

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326722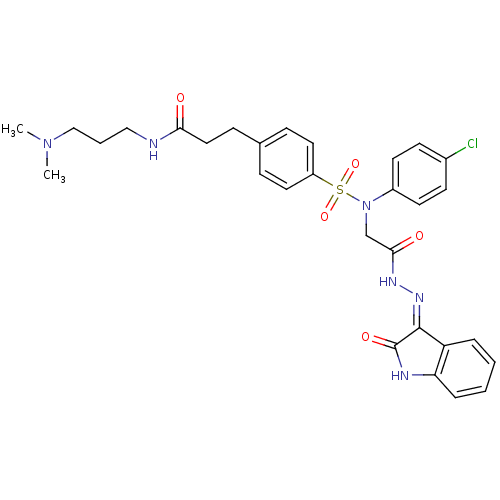 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50326722 ((Z)-3-(4-(N-(4-chlorophenyl)-N-(2-oxo-2-(2-(2-oxoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410618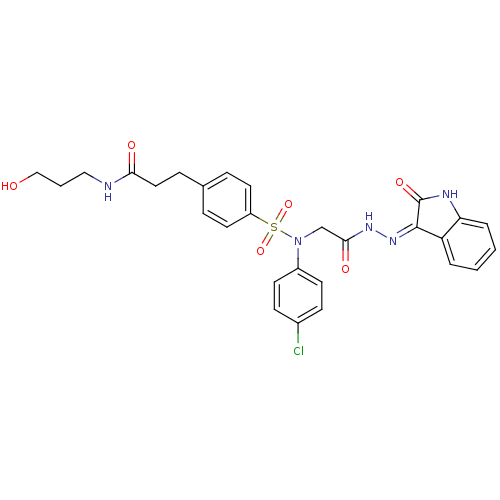 (CHEMBL2113181) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410616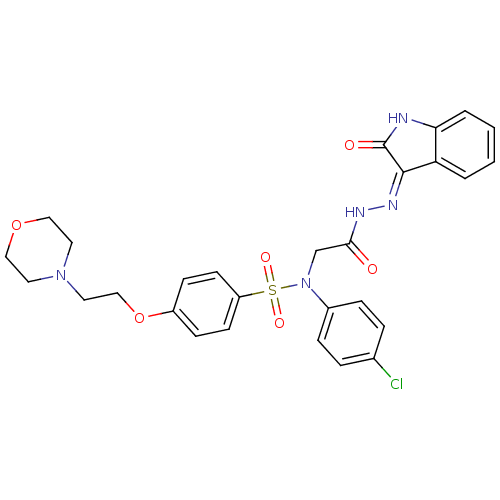 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410625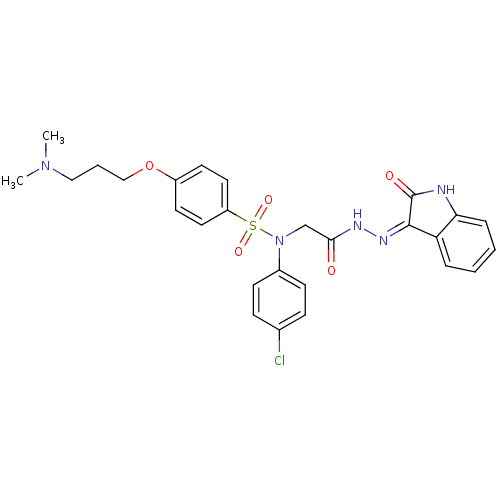 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313357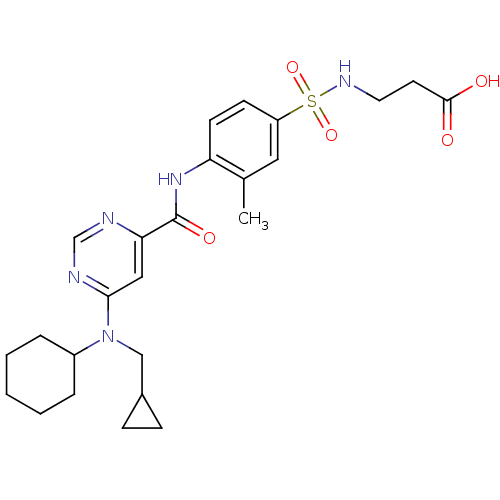 (3-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410622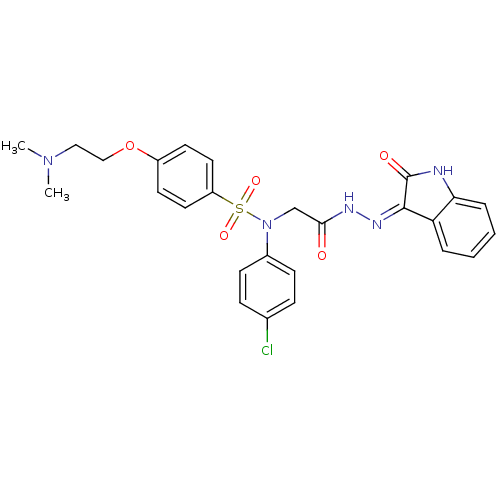 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410634 (CHEMBL2113189) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313351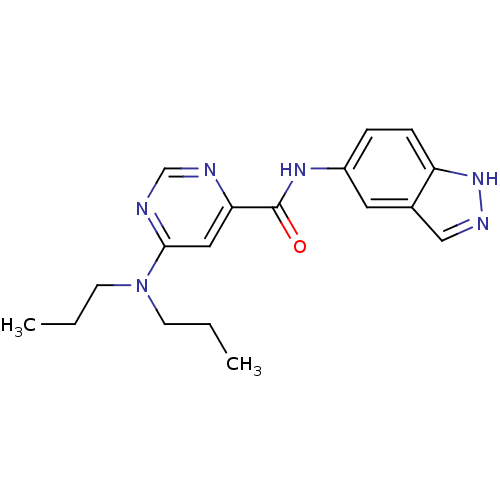 (6-(dipropylamino)-N-(1H-indazol-5-yl)pyrimidine-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410619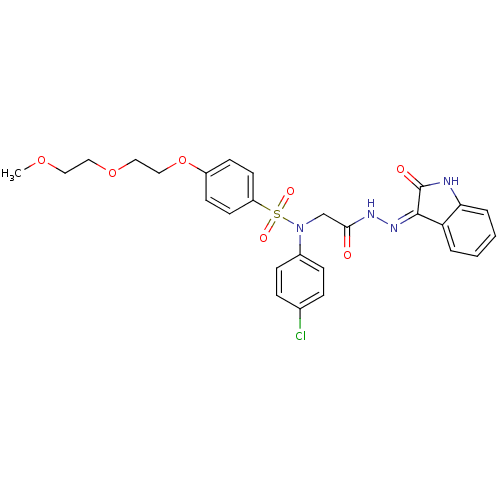 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183667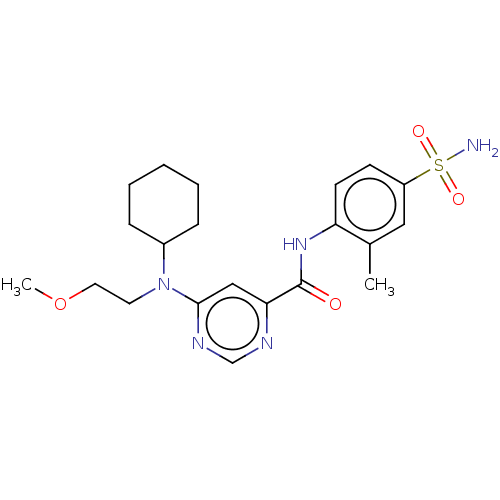 (US9150519, 1-55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.20 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410640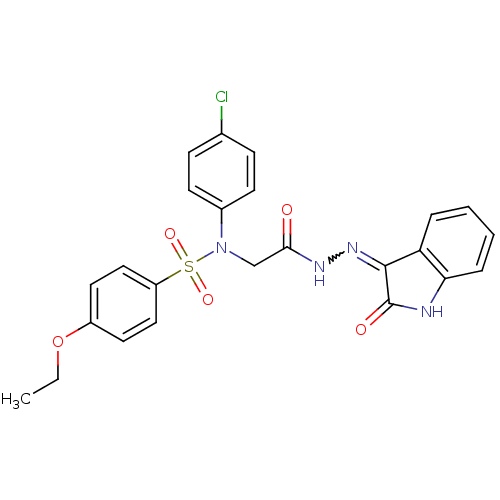 (CHEMBL2113198) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410623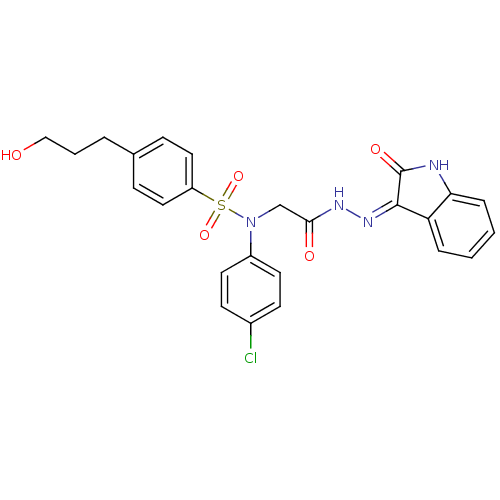 (CHEMBL2113179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410625 (CHEMBL2113201) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313358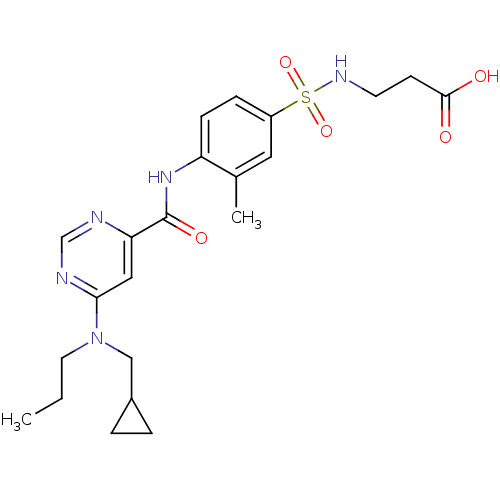 (3-(4-(6-((cyclopropylmethyl)(propyl)amino)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 6 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410635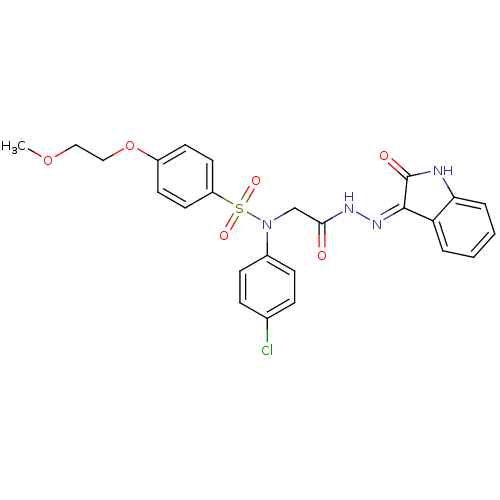 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183686 (US9150519, 1-84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410621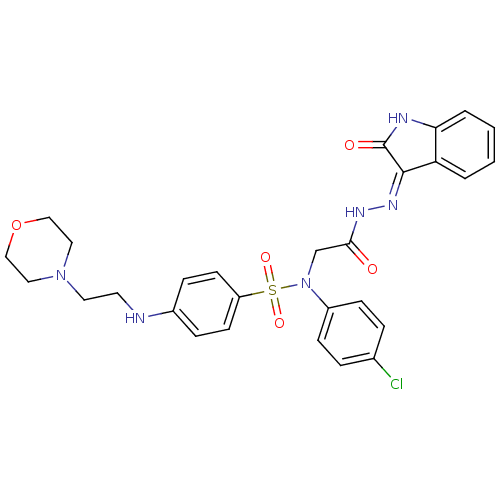 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183668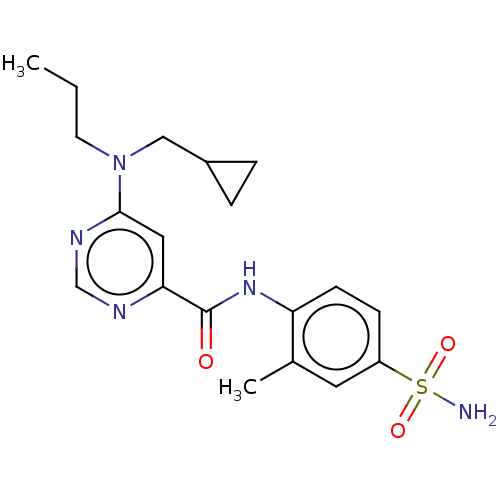 (US9150519, 1-56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313359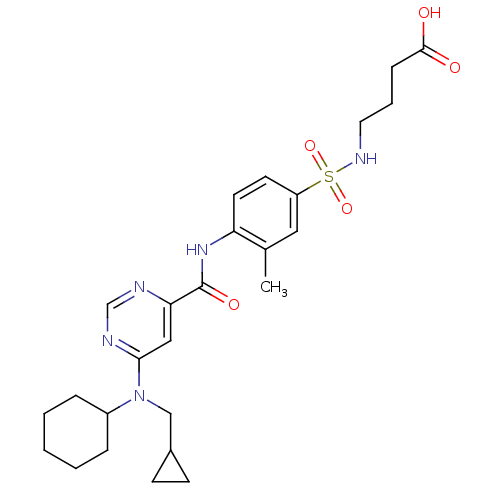 (4-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183701 (US9150519, 1-100) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410616 (CHEMBL2113185) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410638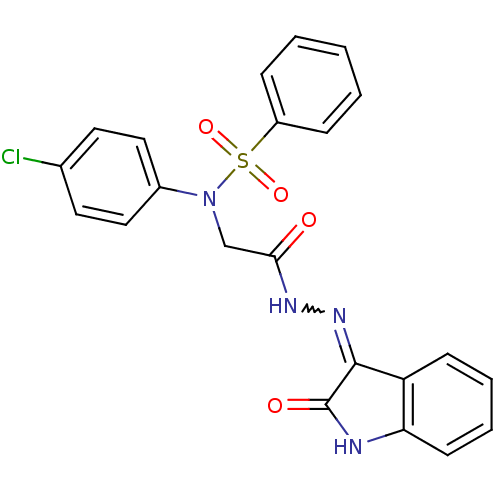 (CHEMBL2113207) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183699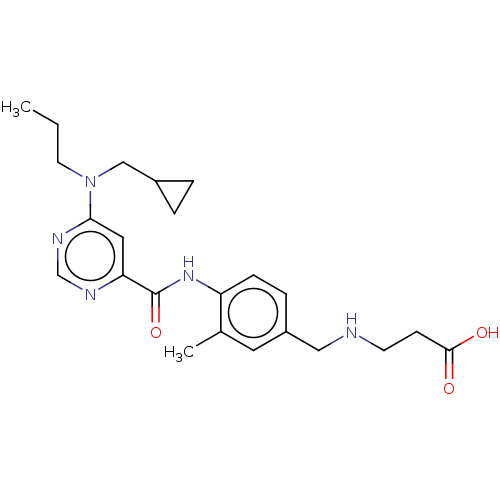 (US9150519, 1-97) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313363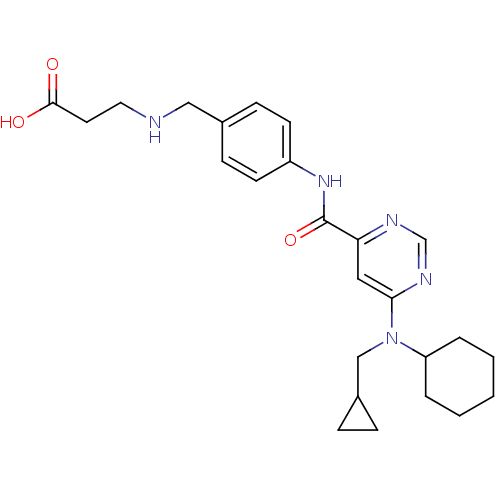 (3-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 9 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410630 (CHEMBL2113209) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183676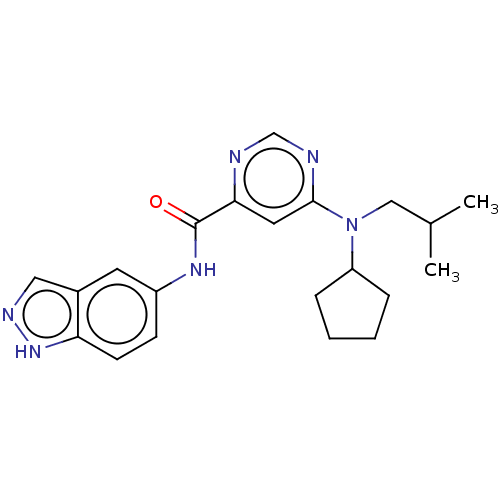 (US9150519, 1-69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410619 (CHEMBL2113208) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313361 (2-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 12 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178153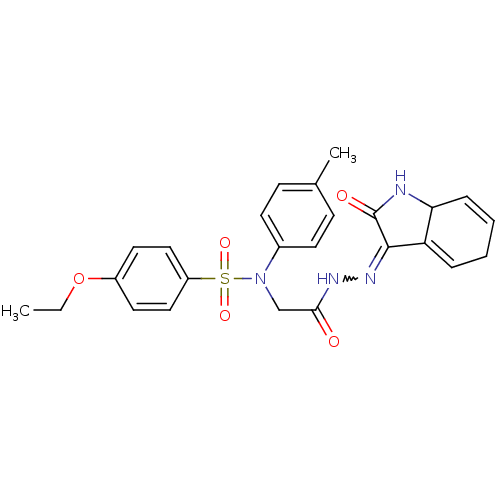 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410622 (CHEMBL2113203) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178186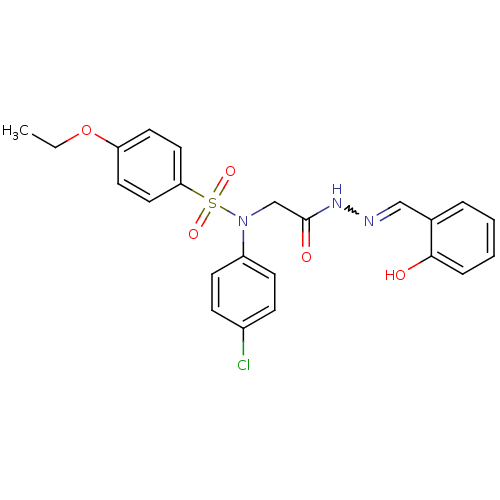 (CHEMBL372122 | N-(4-chlorophenyl)-4-ethoxy-N-{2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410637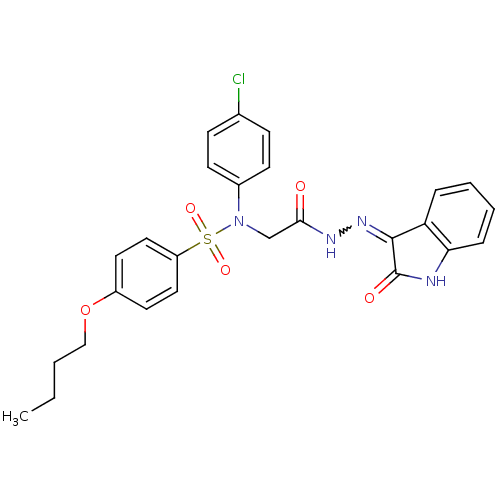 (CHEMBL2113210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178209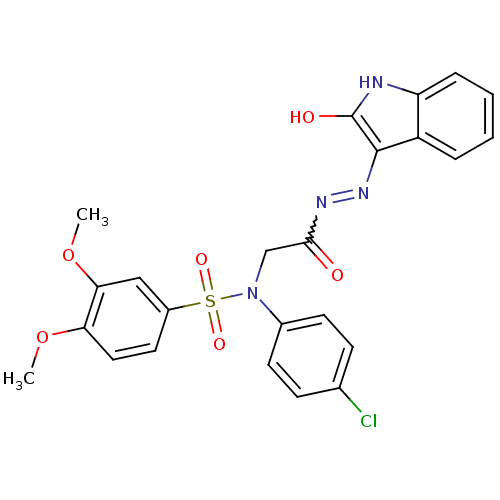 (CHEMBL371223 | N-(4-chlorophenyl)-3,4-dimethoxy-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410624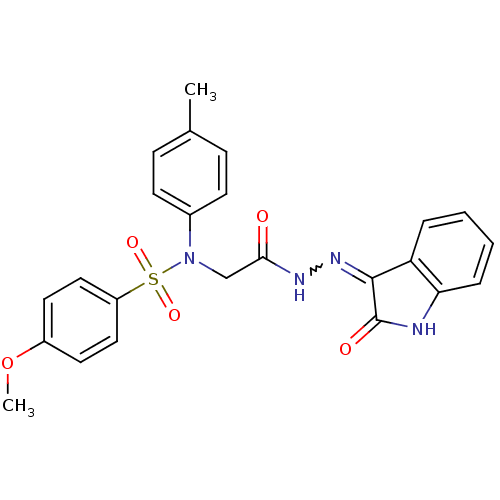 (CHEMBL2113215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410628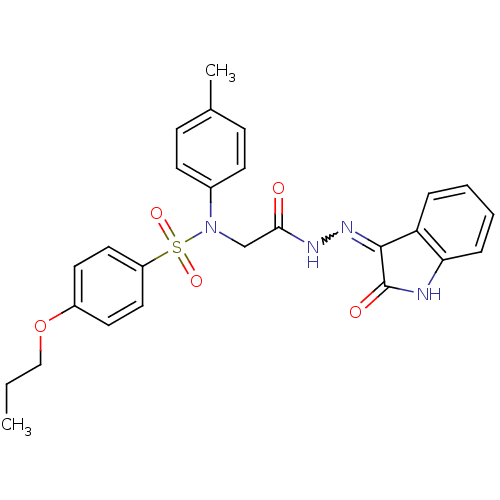 (CHEMBL2113216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183692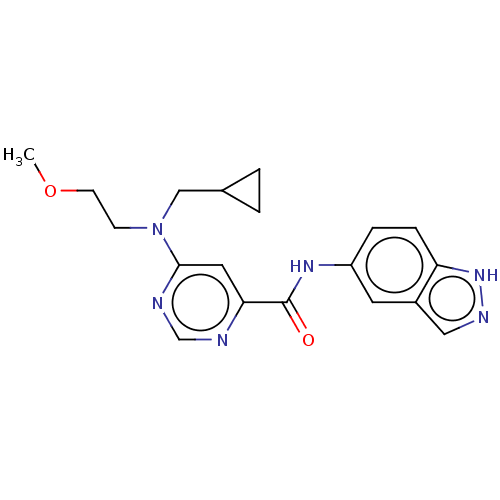 (US9150519, 1-90) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 23 