

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095778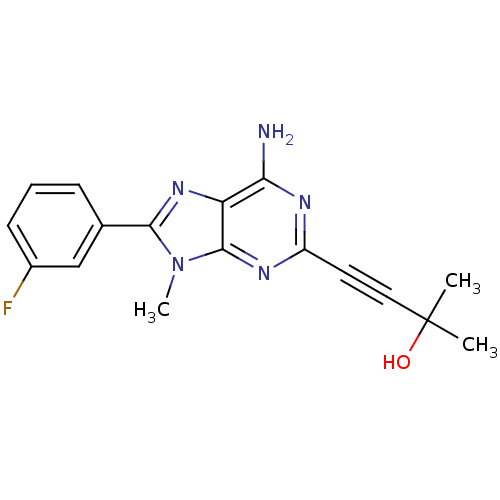 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095790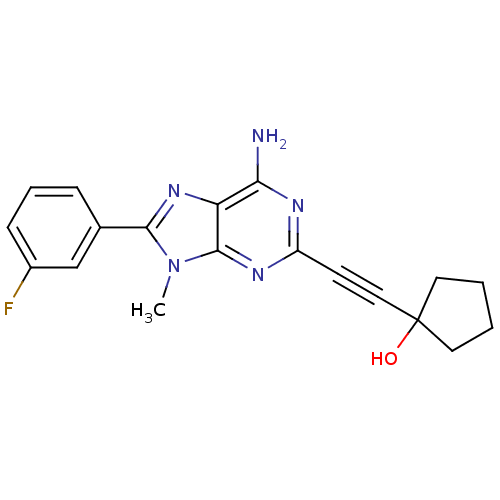 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095786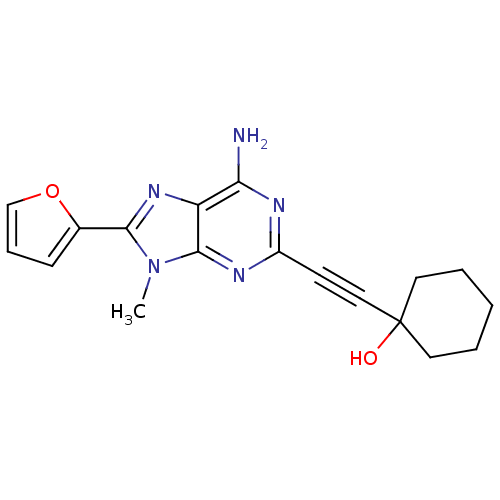 (1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095787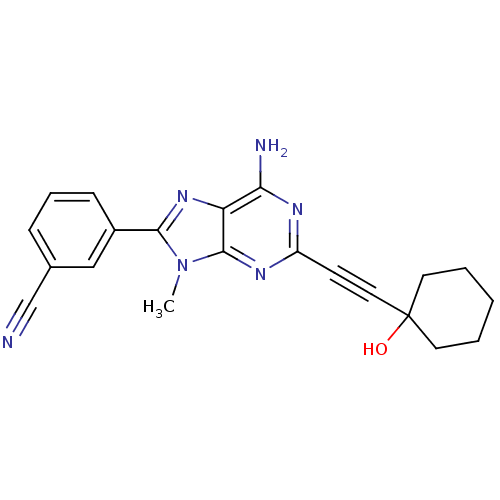 (3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095784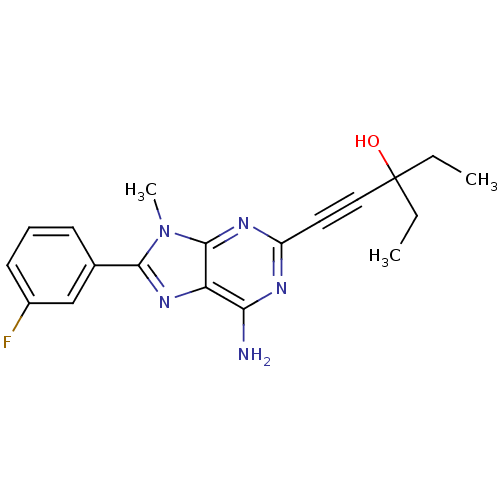 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059376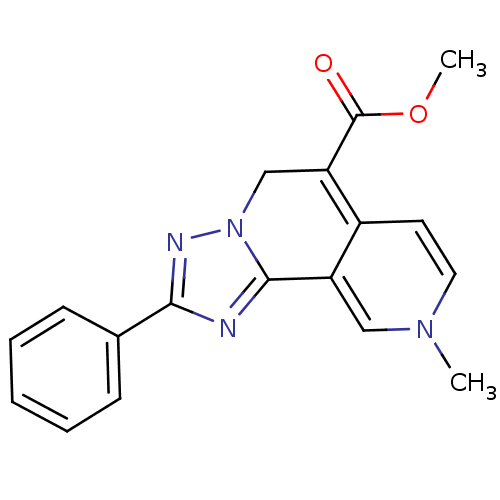 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095793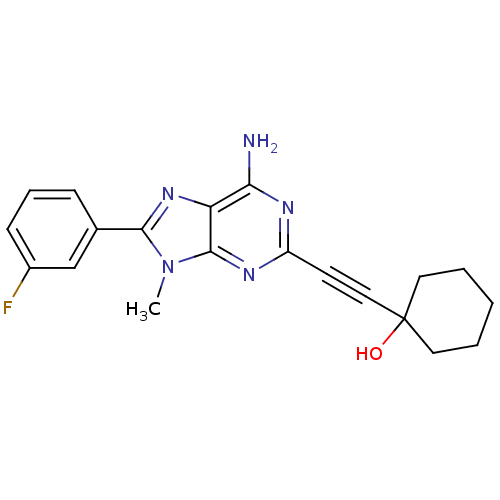 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095788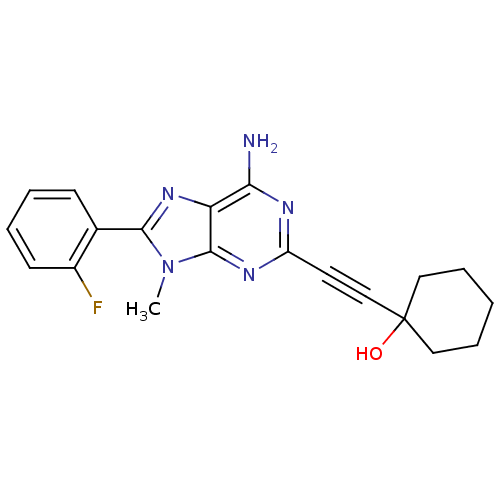 (1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095793 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50079652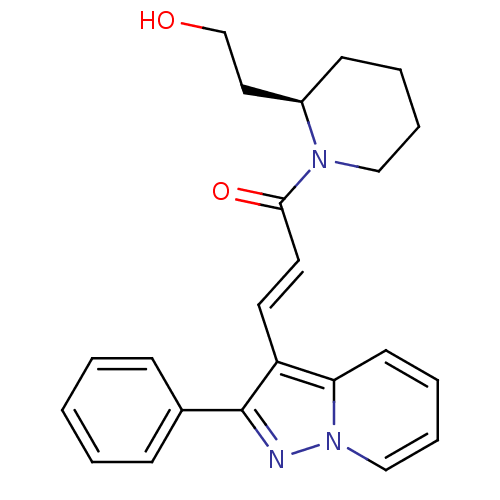 