

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347454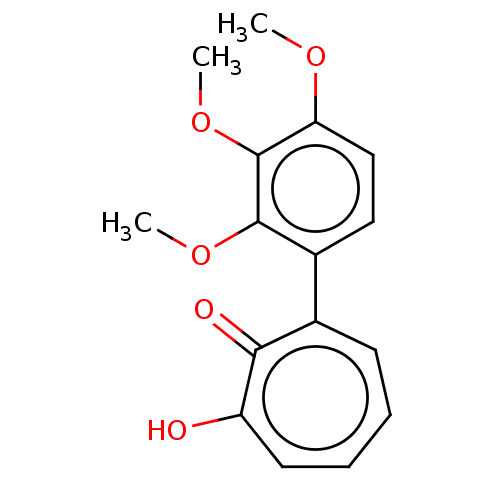 (MO-OH-TM | US9790158, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347461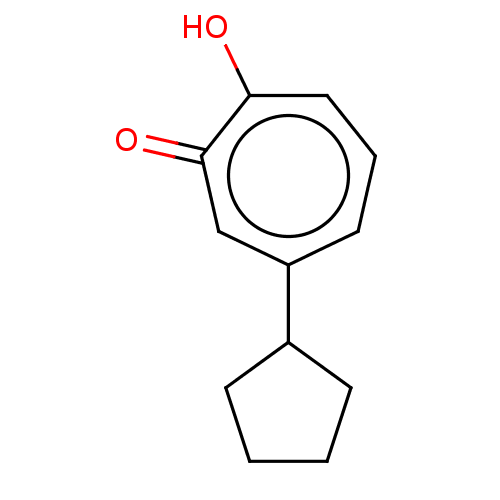 (US9790158, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50492541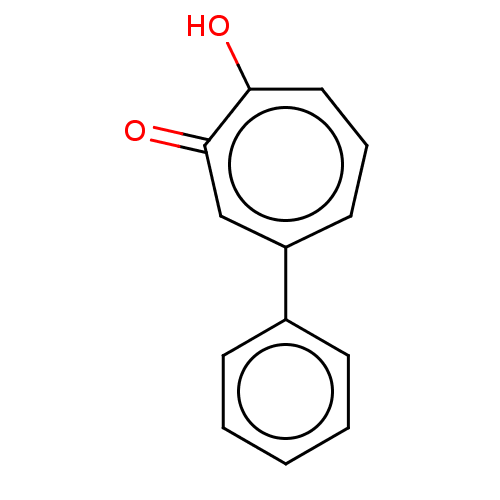 (CHEMBL2408242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347330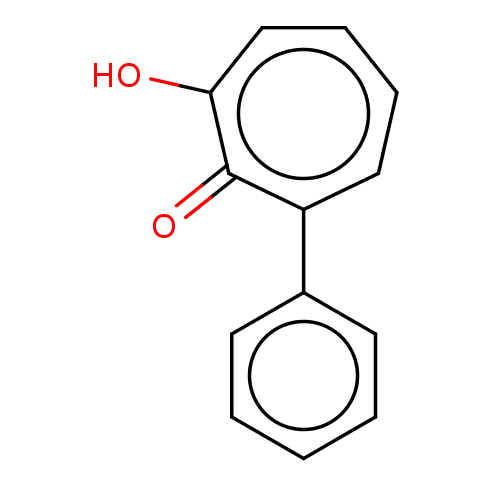 (MO-OH-PHE | US9790158, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21995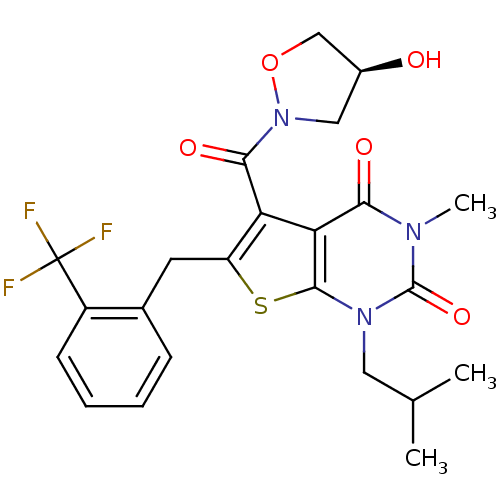 (5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50492540 (CHEMBL2408243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM348884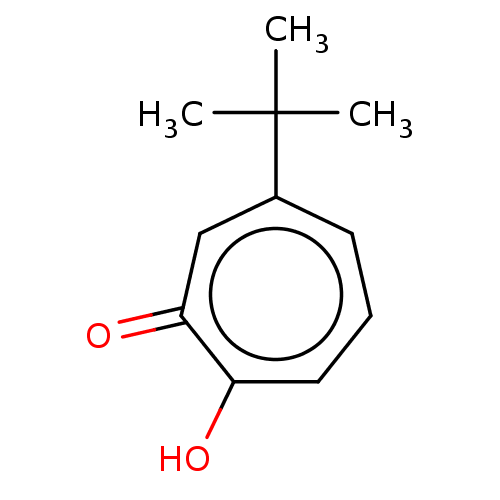 (US9790158, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013943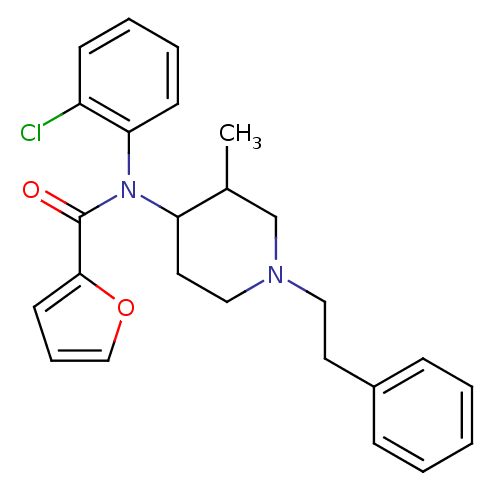 (CHEMBL327270 | Furan-2-carboxylic acid (2-chloro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347452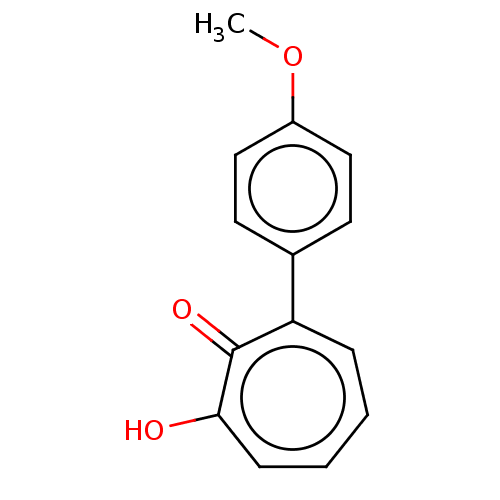 (MO-OH-SM | US9790158, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013945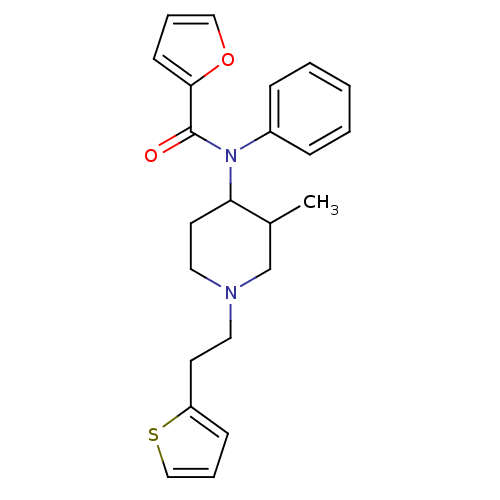 (CHEMBL431047 | Furan-2-carboxylic acid [3-methyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934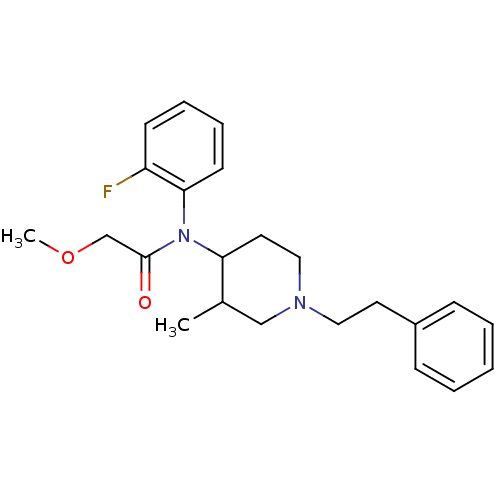 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013934 (CHEMBL319060 | N-(2-Fluoro-phenyl)-2-methoxy-N-(3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347460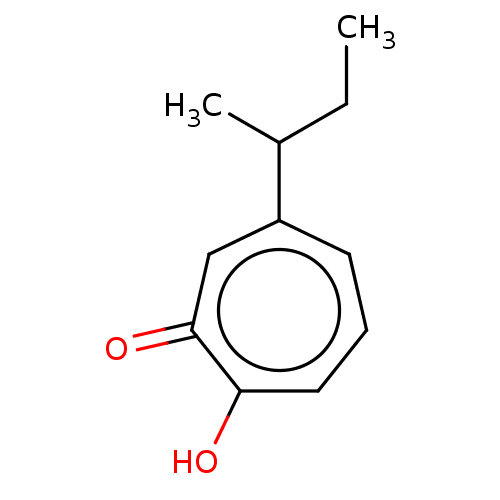 (US9790158, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM94503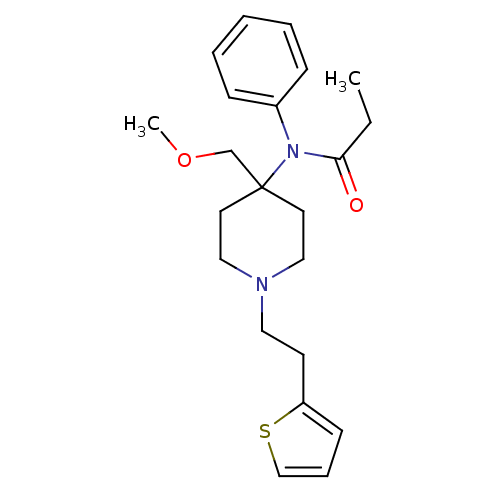 (2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347454 (MO-OH-TM | US9790158, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347451 (MO-OH-NAP | US9790158, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013939 (CHEMBL95247 | Furan-2-carboxylic acid (3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21992 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013938 (2-Methoxy-N-(3-methyl-1-phenethyl-piperidin-4-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347453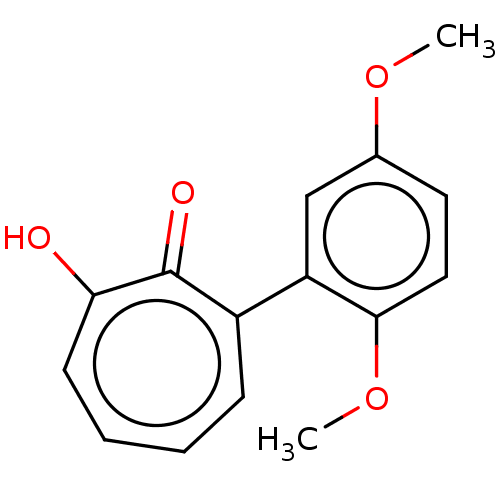 (MO-OH-DM | US9790158, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21996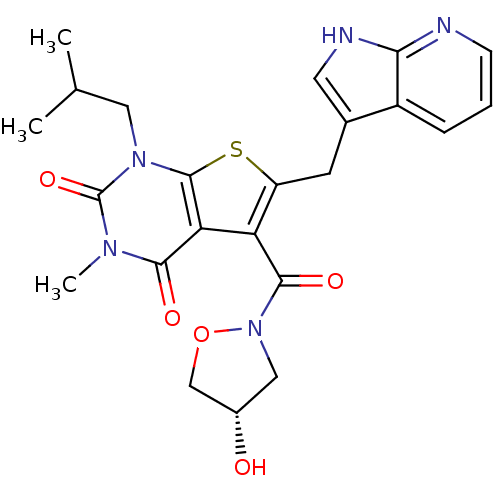 (5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347453 (MO-OH-DM | US9790158, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347457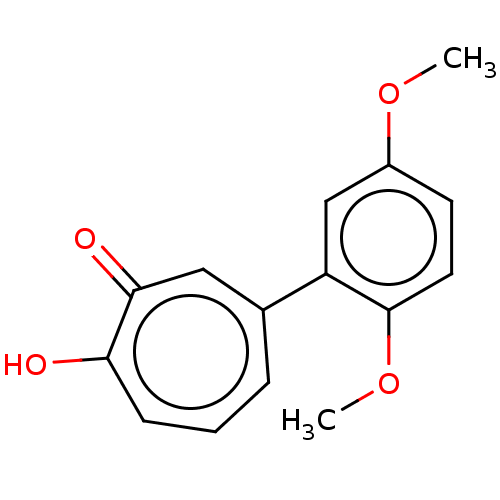 (US9790158, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21994 (5-{[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]carbonyl}-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM347451 (MO-OH-NAP | US9790158, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013949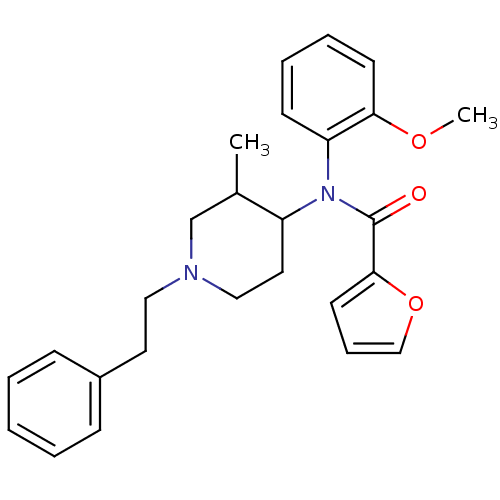 (CHEMBL95248 | Furan-2-carboxylic acid (2-methoxy-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM347452 (MO-OH-SM | US9790158, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21987 (6-[(6-fluoroquinolin-4-yl)methyl]-5-{[(3R)-3-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.810 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM210930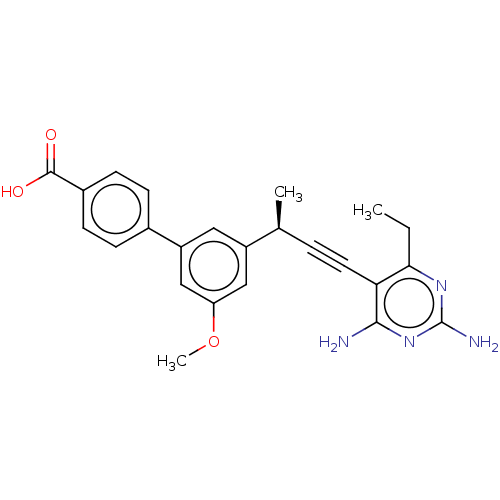 (UCP1173) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347330 (MO-OH-PHE | US9790158, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50005711 (CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50013948 (CHEMBL95909 | N-(2-Chloro-phenyl)-2-methoxy-N-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals Curated by ChEMBL | Assay Description Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes. | J Med Chem 33: 2876-82 (1990) BindingDB Entry DOI: 10.7270/Q2X34WF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GNAT family acetyltransferase (Enterococcus durans) | BDBM50193475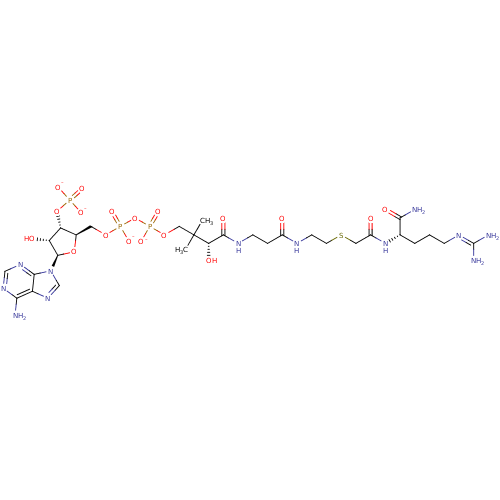 ((3R)-3-{[2-({2-[({[(1S)-1-carbamoyl-4-[(diaminomet...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecium AAC(6')Ii | J Med Chem 49: 5273-81 (2006) Article DOI: 10.1021/jm060732n BindingDB Entry DOI: 10.7270/Q2183789 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21998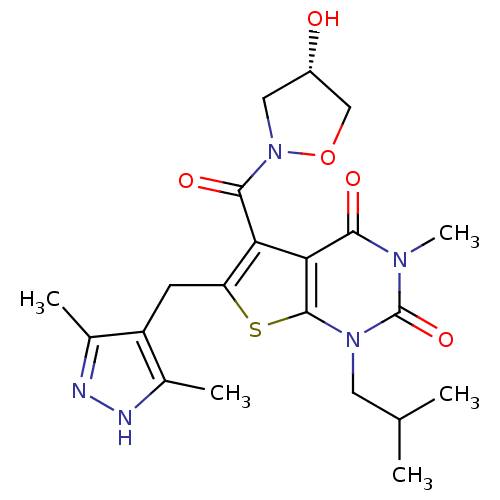 (6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -50.