

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase (Clostridium perfringens) | BDBM50311581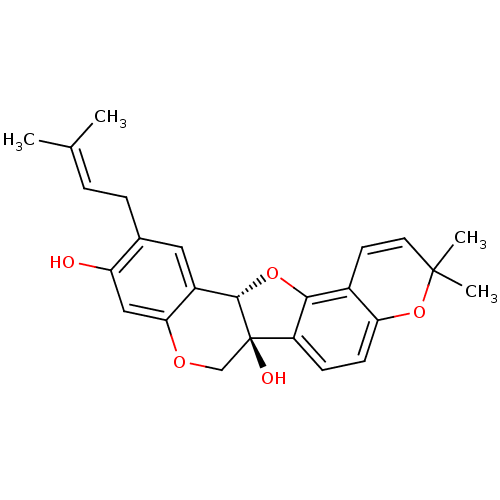 (CHEMBL1086764 | erysubin E) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317430 (CHEMBL454849 | Erythrabyssin I | cristacarpin | cr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317435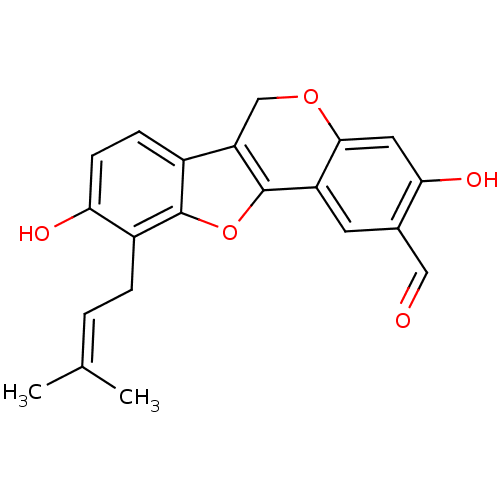 (CHEMBL1096406 | Erythribyssin O) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50311583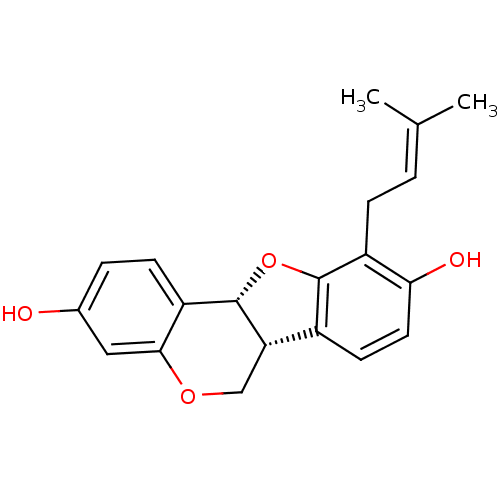 (Abyssinone II | CHEMBL508534 | phaseolidin | phase...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50311586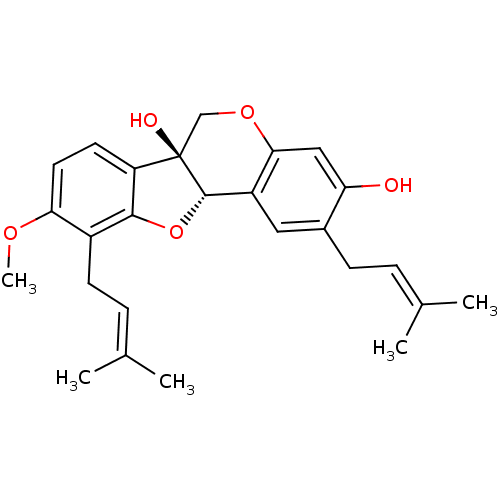 (CHEMBL1088462 | erystagallin A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317436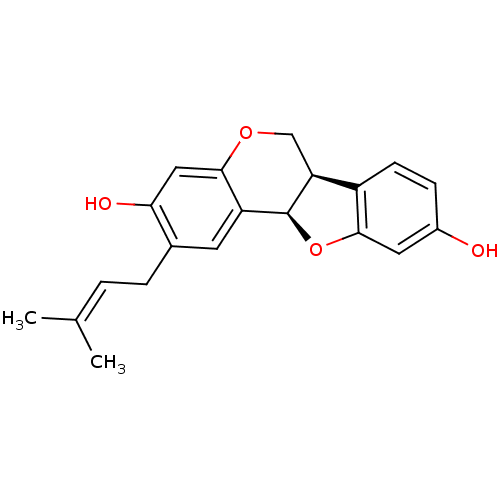 (CHEMBL1096407 | calopocarpin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317434 (CHEMBL1097045 | eryvarin D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317431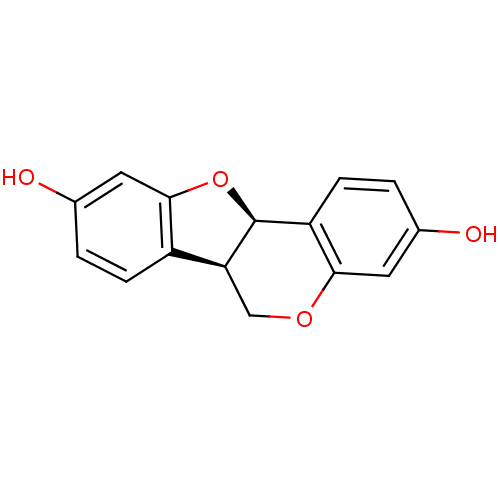 (CHEMBL1098413 | demethylmedicarpin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317437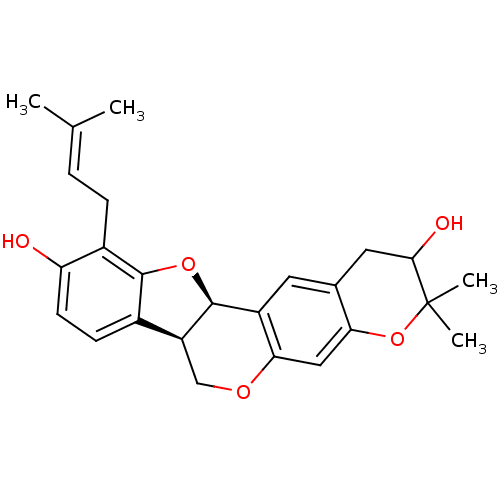 (CHEMBL1096408 | Erythribyssin L) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317438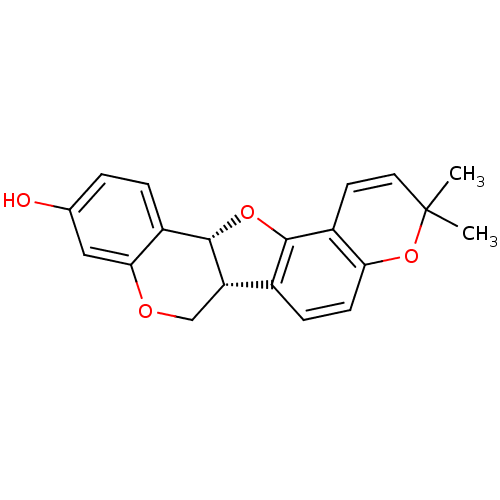 (Abyssinone I | CHEMBL448350 | phaseollin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317433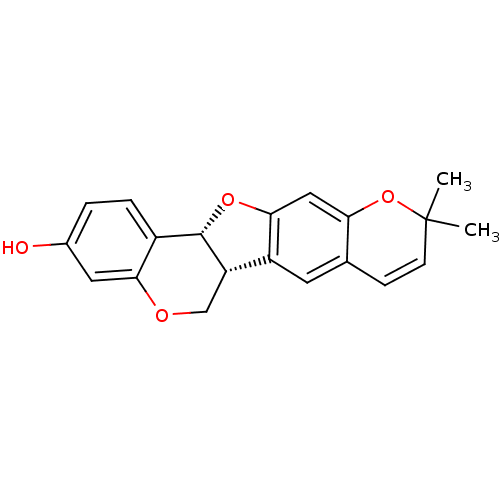 (CHEMBL1098729 | isoneorautenol) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317439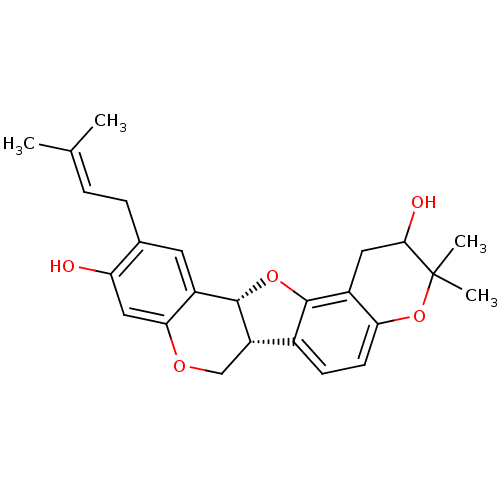 (CHEMBL1095422 | erysubin