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50095781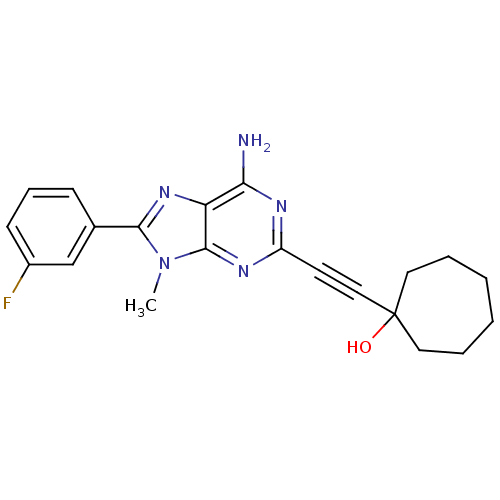 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095788 (1-[6-Amino-8-(2-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095790 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095786 (1-(6-Amino-8-furan-2-yl-9-methyl-9H-purin-2-ylethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095781 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095784 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095778 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50006710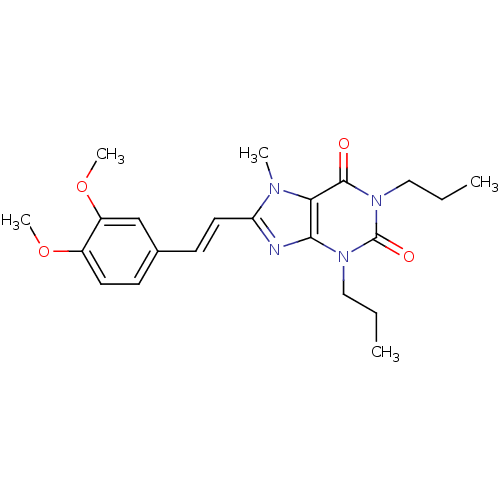 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50095787 (3-[6-Amino-2-(1-hydroxy-cyclohexylethynyl)-9-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095784 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095793 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095790 (1-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50095778 (4-[6-Amino-8-(3-fluoro-phenyl)-9-methyl-9H-purin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50006710 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50059376 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50059376 (9-Methyl-2-phenyl-5,9-dihydro-[1,2,4]triazolo[5,1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680 | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50006710 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPA | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297172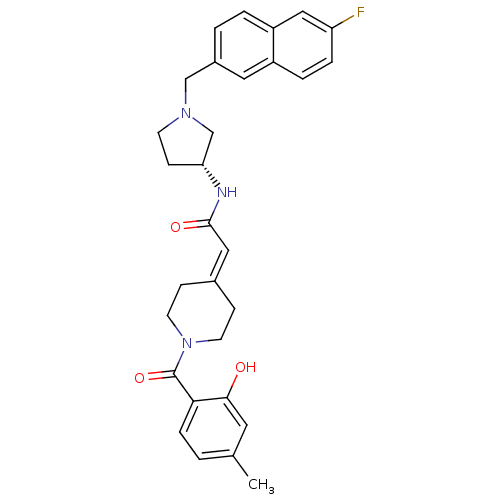 (CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171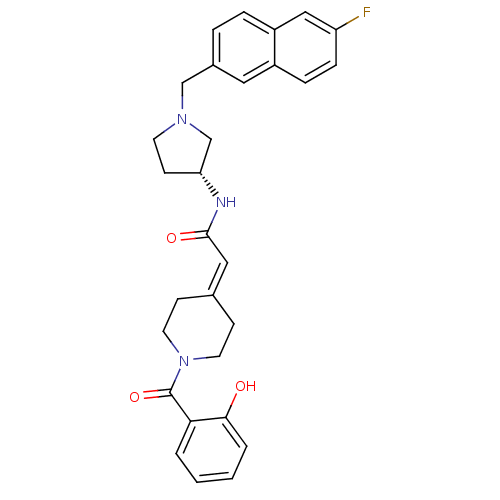 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50006710 (8-[(E)-2-(3,4-Dimethoxy-phenyl)-vinyl]-7-methyl-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against cyclic AMP production in rat Adenosine A2A receptor assay | J Med Chem 44: 170-9 (2001) BindingDB Entry DOI: 