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183710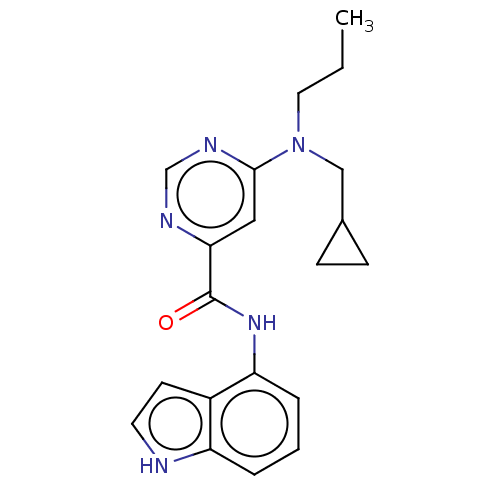 (US9150519, 1-113) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410621 (CHEMBL2113177) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50178153 (4-ethoxy-N-(4-methylphenyl)-N-{2-oxo-2-[2-(2-oxo-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50177595 ((2S)-5-amino-2-{[(2S)-1-{[(4R,7S,10S,13S,16R)-13-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313362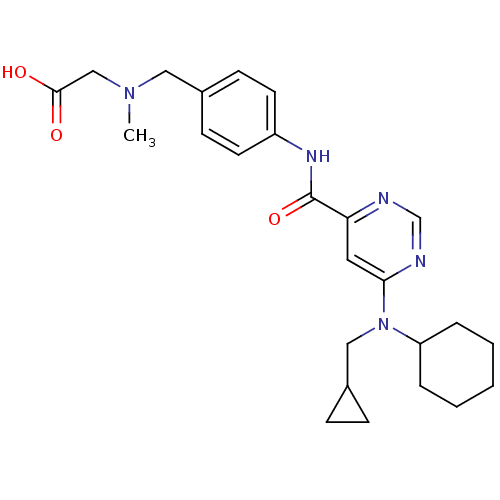 (2-((4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 29 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50410635 (CHEMBL2113211) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178197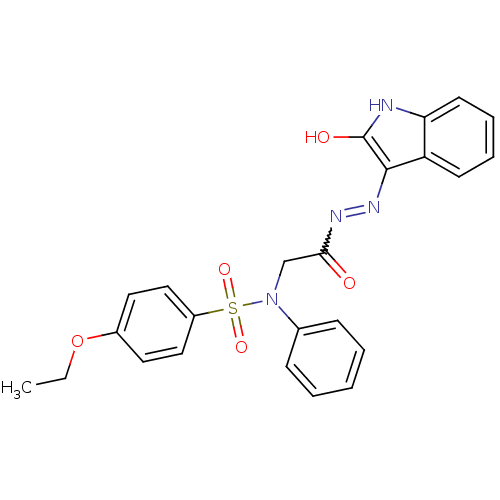 (4-ethoxy-N-{2-oxo-2-[2-(2-oxo-1,2-dihydro-3H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410636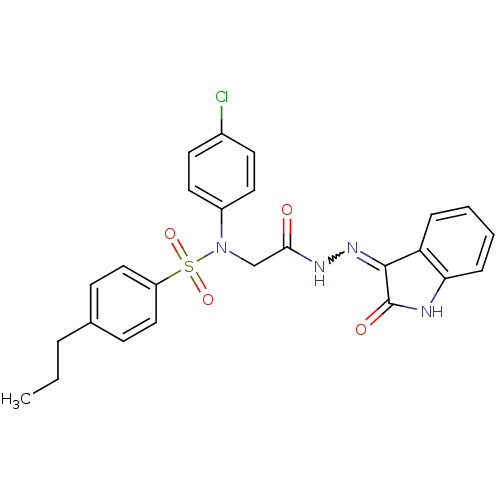 (CHEMBL2113212) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50410631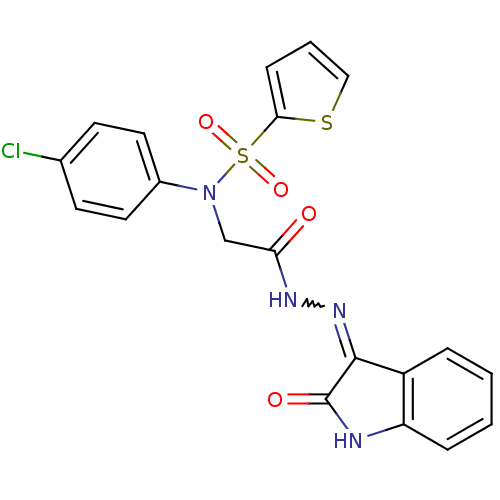 (CHEMBL2113204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183687 (US9150519, 1-85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313345 (6-(cyclohexyl(cyclopropylmethyl)amino)-N-(4-((dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 51 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50178213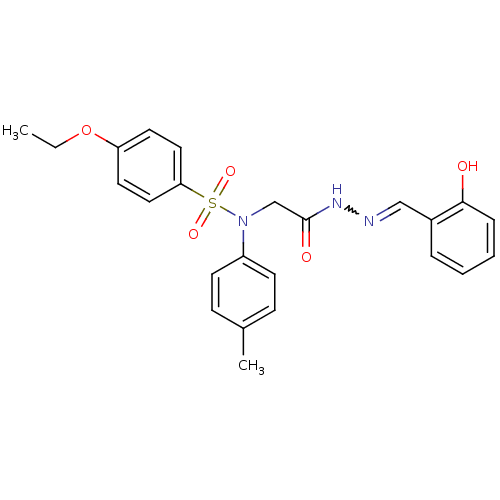 (4-ethoxy-N-{2-[2-(2-hydroxybenzylidene)hydrazino]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cells | J Med Chem 48: 7882-905 (2005) Article DOI: 10.1021/jm050645f BindingDB Entry DOI: 10.7270/Q2QF8TN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 515 total ) | Next | Last >> |