D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317432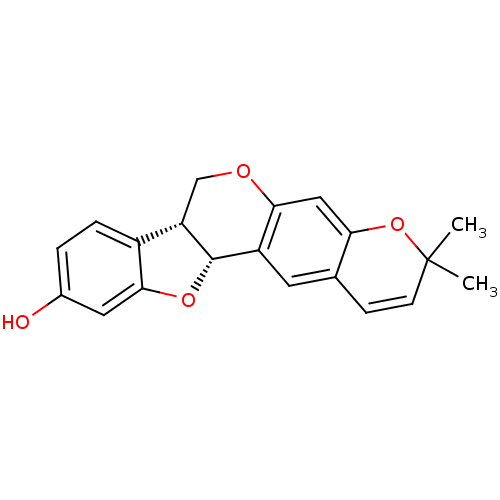 (CHEMBL1098728 | NEORAUTENOL) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Clostridium perfringens neuraminidase | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50483016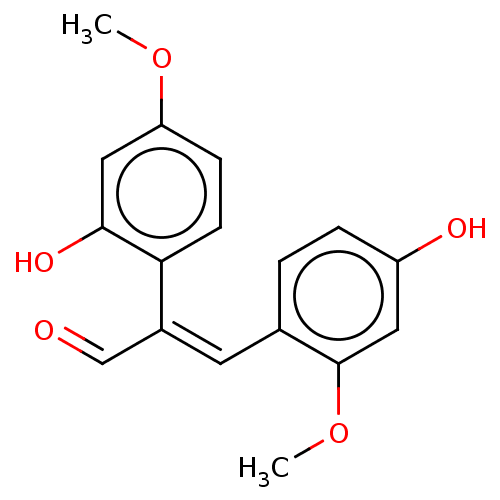 (ERYTHRADDISON B) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50483015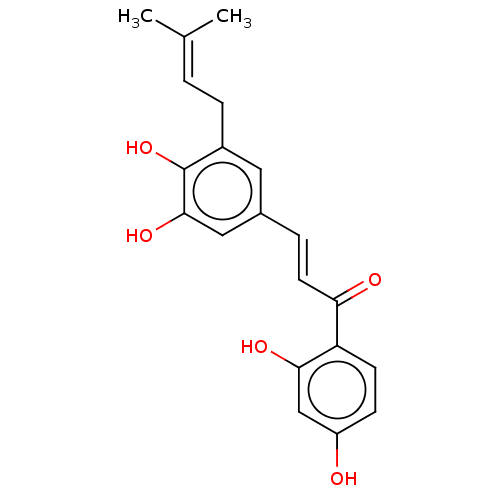 (5''-Prenylbutein | 5'-PRENYLBUTEIN) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50212400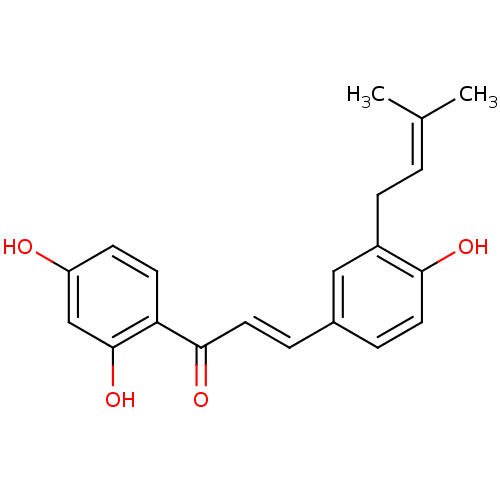 (1-(2,4-Dihydroxyphenyl)-3-[4-hydroxy-3-(3-methylbu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50370984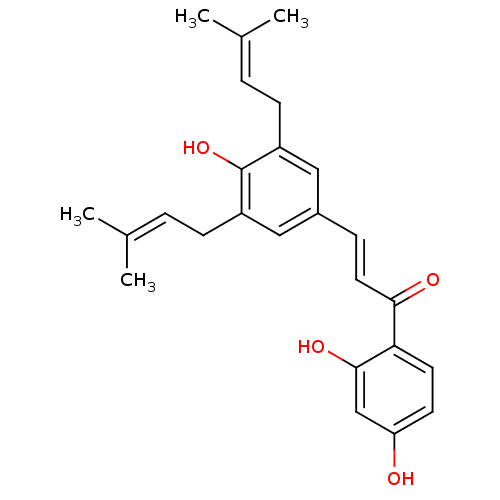 (Abyssinone Vi | CHEMBL508727) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Non-competitive inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate sub... | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H9N2 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of influenza A virus H1N1 Neuraminidase using 4-methylumbelliferyl-alpha-D-N-acetylneuraminic acid sodium salt hydrate substrate | Bioorg Med Chem Lett 20: 6430-4 (2010) Article DOI: 10.1016/j.bmcl.2010.09.077 BindingDB Entry DOI: 10.7270/Q2HQ42Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Vibrio cholerae) | BDBM50317435 (CHEMBL1096406 | Erythribyssin O) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Vibrio cholerae neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 30 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50311581 (CHEMBL1086764 | erysubin E) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317435 (CHEMBL1096406 | Erythribyssin O) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317436 (CHEMBL1096407 | calopocarpin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50311583 (Abyssinone II | CHEMBL508534 | phaseolidin | phase...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50311586 (CHEMBL1088462 | erystagallin A) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317434 (CHEMBL1097045 | eryvarin D) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317430 (CHEMBL454849 | Erythrabyssin I | cristacarpin | cr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317437 (CHEMBL1096408 | Erythribyssin L) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Vibrio cholerae) | BDBM50317434 (CHEMBL1097045 | eryvarin D) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Vibrio cholerae neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 30 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB) Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | Bioorg Med Chem 16: 10356-62 (2008) Article DOI: 10.1016/j.bmc.2008.10.012 BindingDB Entry DOI: 10.7270/Q251404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | J Nat Prod 70: 1039-42 (2007) Article DOI: 10.1021/np060477+ BindingDB Entry DOI: 10.7270/Q2KH0P5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitorphenol production after 30 mins | Bioorg Med Chem 19: 3378-83 (2011) Article DOI: 10.1016/j.bmc.2011.04.037 BindingDB Entry DOI: 10.7270/Q2DF6RKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311577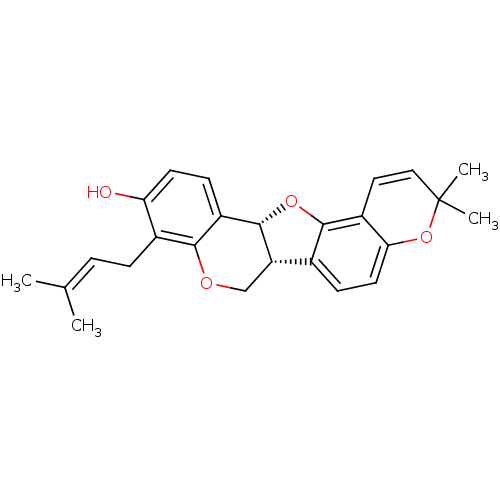 (CHEMBL561967 | Erybreadin B | erybraedin B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50104694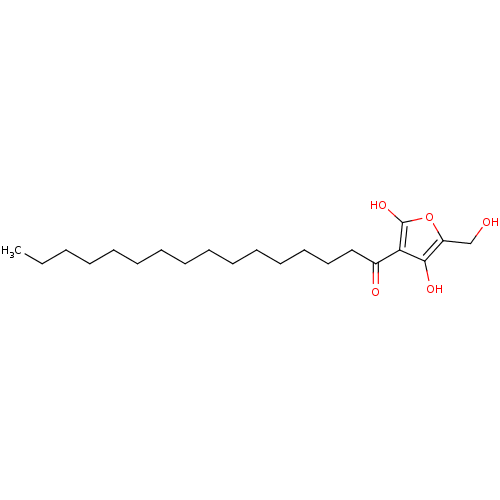 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50104694 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | J Nat Prod 70: 1039-42 (2007) Article DOI: 10.1021/np060477+ BindingDB Entry DOI: 10.7270/Q2KH0P5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50394207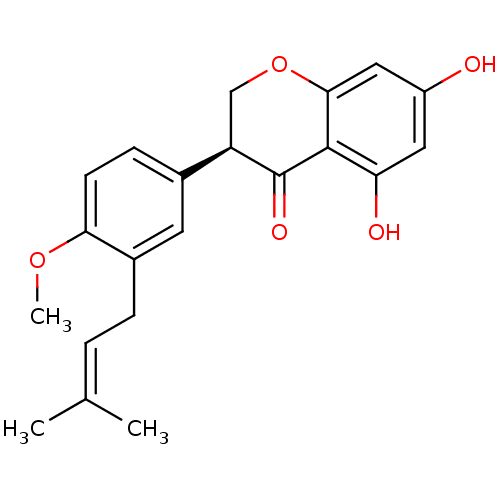 (CHEMBL2159047) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50104694 ((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB) Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | Bioorg Med Chem 16: 10356-62 (2008) Article DOI: 10.1016/j.bmc.2008.10.012 BindingDB Entry DOI: 10.7270/Q251404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Clostridium perfringens) | BDBM50317431 (CHEMBL1098413 | demethylmedicarpin) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Clostridium perfringens neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 10 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311580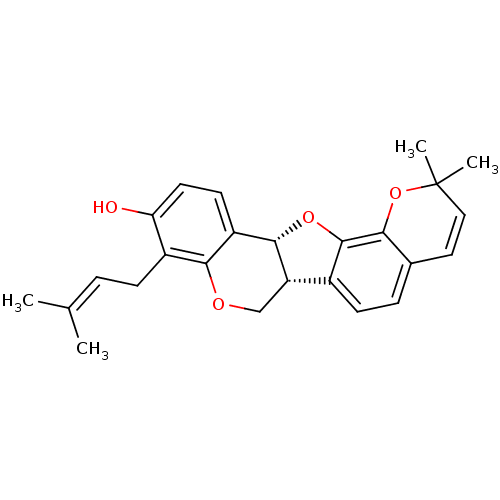 (CHEMBL1087148 | Erybreadin D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311582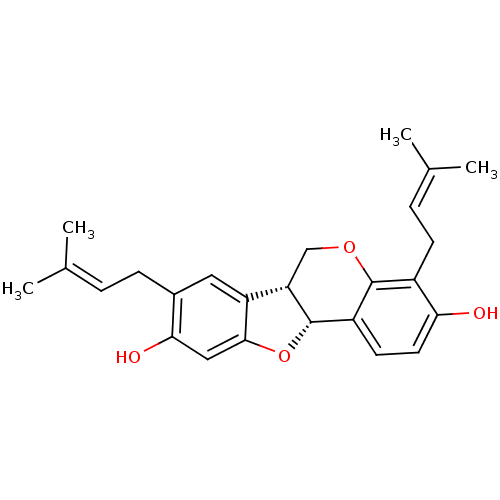 (CHEMBL1086765 | Erybreadin C) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Vibrio cholerae) | BDBM50317436 (CHEMBL1096407 | calopocarpin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Vibrio cholerae neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 30 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50317432 (CHEMBL1098728 | NEORAUTENOL) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50394204 (CHEMBL2159045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311579 (CHEMBL551155 | folitenol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50311581 (CHEMBL1086764 | erysubin E) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B assessed as p-nitrophenol production | Bioorg Med Chem Lett 19: 6745-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.108 BindingDB Entry DOI: 10.7270/Q24B328V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase (Vibrio cholerae) | BDBM50311583 (Abyssinone II | CHEMBL508534 | phaseolidin | phase...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Vibrio cholerae neuraminidase assessed as inhibition of 4-MU-NANA hydrolysis after 30 mins by spectrofluorometry | Bioorg Med Chem 18: 3335-44 (2010) Article DOI: 10.1016/j.bmc.2010.03.005 BindingDB Entry DOI: 10.7270/Q2MW2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50394206 (CHEMBL2159046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-nitrophenyl phosphate as substrate after 30 mins | Bioorg Med Chem 20: 6459-64 (2012) Article DOI: 10.1016/j.bmc.2012.08.024 BindingDB Entry DOI: 10.7270/Q29C6ZHS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50274831 ((S)-5,7,8'-trihydroxy-2',2'-dimethyl-5'-(3-methylb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology (KRIBB) Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B | Bioorg Med Chem 16: 10356-62 (2008) Article DOI: 10.1016/j.bmc.2008.10.012 BindingDB Entry DOI: 10.7270/Q251404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 139 total ) | Next | Last >> |