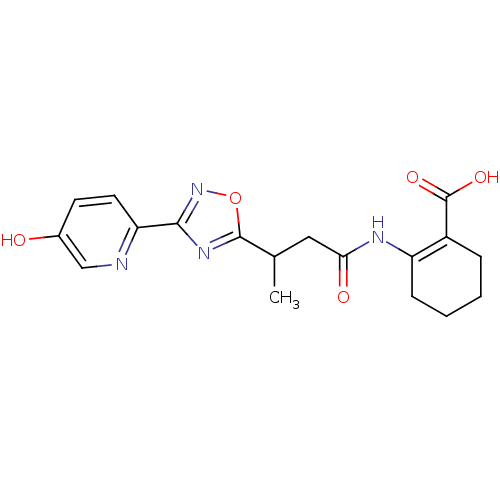 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50489369 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease I50V mutant using Abz-Thr-Ile-Nle-p-nitro-Phe-Gln-Arg-NH2 as substrate incubated for 5 mins prior to substrate addition ... | J Med Chem 56: 1074-83 (2013) Article DOI: 10.1021/jm301519z BindingDB Entry DOI: 10.7270/Q2542RH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyrotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50196457 ((2S)-1-{(2S)-3-(1-ethyl-1H-4-imidazolyl)-2-[(2S)-6...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Displacement of [3H]N(1)-Me-His-TRH from TRHR2 | Bioorg Med Chem 15: 433-43 (2006) Article DOI: 10.1016/j.bmc.2006.09.045 BindingDB Entry DOI: 10.7270/Q2ZW1KJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583527 (CHEMBL5086138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583532 (CHEMBL5079726) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583531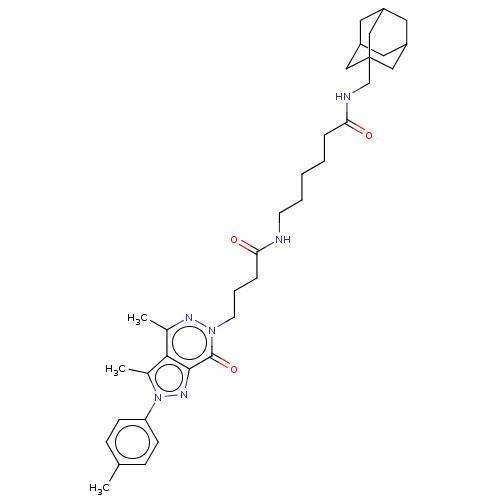 (CHEMBL5083136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583529 (CHEMBL5087129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinal rod rhodopsin-sensitive cGMP 3',5'-cyclic phosphodiesterase subunit delta (Homo sapiens (Human)) | BDBM50583526 (CHEMBL5072868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to PDE delta (unknown origin) assessed as dissociation constant measured after 2 hrs by fluorescence polarization assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00670 BindingDB Entry DOI: 10.7270/Q2PC368T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50449913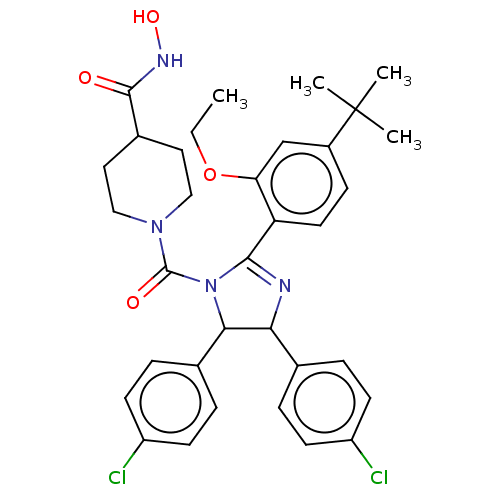 (CHEMBL4170056) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay | J Med Chem 61: 7245-7260 (2018) Article DOI: 10.1021/acs.jmedchem.8b00664 BindingDB Entry DOI: 10.7270/Q2R213Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50449907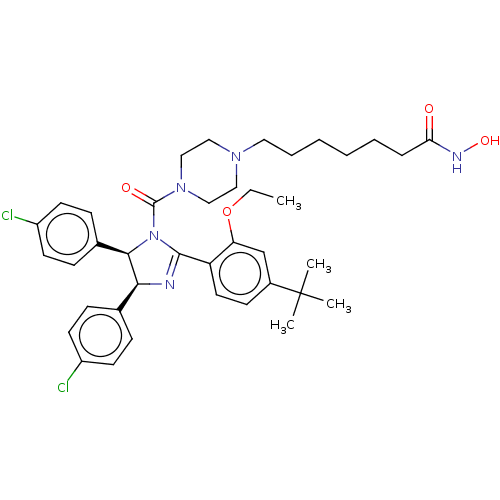 (CHEMBL4163675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay | J Med Chem 61: 7245-7260 (2018) Article DOI: 10.1021/acs.jmedchem.8b00664 BindingDB Entry DOI: 10.7270/Q2R213Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50449901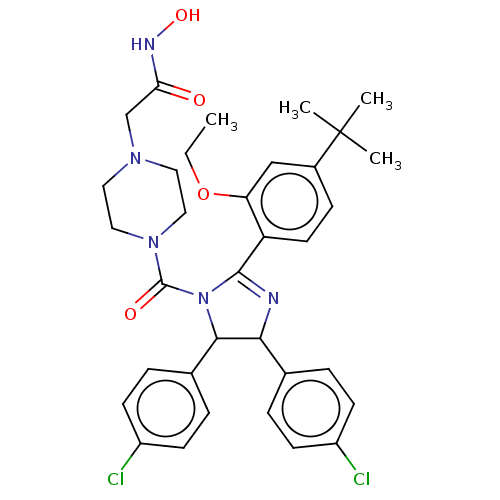 (CHEMBL4160754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay | J Med Chem 61: 7245-7260 (2018) Article DOI: 10.1021/acs.jmedchem.8b00664 BindingDB Entry DOI: 10.7270/Q2R213Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 4846 total ) | Next | Last >> |