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | J Med Chem 50: 254-63 (2007) Article DOI: 10.1021/jm060995h BindingDB Entry DOI: 10.7270/Q2GT5KGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih... | ACS Med Chem Lett 7: 692-6 (2016) Article DOI: 10.1021/acsmedchemlett.6b00120 BindingDB Entry DOI: 10.7270/Q2DJ5HKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM210930 (UCP1173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy... | J Med Chem 59: 6493-500 (2016) Article DOI: 10.1021/acs.jmedchem.6b00688 BindingDB Entry DOI: 10.7270/Q2MW2MPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347456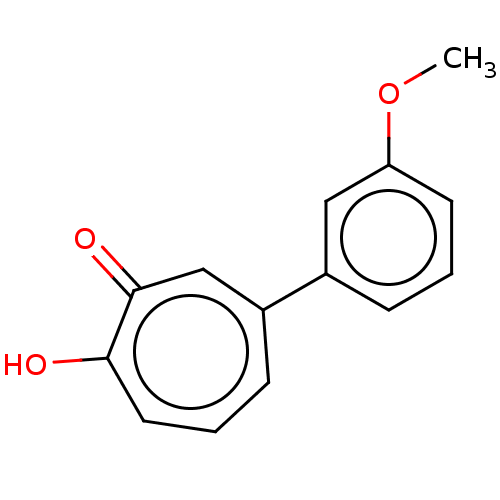 (US9790158, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM347457 (US9790158, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina... | US Patent US9790158 (2017) BindingDB Entry DOI: 10.7270/Q2QZ2D3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50492541 (CHEMBL2408242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis | ACS Med Chem Lett 4: 757-61 (2013) Article DOI: 10.1021/ml400158k BindingDB Entry DOI: 10.7270/Q28055HS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2421 total ) | Next | Last >> |