 Found 391 hits with Last Name = 'tsao' and Initial = 'h'
Found 391 hits with Last Name = 'tsao' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A-interacting protein
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora C |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM25558

(4-arylamino-3-pyridinecarbonitrile, 4p | 5-(3,4-di...)Show SMILES COc1ccc(cc1OC)-c1cncc(C#N)c1Nc1ccc2[nH]ccc2c1 Show InChI InChI=1S/C22H18N4O2/c1-27-20-6-3-14(10-21(20)28-2)18-13-24-12-16(11-23)22(18)26-17-4-5-19-15(9-17)7-8-25-19/h3-10,12-13,25H,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | -40.1 | 70 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research
| Assay Description
All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... |
J Med Chem 51: 5958-63 (2008)
Article DOI: 10.1021/jm800214a
BindingDB Entry DOI: 10.7270/Q21V5C8K |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM25543
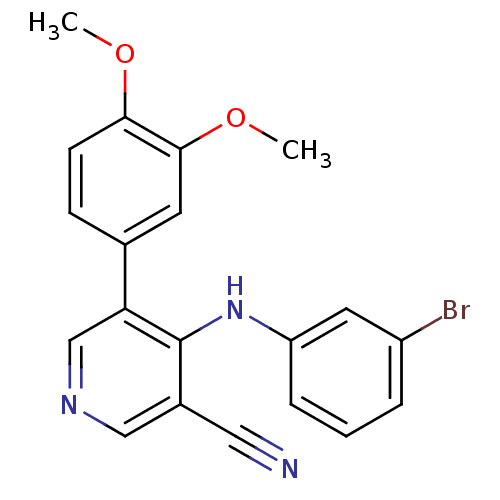
(4-[(3-bromophenyl)amino]-5-(3,4-dimethoxyphenyl)py...)Show InChI InChI=1S/C20H16BrN3O2/c1-25-18-7-6-13(8-19(18)26-2)17-12-23-11-14(10-22)20(17)24-16-5-3-4-15(21)9-16/h3-9,11-12H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90E+3 | -30.0 | 4.60E+3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research
| Assay Description
All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... |
J Med Chem 51: 5958-63 (2008)
Article DOI: 10.1021/jm800214a
BindingDB Entry DOI: 10.7270/Q21V5C8K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Fes/Fps
(Homo sapiens (Human)) | BDBM50242740
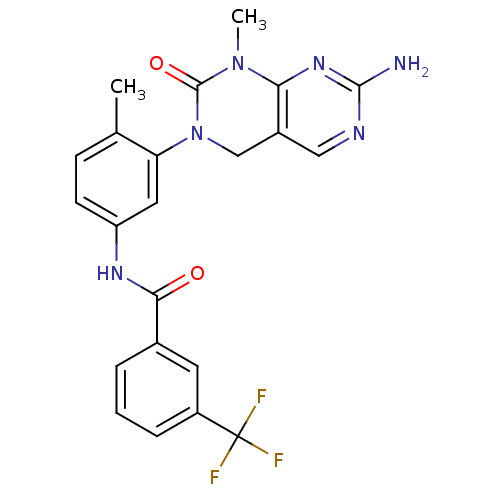
(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Fes |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Bmx |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM482158
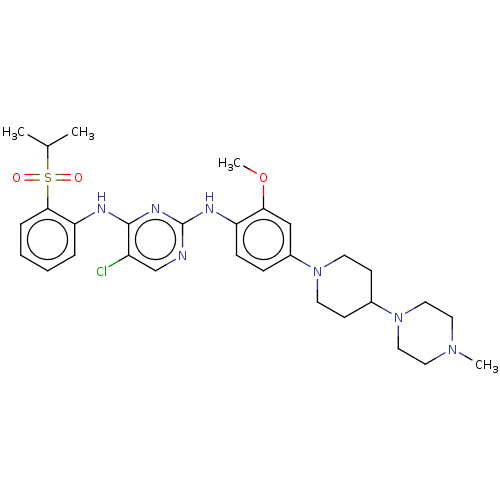
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50378568
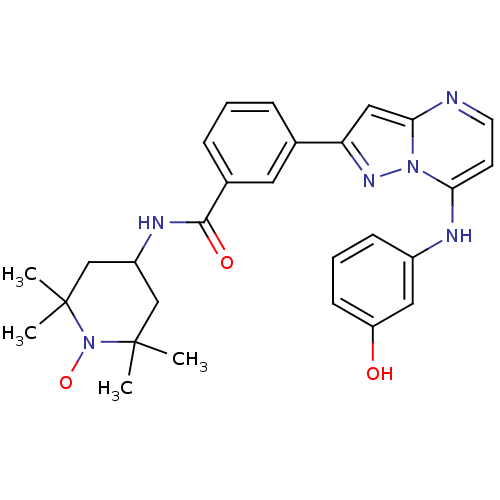
(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Yes |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Lck by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Abl 1 |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Fyn |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of src kinase |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201921
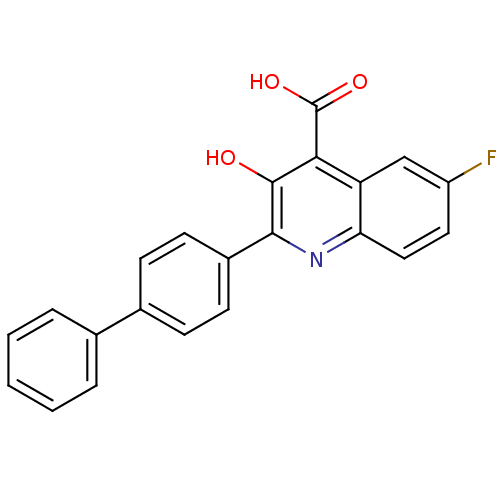
(2-Biphenyl-4-yl-6-fluoro-3-hydroxy-quinoline-4-car...)Show SMILES OC(=O)c1c(O)c(nc2ccc(F)cc12)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H14FNO3/c23-16-10-11-18-17(12-16)19(22(26)27)21(25)20(24-18)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-12,25H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201923

(3-hydroxy-2-(4'-hydroxy-biphenyl-4-yl)-quinoline-4...)Show SMILES OC(=O)c1c(O)c(nc2ccccc12)-c1ccc(cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H15NO4/c24-16-11-9-14(10-12-16)13-5-7-15(8-6-13)20-21(25)19(22(26)27)17-3-1-2-4-18(17)23-20/h1-12,24-25H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201945

(6-fluoro-3-hydroxy-2-(4-phenoxyphenyl)quinoline-4-...)Show SMILES OC(=O)c1c(O)c(nc2ccc(F)cc12)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H14FNO4/c23-14-8-11-18-17(12-14)19(22(26)27)21(25)20(24-18)13-6-9-16(10-7-13)28-15-4-2-1-3-5-15/h1-12,25H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201905
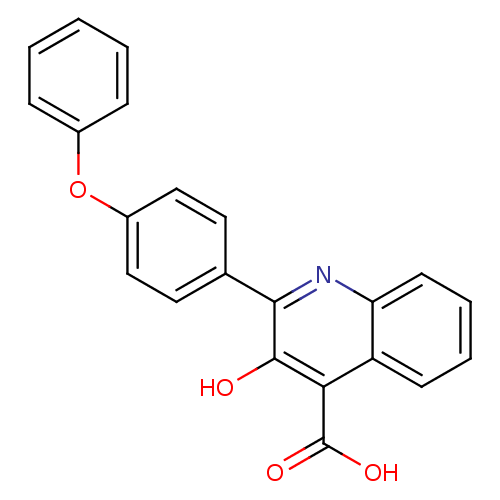
(3-hydroxy-2-(4-phenoxyphenyl)quinoline-4-carboxyli...)Show SMILES OC(=O)c1c(O)c(nc2ccccc12)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C22H15NO4/c24-21-19(22(25)26)17-8-4-5-9-18(17)23-20(21)14-10-12-16(13-11-14)27-15-6-2-1-3-7-15/h1-13,24H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201919
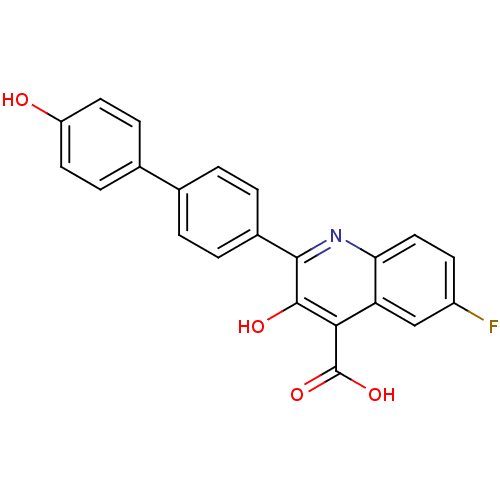
(6-fluoro-3-hydroxy-2-(4'-hydroxy-biphenyl-4-yl)-qu...)Show SMILES OC(=O)c1c(O)c(nc2ccc(F)cc12)-c1ccc(cc1)-c1ccc(O)cc1 Show InChI InChI=1S/C22H14FNO4/c23-15-7-10-18-17(11-15)19(22(27)28)21(26)20(24-18)14-3-1-12(2-4-14)13-5-8-16(25)9-6-13/h1-11,25-26H,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201925
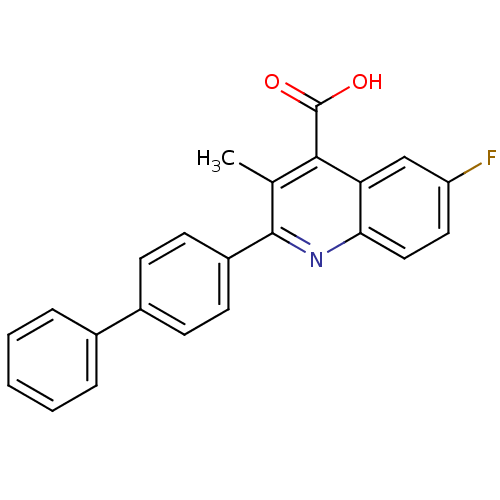
(2-BIPHENYL-4-YL-6-FLUORO-3-METHYL-QUINOLINE-4-CARB...)Show SMILES Cc1c(nc2ccc(F)cc2c1C(O)=O)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C23H16FNO2/c1-14-21(23(26)27)19-13-18(24)11-12-20(19)25-22(14)17-9-7-16(8-10-17)15-5-3-2-4-6-15/h2-13H,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Met by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50242737

((R,Z)-5-(2,6-dichlorobenzylsulfonyl)-3-((3,5-dimet...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(cc23)S(=O)(=O)Cc2c(Cl)cccc2Cl)c(C)c1C(=O)N1CCC[C@@H]1CN1CCCC1 |r| Show InChI InChI=1S/C32H34Cl2N4O4S/c1-19-29(35-20(2)30(19)32(40)38-14-6-7-21(38)17-37-12-3-4-13-37)16-24-23-15-22(10-11-28(23)36-31(24)39)43(41,42)18-25-26(33)8-5-9-27(25)34/h5,8-11,15-16,21,35H,3-4,6-7,12-14,17-18H2,1-2H3,(H,36,39)/b24-16-/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Met by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201949

(2-biphenyl-4-yl-3-hydroxy-quinoline-4-carboxylic a...)Show SMILES OC(=O)c1c(O)c(nc2ccccc12)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H15NO3/c24-21-19(22(25)26)17-8-4-5-9-18(17)23-20(21)16-12-10-15(11-13-16)14-6-2-1-3-7-14/h1-13,24H,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM482158

(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201918
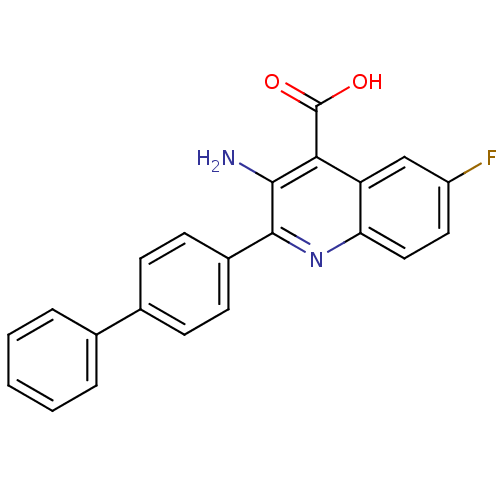
(3-amino-2-biphenyl-4-yl-6-fluoro-quinoline-4-carbo...)Show SMILES Nc1c(nc2ccc(F)cc2c1C(O)=O)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H15FN2O2/c23-16-10-11-18-17(12-16)19(22(26)27)20(24)21(25-18)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-12H,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of Hck |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201939
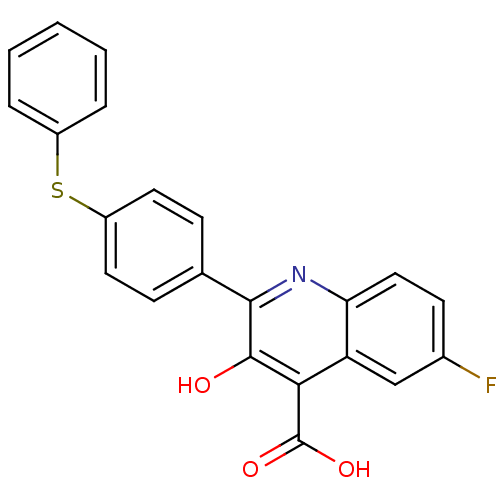
(6-fluoro-3-hydroxy-2-(4-(phenylthio)phenyl)quinoli...)Show SMILES OC(=O)c1c(O)c(nc2ccc(F)cc12)-c1ccc(Sc2ccccc2)cc1 Show InChI InChI=1S/C22H14FNO3S/c23-14-8-11-18-17(12-14)19(22(26)27)21(25)20(24-18)13-6-9-16(10-7-13)28-15-4-2-1-3-5-15/h1-12,25H,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM482158

(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of InsR |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of ALK by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 2
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of GCK |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of KDR by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Abl |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM6190
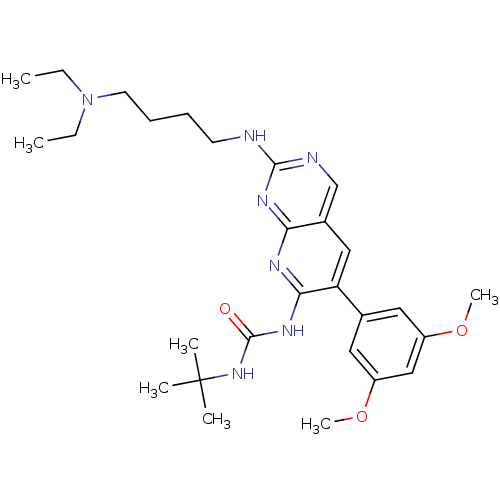
(3-tert-butyl-1-(2-{[4-(diethylamino)butyl]amino}-6...)Show SMILES CCN(CC)CCCCNc1ncc2cc(c(NC(=O)NC(C)(C)C)nc2n1)-c1cc(OC)cc(OC)c1 Show InChI InChI=1S/C28H41N7O3/c1-8-35(9-2)13-11-10-12-29-26-30-18-20-16-23(19-14-21(37-6)17-22(15-19)38-7)25(31-24(20)32-26)33-27(36)34-28(3,4)5/h14-18H,8-13H2,1-7H3,(H3,29,30,31,32,33,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of LCK by LANCE FRET assay |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50378568

(CHEMBL1163016)Show SMILES CC1(C)CC(CC(C)(C)N1[O])NC(=O)c1cccc(c1)-c1cc2nccc(Nc3cccc(O)c3)n2n1 |^1:10| Show InChI InChI=1S/C28H31N6O3/c1-27(2)16-21(17-28(3,4)34(27)37)31-26(36)19-8-5-7-18(13-19)23-15-25-29-12-11-24(33(25)32-23)30-20-9-6-10-22(35)14-20/h5-15,21,30,35H,16-17H2,1-4H3,(H,31,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha |
J Med Chem 53: 1238-49 (2010)
Article DOI: 10.1021/jm901525b
BindingDB Entry DOI: 10.7270/Q2QJ7J72 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50161957
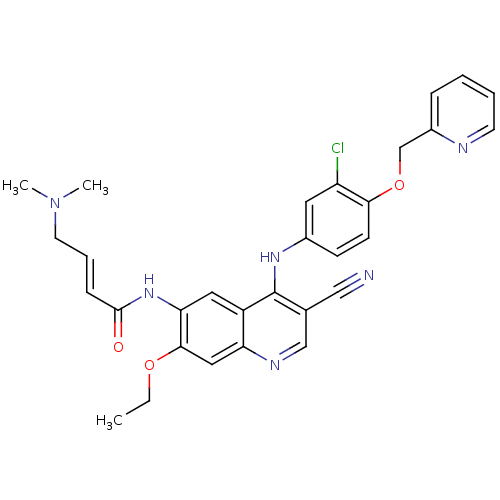
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of human Her2 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50242737

((R,Z)-5-(2,6-dichlorobenzylsulfonyl)-3-((3,5-dimet...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(cc23)S(=O)(=O)Cc2c(Cl)cccc2Cl)c(C)c1C(=O)N1CCC[C@@H]1CN1CCCC1 |r| Show InChI InChI=1S/C32H34Cl2N4O4S/c1-19-29(35-20(2)30(19)32(40)38-14-6-7-21(38)17-37-12-3-4-13-37)16-24-23-15-22(10-11-28(23)36-31(24)39)43(41,42)18-25-26(33)8-5-9-27(25)34/h5,8-11,15-16,21,35H,3-4,6-7,12-14,17-18H2,1-2H3,(H,36,39)/b24-16-/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Ron by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM25553
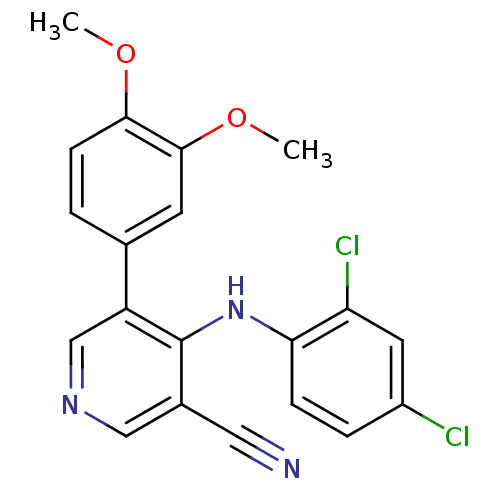
(4-[(2,4-dichlorophenyl)amino]-5-(3,4-dimethoxyphen...)Show SMILES COc1ccc(cc1OC)-c1cncc(C#N)c1Nc1ccc(Cl)cc1Cl Show InChI InChI=1S/C20H15Cl2N3O2/c1-26-18-6-3-12(7-19(18)27-2)15-11-24-10-13(9-23)20(15)25-17-5-4-14(21)8-16(17)22/h3-8,10-11H,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research
| Assay Description
All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... |
J Med Chem 51: 5958-63 (2008)
Article DOI: 10.1021/jm800214a
BindingDB Entry DOI: 10.7270/Q21V5C8K |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM25554
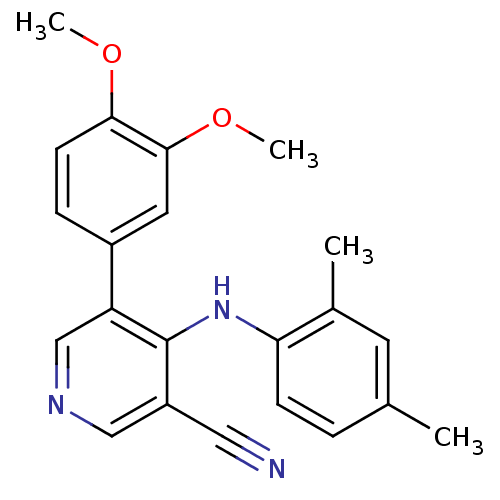
(4-arylamino-3-pyridinecarbonitrile, 4l | 5-(3,4-di...)Show InChI InChI=1S/C22H21N3O2/c1-14-5-7-19(15(2)9-14)25-22-17(11-23)12-24-13-18(22)16-6-8-20(26-3)21(10-16)27-4/h5-10,12-13H,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Wyeth Research
| Assay Description
All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl... |
J Med Chem 51: 5958-63 (2008)
Article DOI: 10.1021/jm800214a
BindingDB Entry DOI: 10.7270/Q21V5C8K |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of B-raf |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50242737

((R,Z)-5-(2,6-dichlorobenzylsulfonyl)-3-((3,5-dimet...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(cc23)S(=O)(=O)Cc2c(Cl)cccc2Cl)c(C)c1C(=O)N1CCC[C@@H]1CN1CCCC1 |r| Show InChI InChI=1S/C32H34Cl2N4O4S/c1-19-29(35-20(2)30(19)32(40)38-14-6-7-21(38)17-37-12-3-4-13-37)16-24-23-15-22(10-11-28(23)36-31(24)39)43(41,42)18-25-26(33)8-5-9-27(25)34/h5,8-11,15-16,21,35H,3-4,6-7,12-14,17-18H2,1-2H3,(H,36,39)/b24-16-/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Flk1 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM12255

(AZD0530 | CHEMBL217092 | Compound 33 | N-(5-chloro...)Show SMILES CN1CCN(CCOc2cc(OC3CCOCC3)c3c(Nc4c5OCOc5ccc4Cl)ncnc3c2)CC1 Show InChI InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit by cellular assay |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Homo sapiens (Human)) | BDBM50378795
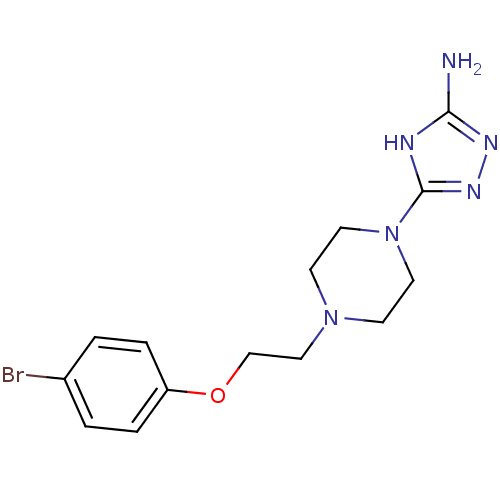
(CHEMBL1215473)Show InChI InChI=1S/C14H19BrN6O/c15-11-1-3-12(4-2-11)22-10-9-20-5-7-21(8-6-20)14-17-13(16)18-19-14/h1-4H,5-10H2,(H3,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of acidic mammalian chitinase after 60 mins |
J Med Chem 53: 6122-8 (2010)
Article DOI: 10.1021/jm100533p
BindingDB Entry DOI: 10.7270/Q22N538H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50201900

(6-bromo-2-(4-chlorophenyl)-3-hydroxyquinoline-4-ca...)Show InChI InChI=1S/C16H9BrClNO3/c17-9-3-6-12-11(7-9)13(16(21)22)15(20)14(19-12)8-1-4-10(18)5-2-8/h1-7,20H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DHOD expressed in Escherichia coli |
J Med Chem 50: 21-39 (2007)
Article DOI: 10.1021/jm0602256
BindingDB Entry DOI: 10.7270/Q2Z60PVV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data