

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354644 (CHEMBL1834218) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354647 (CHEMBL1834244) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025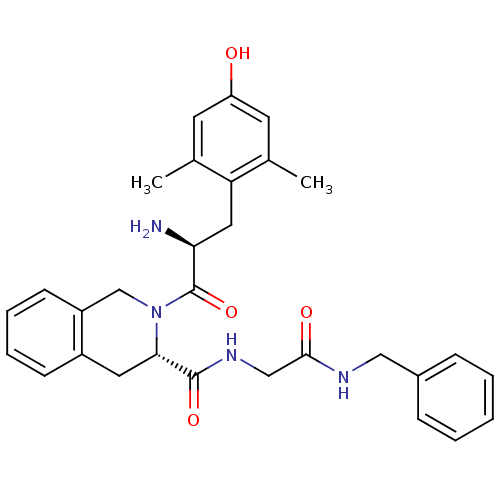 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354650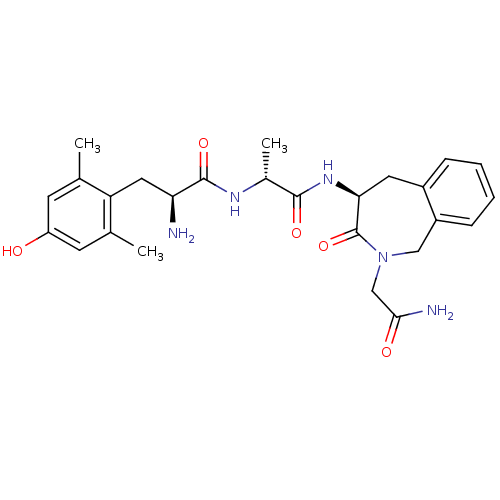 (CHEMBL1834247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354649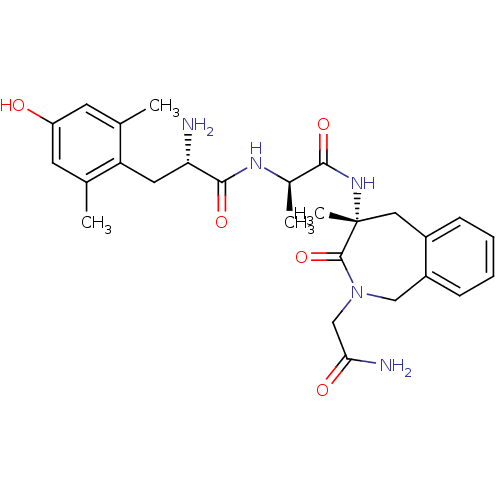 (CHEMBL1834246) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354651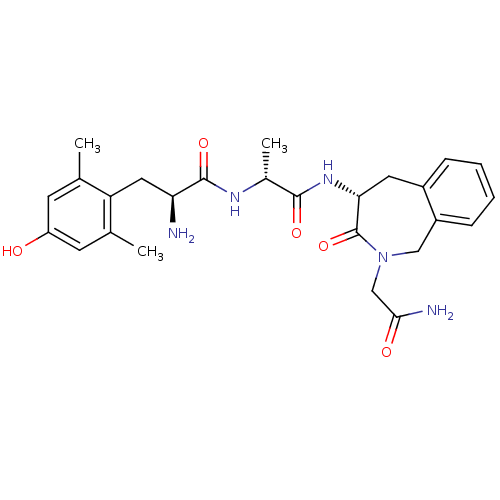 (CHEMBL1834248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354645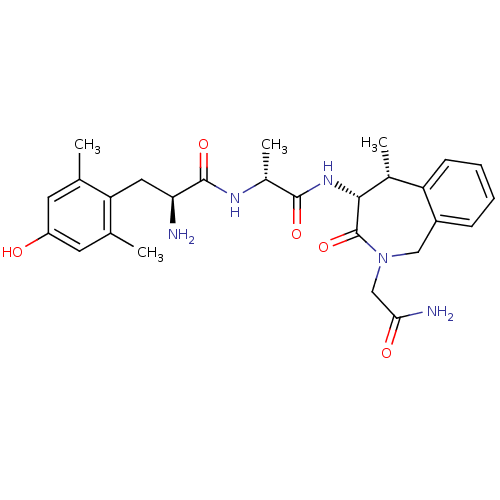 (CHEMBL1834219) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50189920 ((S)-2-amino-N-((S)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354644 (CHEMBL1834218) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fe(3+)-pyochelin receptor (Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444965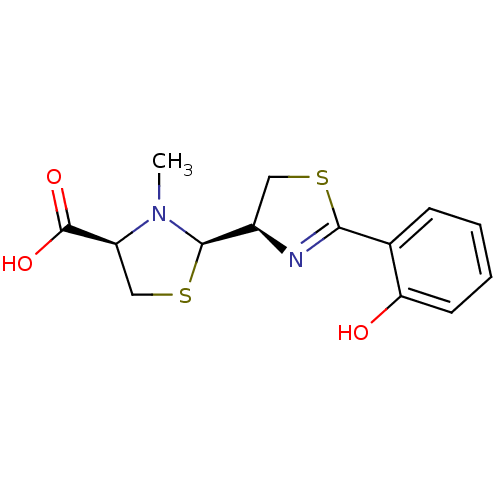 (CHEMBL3099904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP | Bioorg Med Chem Lett 24: 132-5 (2013) Article DOI: 10.1016/j.bmcl.2013.11.054 BindingDB Entry DOI: 