10.7270/Q2R49Q0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297181 (2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297183 (CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297177 (CHEMBL561535 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297182 (2-[1-(3,4-Dimethoxybenzoyl)piperidin-4-ylidene]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297190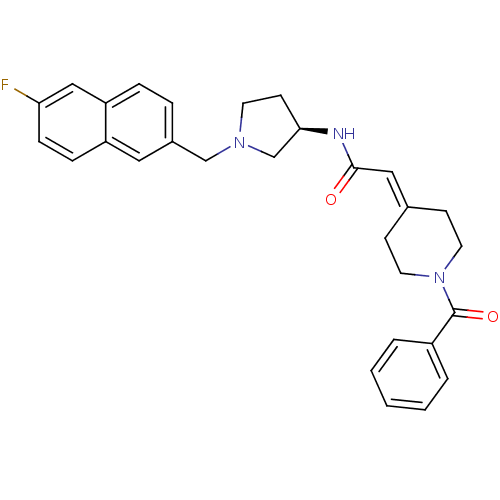 (2-(1-Benzoylpiperidin-4-ylidene)-N-{(3R)-1-[(6-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297188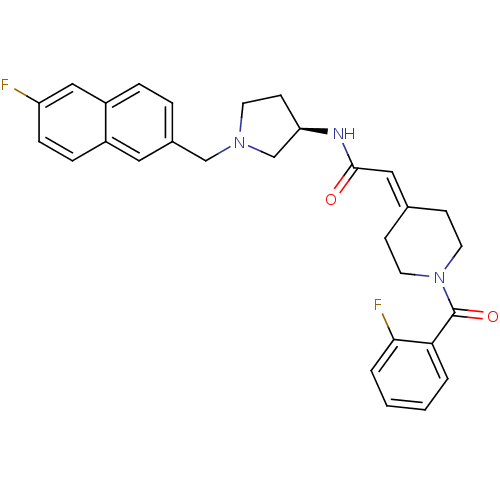 (2-[1-(2-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297180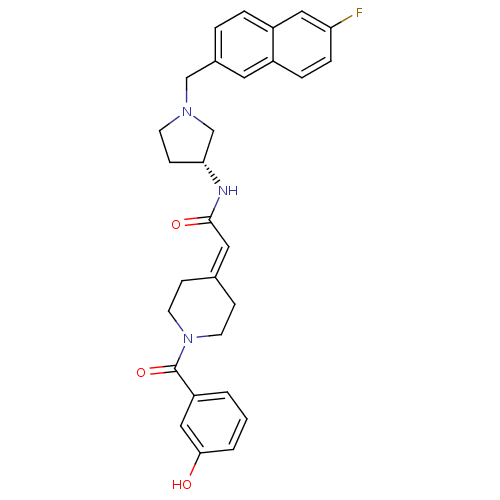 (CHEMBL556916 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297185 (CHEMBL556227 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297179 (CHEMBL562923 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297184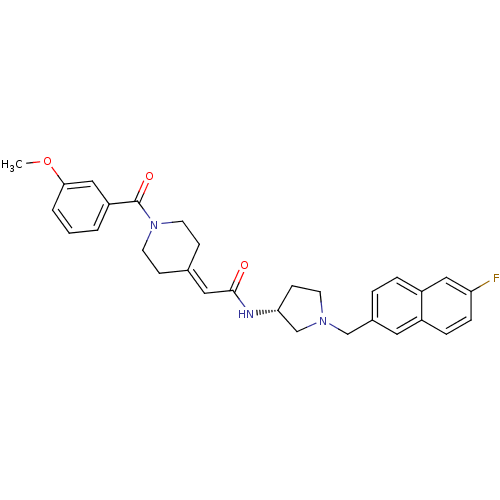 (CHEMBL557118 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297174 (2-[1-(4-Fluoro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297173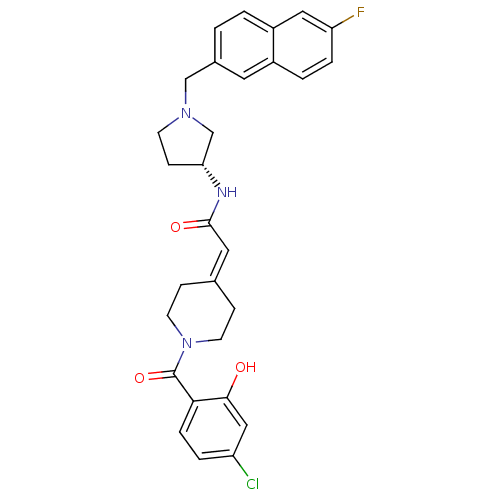 (2-[1-(4-Chloro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264210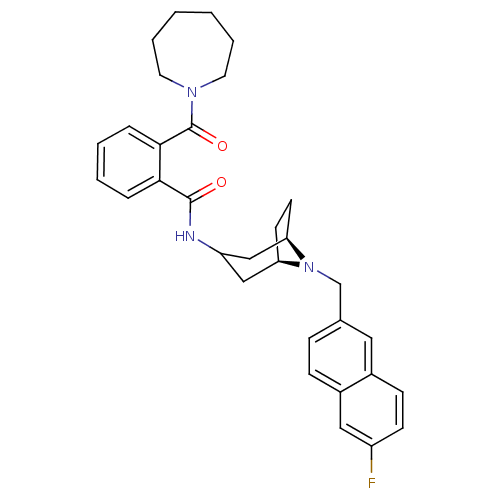 (2-(Azepan-1-ylcarbonyl)-N-{(3-exo)-8-[(6-fluoro-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297187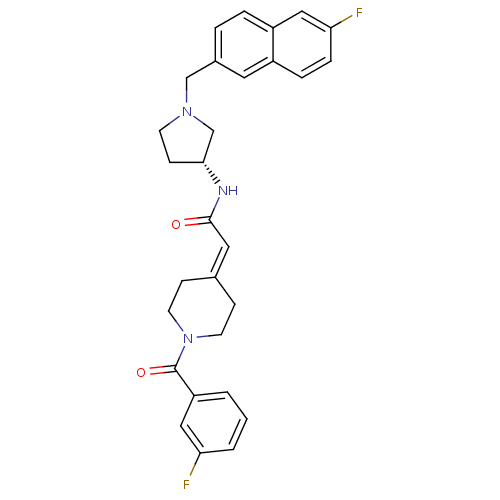 ((R)-2-(1-(3-fluorobenzoyl)piperidin-4-ylidene)-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264161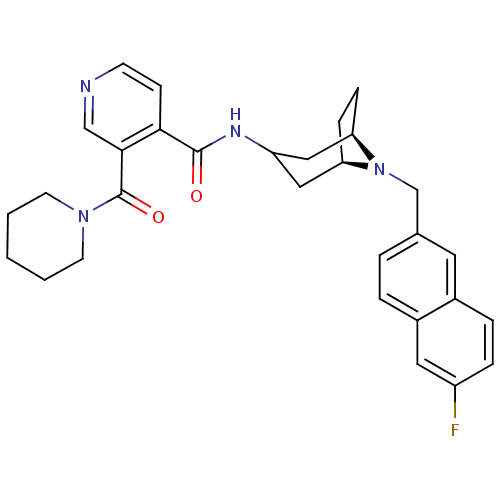 (CHEMBL488929 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry | Bioorg Med Chem 16: 8607-18 (2008) Article DOI: 10.1016/j.bmc.2008.08.006 BindingDB Entry DOI: 10.7270/Q21J9BPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50615151 (CHEMBL5276087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297176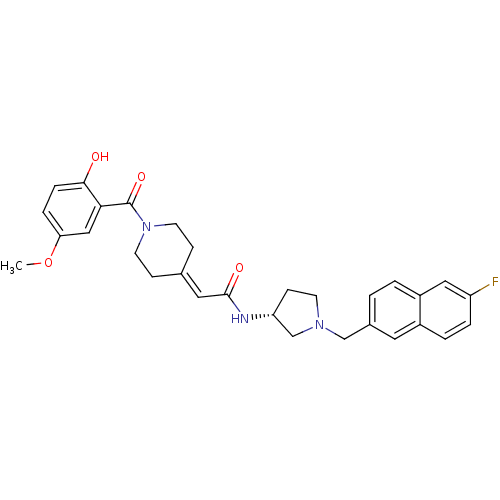 (CHEMBL562574 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |