

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50094037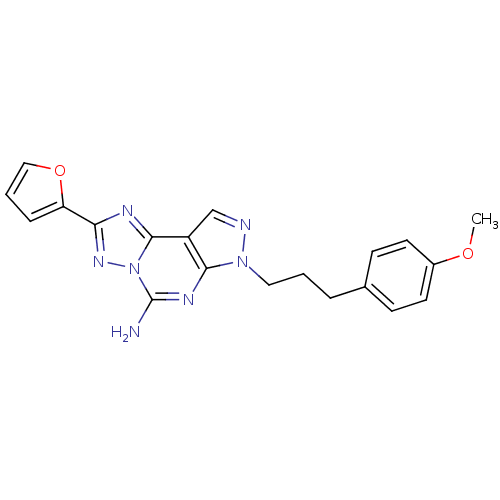 (2-Furan-2-yl-7-[3-(4-methoxy-phenyl)-propyl]-7H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A2A receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677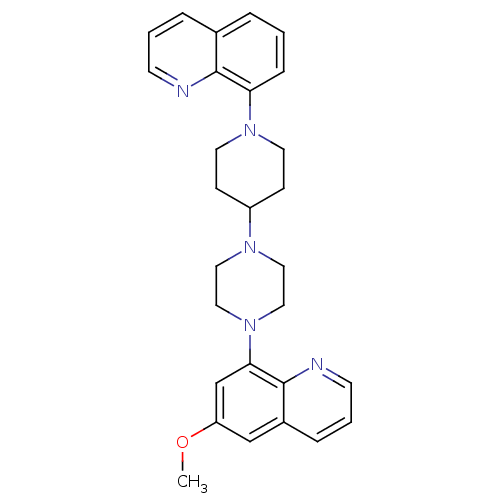 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673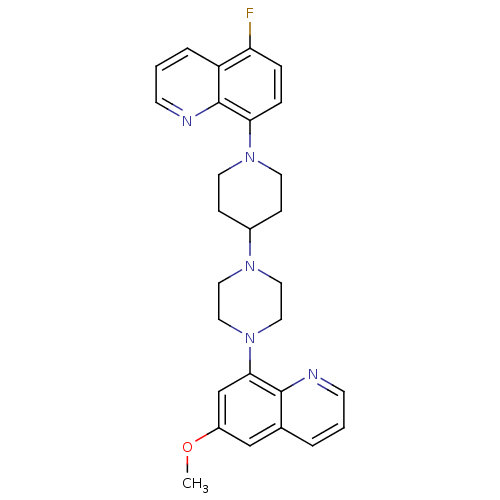 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316680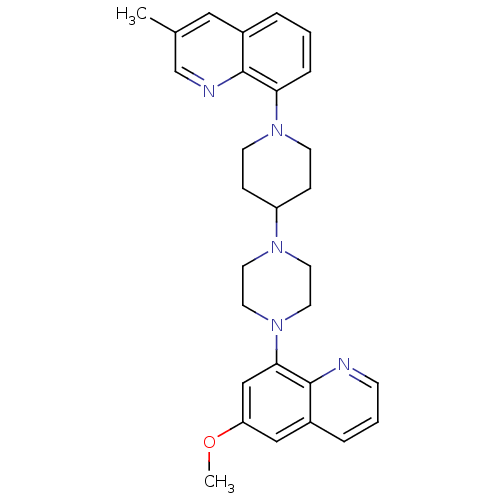 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316684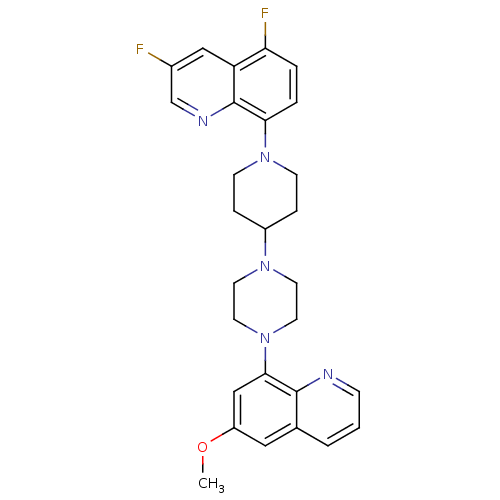 (3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581955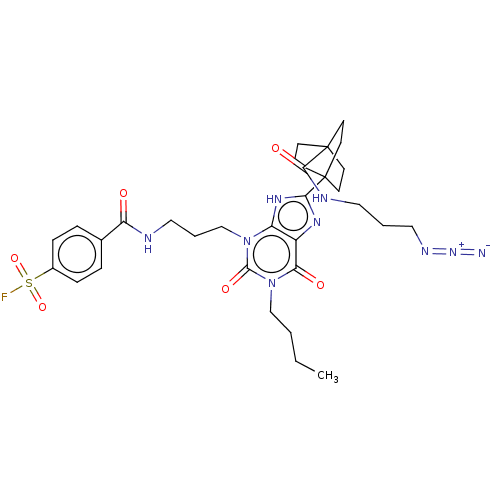 (CHEMBL5073669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316688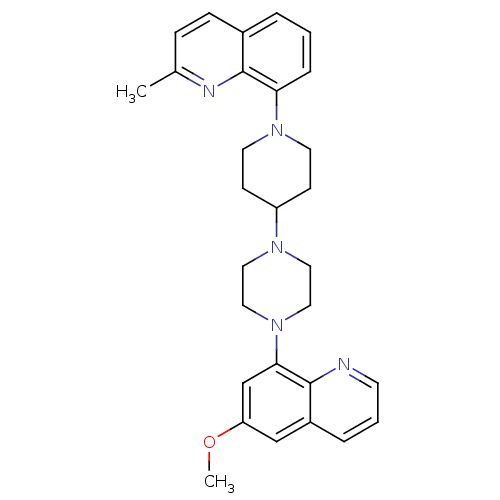 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581950 (CHEMBL4204703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50366779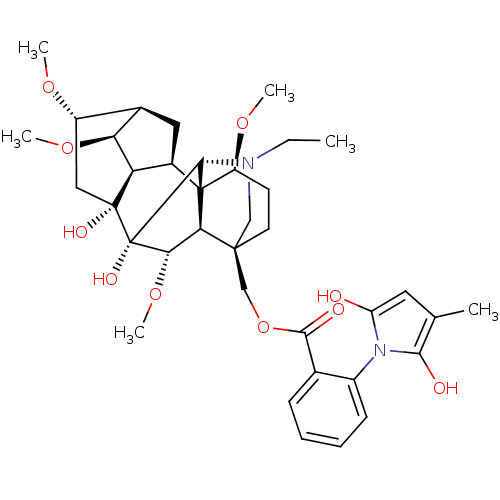 (METHYLLYCACONITINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against alpha7 nAChR (nicotinic acetylcholine receptor) using [3H]-MLA as a radioligand relative to alpha4-beta2 | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957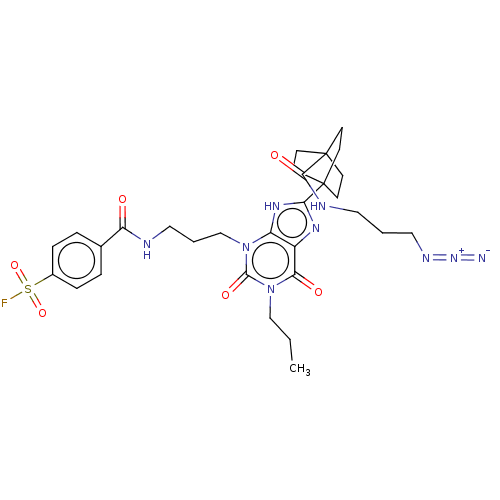 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964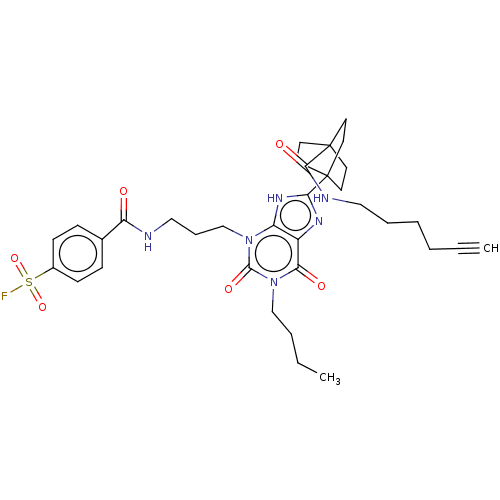 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316690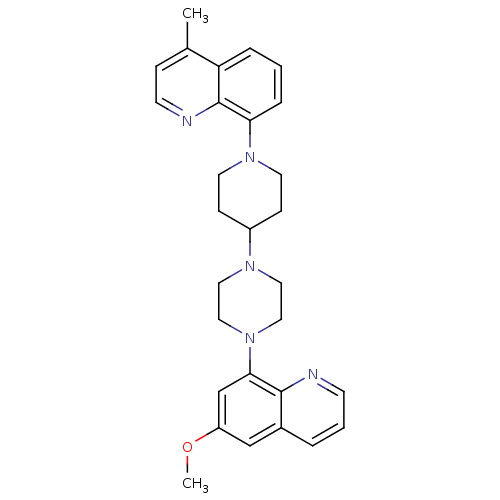 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316682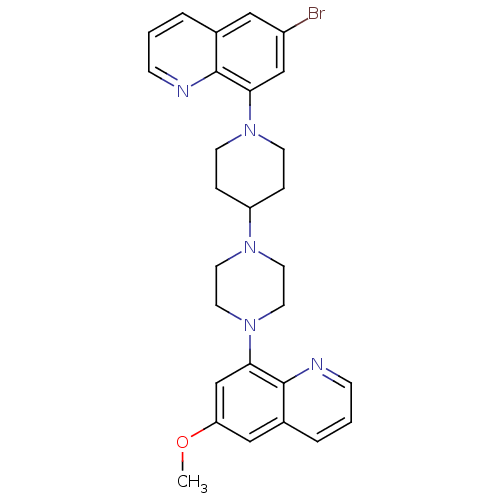 (6-Bromo-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316678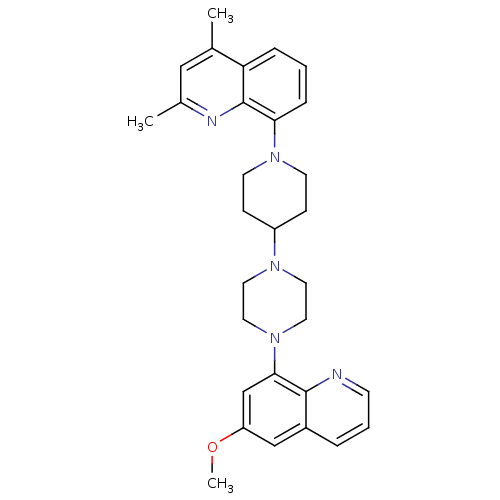 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316679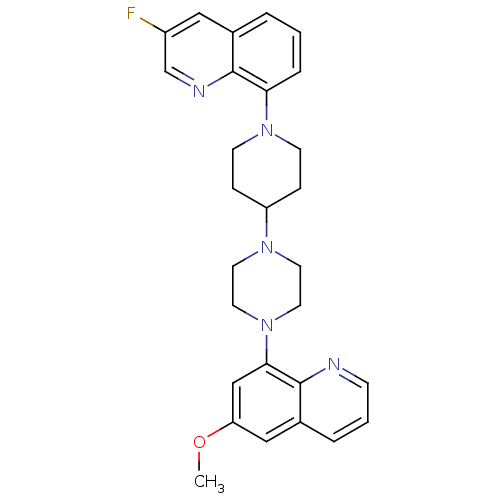 (3-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50366779 (METHYLLYCACONITINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-7 nAChR (nicotinic acetylcholine receptor) using [125I]-alpha-BGT as a radioligand relative to alpha4-beta2 | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316683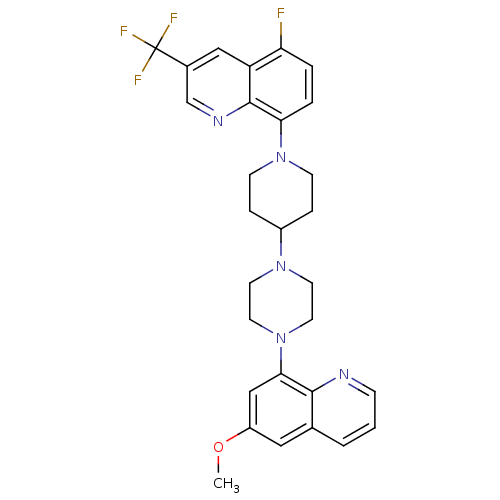 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50472784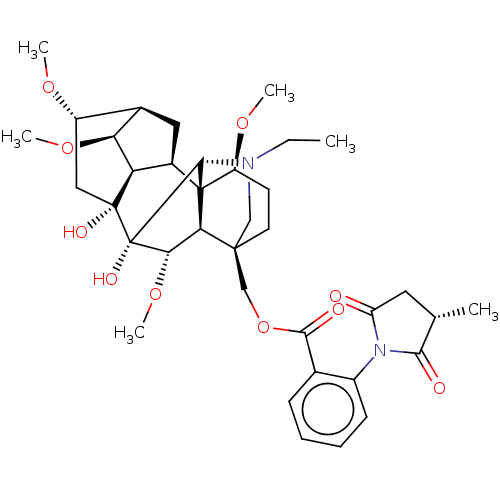 (METHYLLYCACONITINE[3H] MLA | [3H]-MLA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]MLA binding in presence of methylcaconitine. | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316686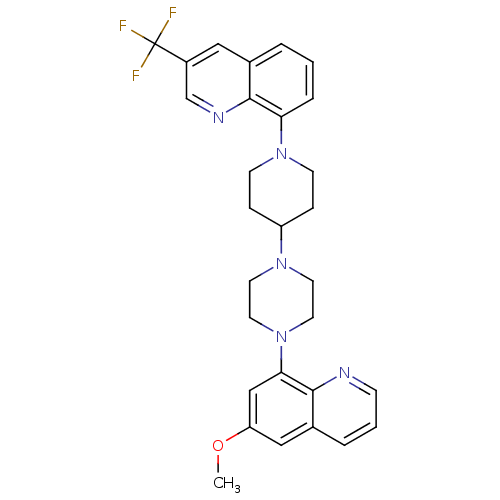 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50268232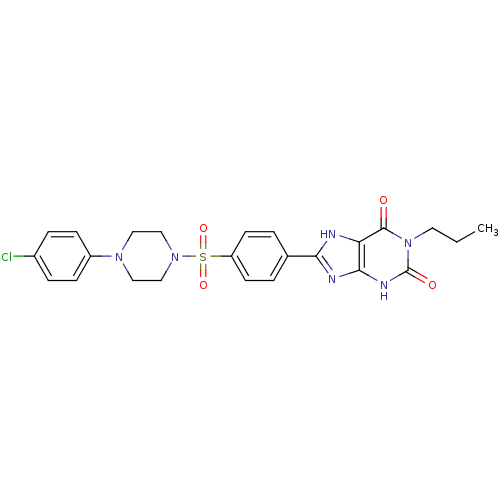 (8-(4-(4-(4-Chlorophenyl)piperazine-1-sulfonyl)phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A2B receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50472783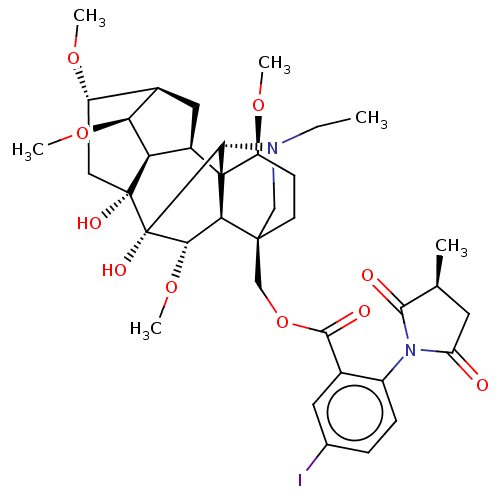 (CHEMBL2112068) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-7 nAChR (nicotinic acetylcholine receptor) using [125I]-alpha-BGT as a radioligand relative to alpha4-beta2 | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581960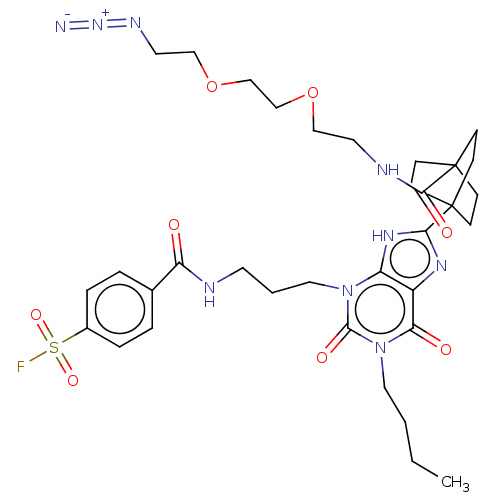 (CHEMBL5074992) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581964 (CHEMBL5092788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50472783 (CHEMBL2112068) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against alpha7 nAChR (nicotinic acetylcholine receptor) using [3H]-MLA as a radioligand relative to alpha4-beta2 | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316685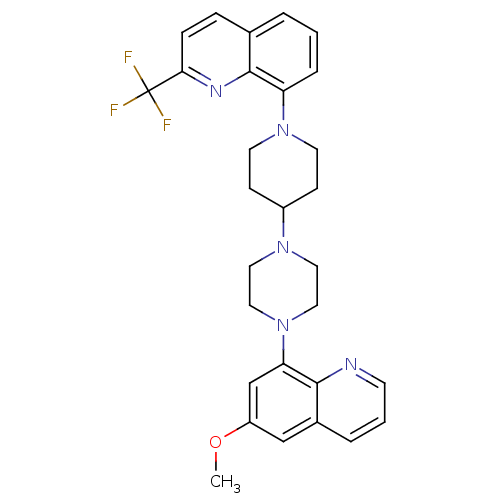 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50472784 (METHYLLYCACONITINE[3H] MLA | [3H]-MLA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]MLA binding in presence of alpha-bungarotoxin | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50001942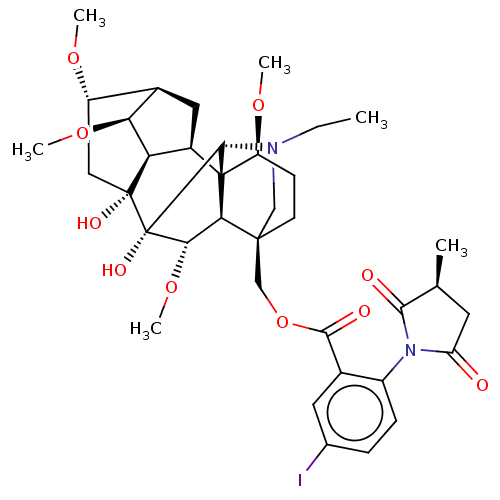 (CHEMBL2113668 | Iodo-MLA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [125I]iodo-MLA binding in presence of alpha-bungarotoxin | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316681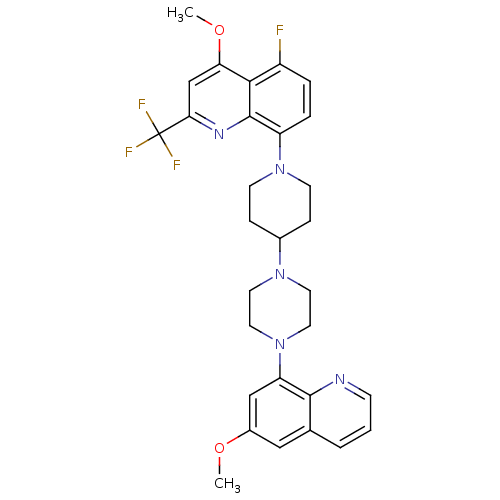 (5-Fluoro-4-methoxy-8-{4-[4-(6-methoxyquinolin-8-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50271706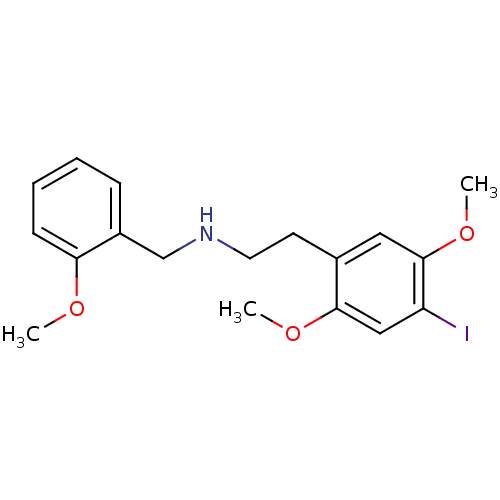 (CHEMBL482496 | N-(2-[3H-OCH3]benzyl)-2-(2-[3H-OCH3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to 5HT2C receptor | Bioorg Med Chem 16: 6116-23 (2008) Article DOI: 10.1016/j.bmc.2008.04.050 BindingDB Entry DOI: 10.7270/Q2G73FND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316687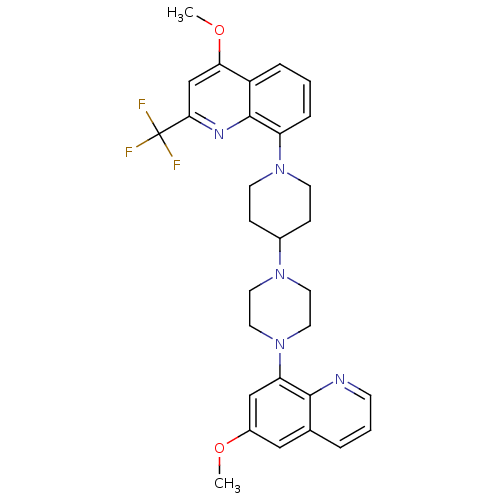 (4-Methoxy-8-{4-[4-(6-methoxyquinolin-8-yl)piperazi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581961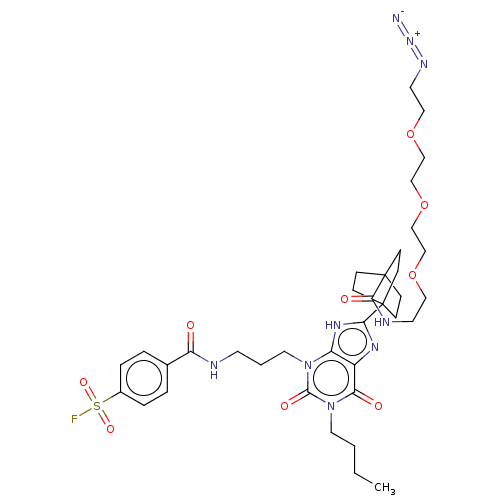 (CHEMBL5088372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581959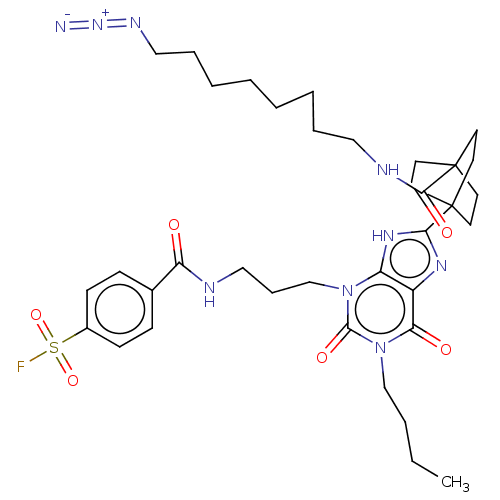 (CHEMBL5076192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21342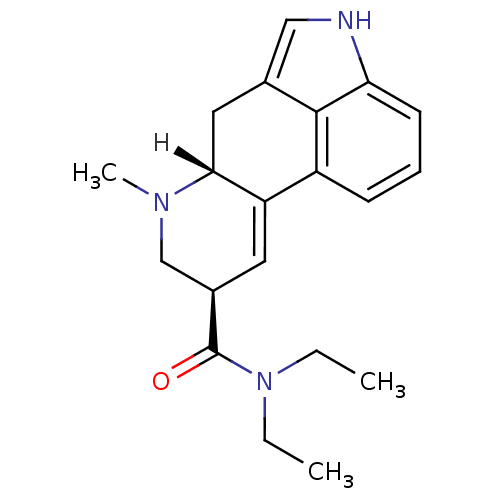 ((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in human A549 cells | Bioorg Med Chem 16: 6116-23 (2008) Article DOI: 10.1016/j.bmc.2008.04.050 BindingDB Entry DOI: 10.7270/Q2G73FND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50581959 (CHEMBL5076192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A3 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21342 ((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2A receptor expressed in human HEK293 cells | Bioorg Med Chem 16: 6116-23 (2008) Article DOI: 10.1016/j.bmc.2008.04.050 BindingDB Entry DOI: 10.7270/Q2G73FND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50001942 (CHEMBL2113668 | Iodo-MLA) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [125I]iodo-MLA binding in presence of methylcaconitine. | J Med Chem 43: 142-5 (2000) Article DOI: 10.1021/jm990544f BindingDB Entry DOI: 10.7270/Q2G163KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581961 (CHEMBL5088372) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581954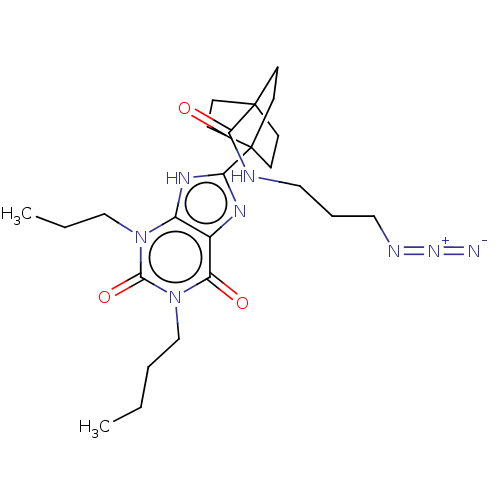 (CHEMBL5087305) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of forskolin-stimulated cAMP accumula... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316675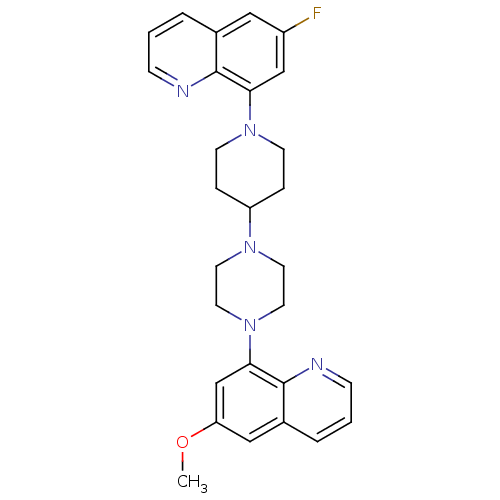 (6-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM21342 ((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor expressed in human A549 cells | Bioorg Med Chem 16: 6116-23 (2008) Article DOI: 10.1016/j.bmc.2008.04.050 BindingDB Entry DOI: 10.7270/Q2G73FND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581960 (CHEMBL5074992) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human adenosine A1 receptor expressed in Flp-In-CHO cell membrane assessed as inhibition of NECA-induced cAMP accumulation in ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50581957 (CHEMBL5080823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H] DPCPX from human A1 adenosine receptor expressed in Flp-In-CHO cell membrane incubated for 4 hrs by microbeta scintillation coun... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02169 BindingDB Entry DOI: 10.7270/Q23200R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316676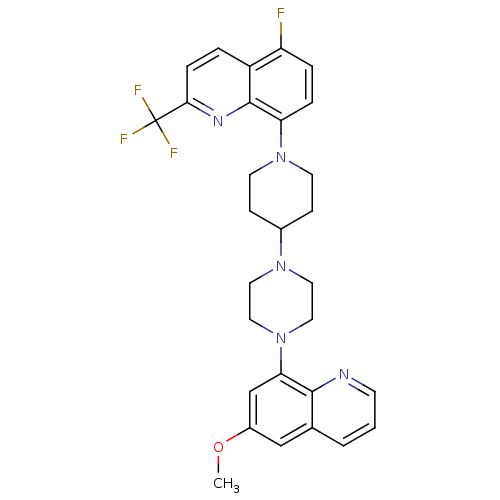 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1075 total ) | Next | Last >> |