

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at dopamine D1 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at dopamine D5 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50004822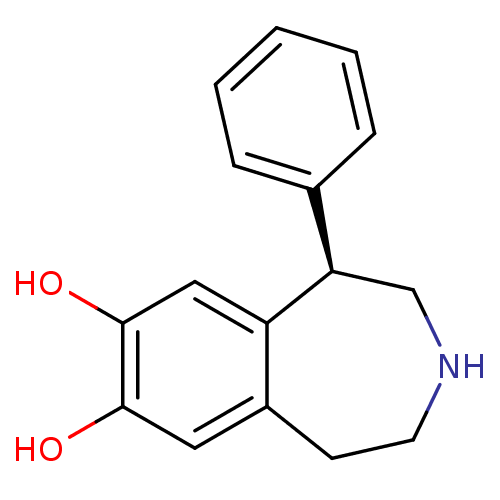 ((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Agonist activity at dopamine D5 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human 5HT1A receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50487259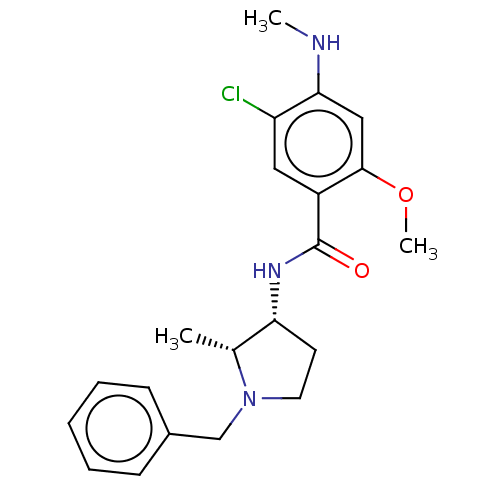 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-N-Methylspiperone from human dopamine D4 receptor expressed in stable HEK cells incubated for 90 mins by microbeta counting meth... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004822 ((R)-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]azepine...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Agonist activity at dopamine D1 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50087033 ((1R,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from human sigma1 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50487259 (CHEBI:64219 | [3H]NEMONAPRIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-N-Methylspiperone from dopamine D3 receptor (unknown origin) incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210757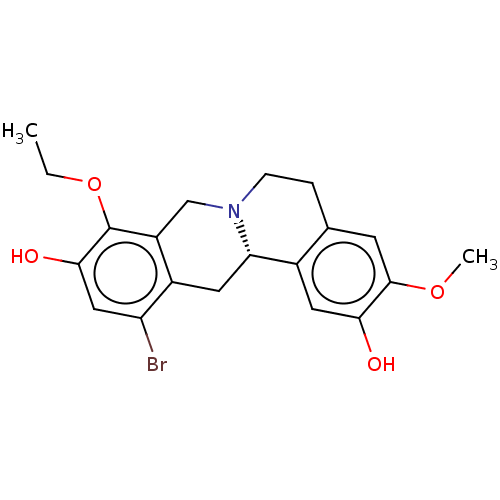 (CHEMBL3921278) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human D1 receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human