10.7270/Q2J38V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354650 (CHEMBL1834247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266060 ((S)-2-amino-N-((R)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354648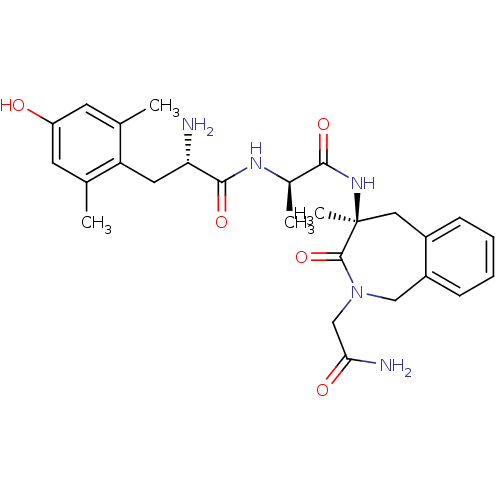 (CHEMBL1834245) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fe(3+)-pyochelin receptor (Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444963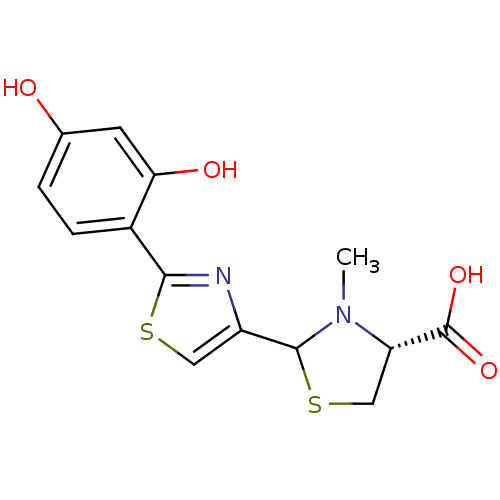 (CHEMBL3099907) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP | Bioorg Med Chem Lett 24: 132-5 (2013) Article DOI: 10.1016/j.bmcl.2013.11.054 BindingDB Entry DOI: 10.7270/Q2J38V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266063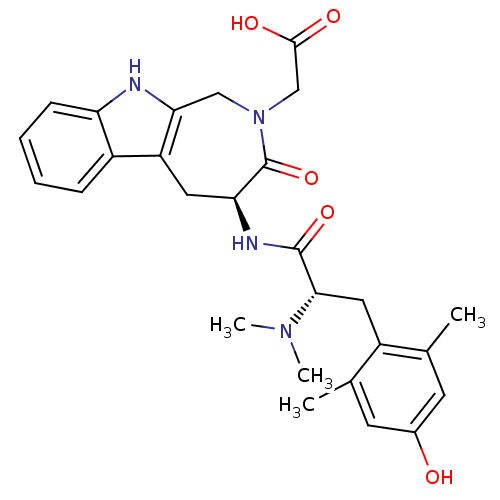 (2-((S)-4-((S)-2-(dimethylamino)-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fe(3+)-pyochelin receptor (Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444964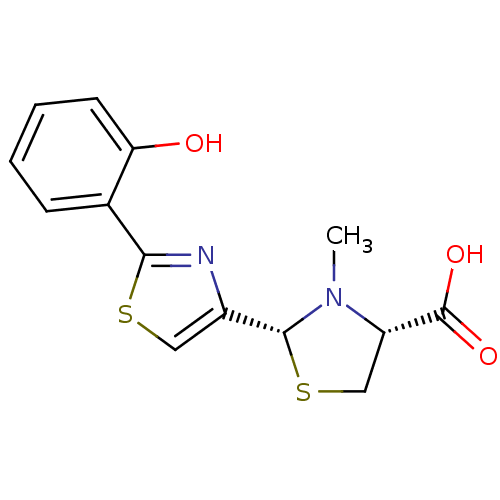 (CHEMBL3099905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP | Bioorg Med Chem Lett 24: 132-5 (2013) Article DOI: 10.1016/j.bmcl.2013.11.054 BindingDB Entry DOI: 10.7270/Q2J38V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266026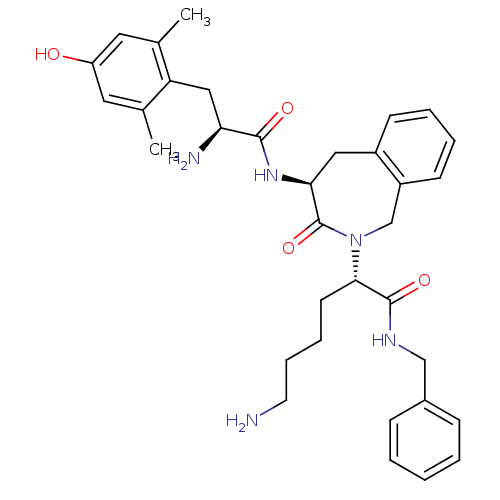 ((S)-6-amino-2-((S)-4-((S)-2-amino-3-(4-hydroxy-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fe(3+)-pyochelin receptor (Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444962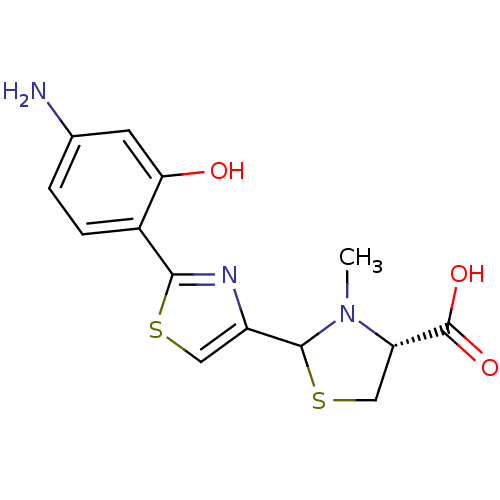 (CHEMBL3099909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg Curated by ChEMBL | Assay Description Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP | Bioorg Med Chem Lett 24: 132-5 (2013) Article DOI: 10.1016/j.bmcl.2013.11.054 BindingDB Entry DOI: 10.7270/Q2J38V1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50189920 ((S)-2-amino-N-((S)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266056 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-N-((S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354646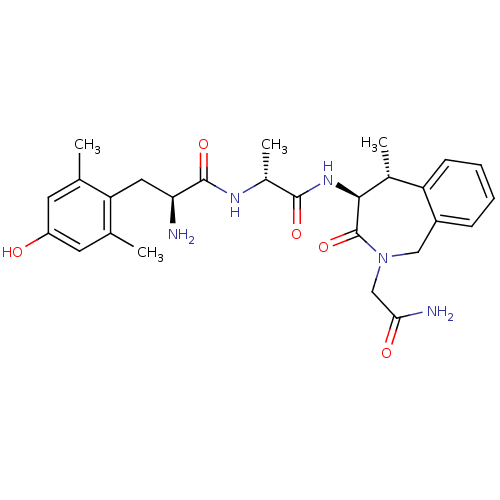 (CHEMBL1834220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat brain Mu-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266057 ((S)-2-(dimethylamino)-3-(4-hydroxy-2,6-dimethylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354647 (CHEMBL1834244) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369245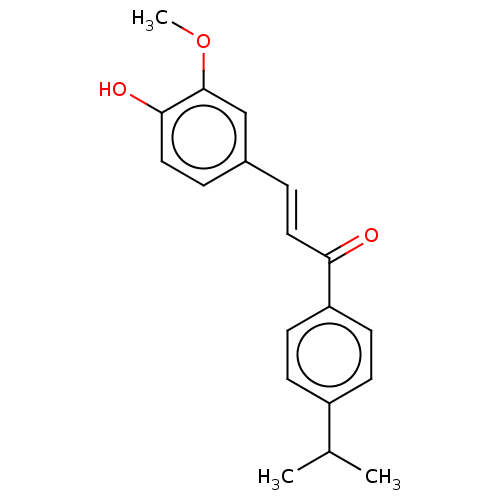 (CHEMBL4170392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369246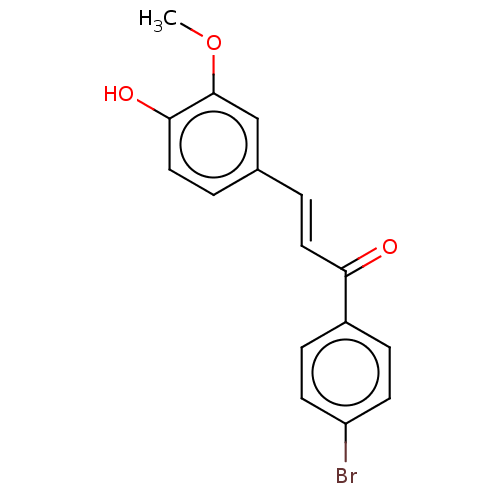 (CHEMBL4160309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369285 (CHEMBL4160346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266058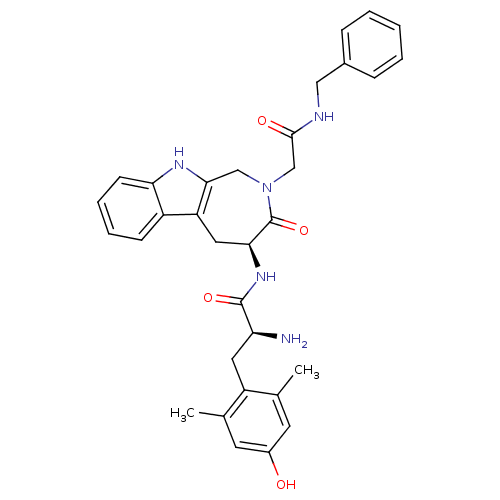 ((S)-2-amino-N-((S)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369286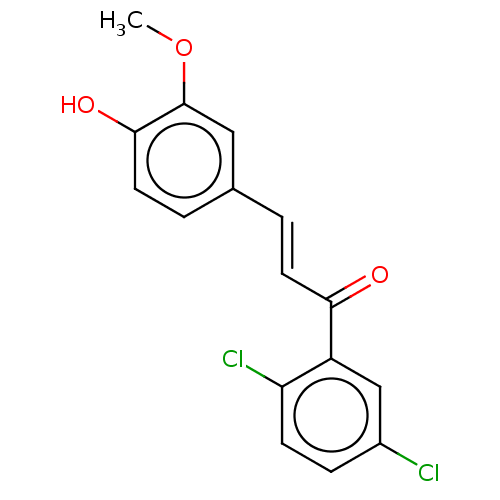 (CHEMBL4167574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50042947 (1-(4-Chloro-phenyl)-3-(4-hydroxy-3-methoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354649 (CHEMBL1834246) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266057 ((S)-2-(dimethylamino)-3-(4-hydroxy-2,6-dimethylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 64.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369256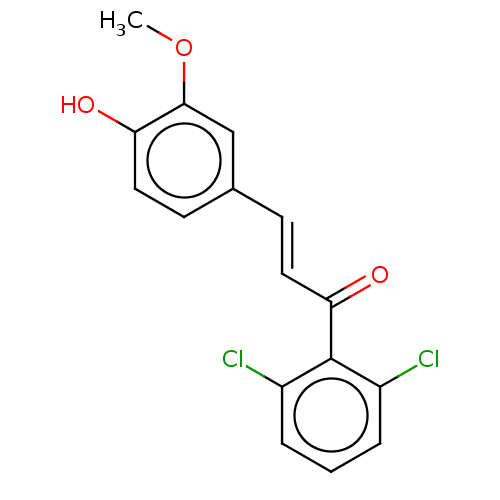 (CHEMBL4175451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369250 (CHEMBL4168923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369280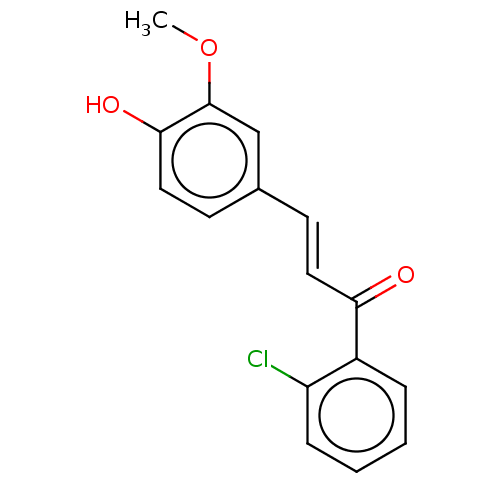 (CHEMBL4166275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369248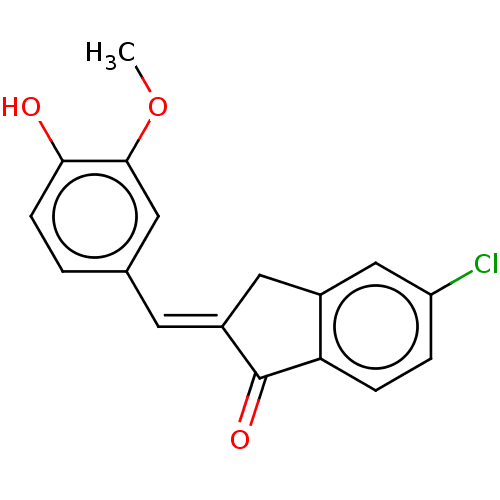 (CHEMBL4163005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369244 (CHEMBL4169628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369321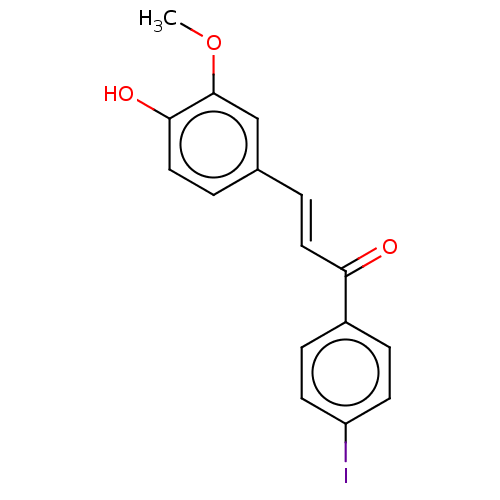 (CHEMBL4168220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266061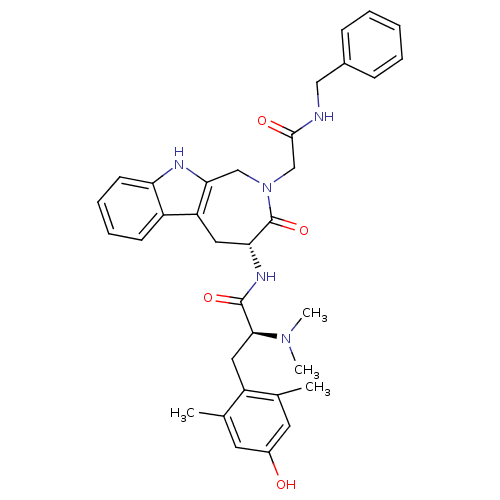 ((S)-N-((R)-2-(2-(benzylamino)-2-oxoethyl)-3-oxo-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266060 ((S)-2-amino-N-((R)-2-(2-(benzylamino)-2-oxoethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369330 (CHEMBL4160583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266059 (CHEMBL501023 | benzyl 2-((S)-4-((S)-2-amino-3-(4-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369279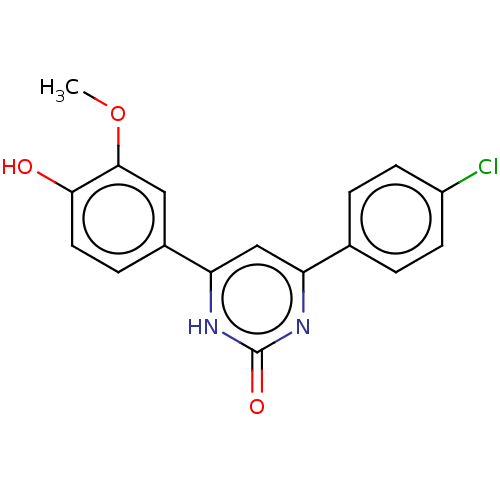 (CHEMBL4166454) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 267 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369249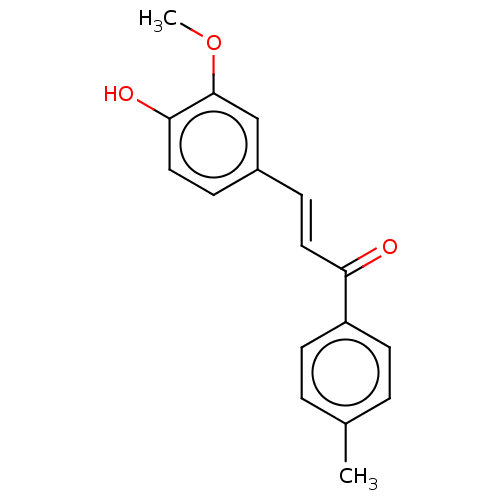 (CHEMBL4173832) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266026 ((S)-6-amino-2-((S)-4-((S)-2-amino-3-(4-hydroxy-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in Sprague-Dawley rat brain synaptosomal membranes | Bioorg Med Chem Lett 19: 433-7 (2008) Article DOI: 10.1016/j.bmcl.2008.11.051 BindingDB Entry DOI: 10.7270/Q2K64HZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50354651 (CHEMBL1834248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 337 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel Curated by ChEMBL | Assay Description Displacement of [3H]Ile-deltorphin-2 from rat brain delta-type opioid receptor by liquid scintillation counting | J Med Chem 54: 6538-47 (2011) Article DOI: 10.1021/jm2003574 BindingDB Entry DOI: 10.7270/Q24Q7VC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50042973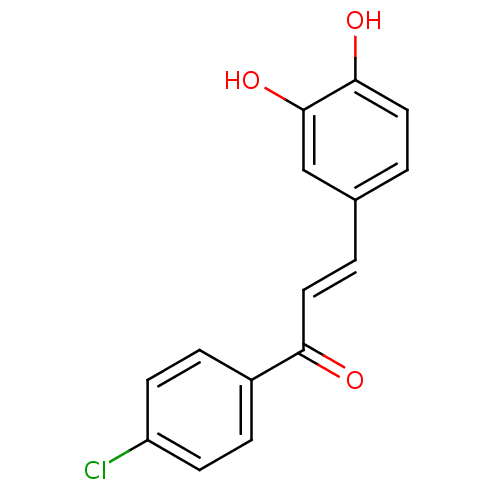 (1-(4-Chloro-phenyl)-3-(3,4-dihydroxy-phenyl)-prope...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369292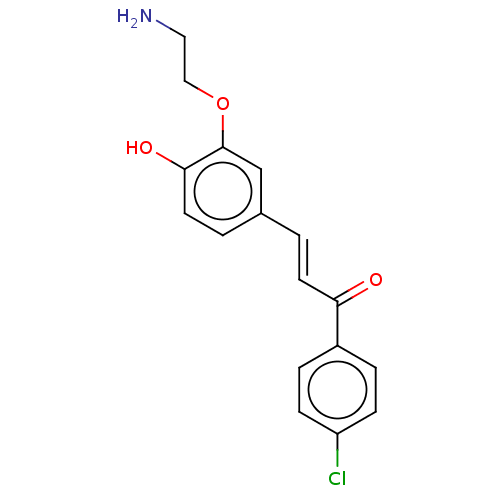 (CHEMBL4175213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369278 (CHEMBL4162605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromal cell-derived factor 1 (Homo sapiens (Human)) | BDBM50369322 (CHEMBL4170951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR7200 CNRS/Universit£ de Strasbourg Curated by ChEMBL | Assay Description Binding affinity to Texas Red-labelled CXCL12 (unknown origin) assessed as inhibition of CXCL12-Texas Red binding to EGFP-labeled human CXCR4 express... | J Med Chem 61: 7671-7686 (2018) Article DOI: 10.1021/acs.jmedchem.8b00657 BindingDB Entry DOI: 10.7270/Q2QF8WDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 258 total ) | Next | Last >> |