D2 receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210764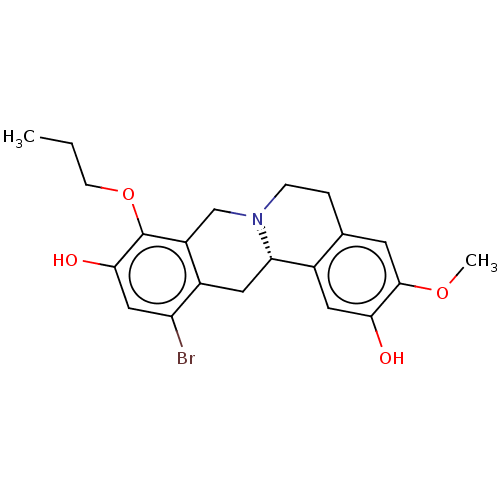 (CHEMBL3976659) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50010301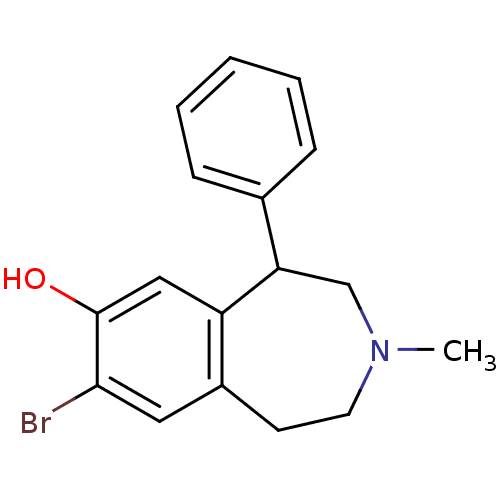 (8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human D5 receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210759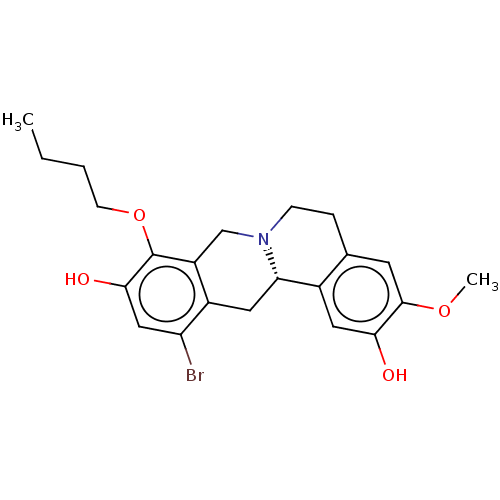 (CHEMBL3905947) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH2390 from human dopamine D1 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175545 (CHEMBL197685 | N-Hydroxycarbamoylmethyl-4-trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Binding affinity to human 5HT2A receptor | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127053 BindingDB Entry DOI: 10.7270/Q2H135KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175544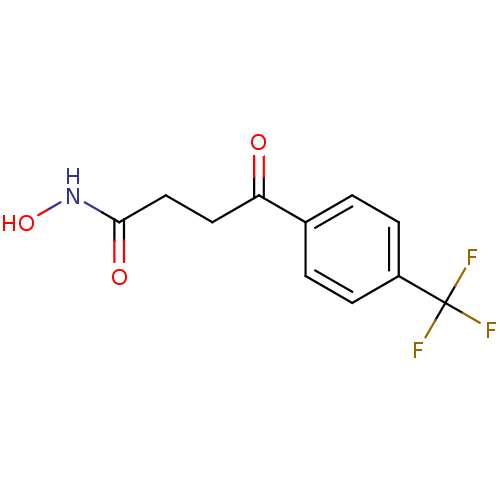 (CHEMBL198664 | N-Hydroxy-4-oxo-4-(4-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175531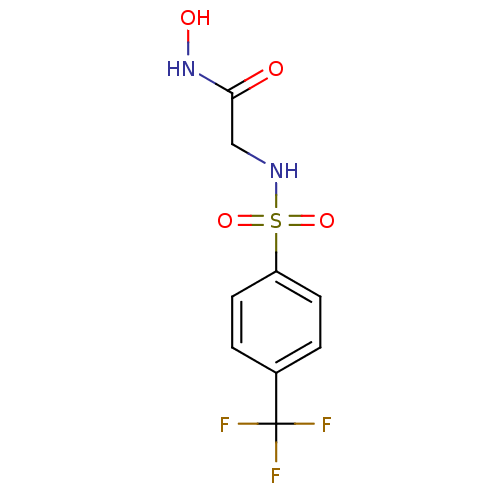 (CHEMBL199156 | N-Hydroxy-2-(4-trifluoromethyl-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50146629 (CHEMBL330040 | N-Hydroxy-2-[(4-nitro-benzyl)-(4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175561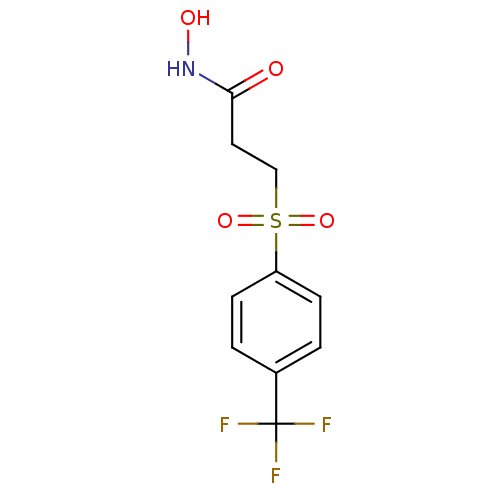 (CHEMBL198988 | N-Hydroxy-3-(4-trifluoromethyl-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-Ketanserin from 5-HT2A receptor (unknown origin) incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098549 ((6aR,aR)-3-Methyl-2-(6-methyl-5,6,6a,7-tetrahydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6 receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50010301 (8-Bromo-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D5 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210767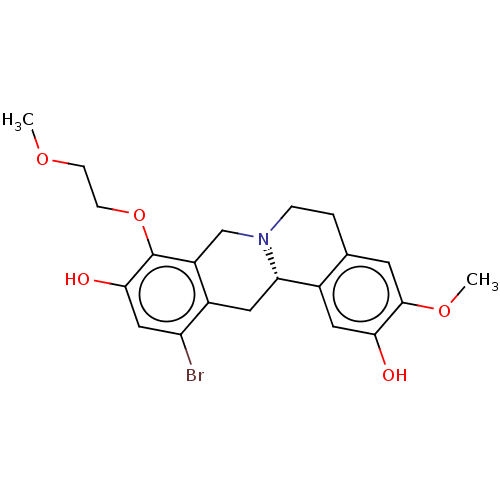 (CHEMBL3945691) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in HEKT cells incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50544133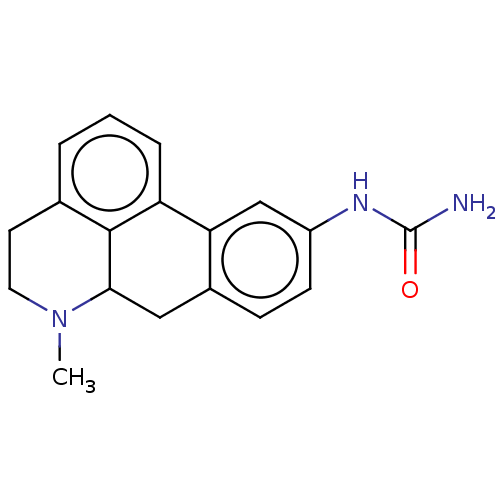 (CHEMBL4636148) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50088118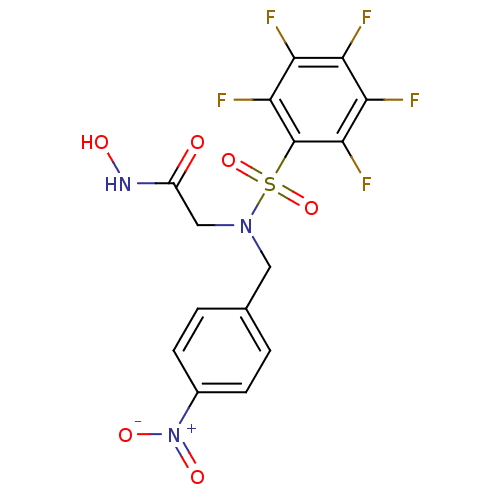 (2-(N-(4-nitrobenzyl)-2,3,4,5,6-pentafluorophenylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210761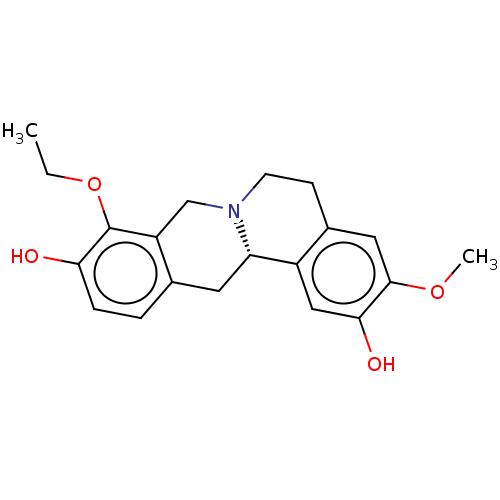 (CHEMBL3961827) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-N-Methylspiperone from human dopamine D2 receptor expressed in stable fibroblast cells incubated for 90 mins by microbeta counti... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50152837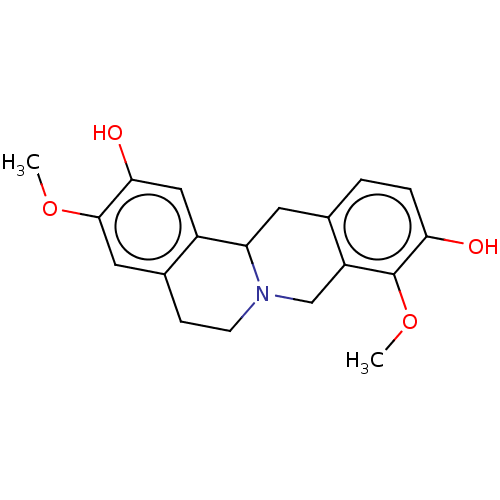 (CHEMBL595489) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH2390 from human dopamine D1 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50378584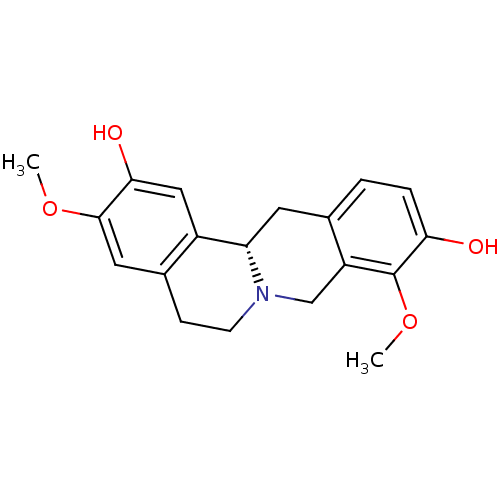 (STEPHOLIDINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50544139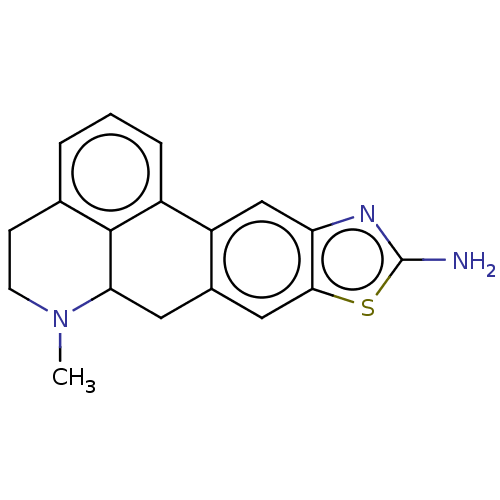 (CHEMBL4633786) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50152842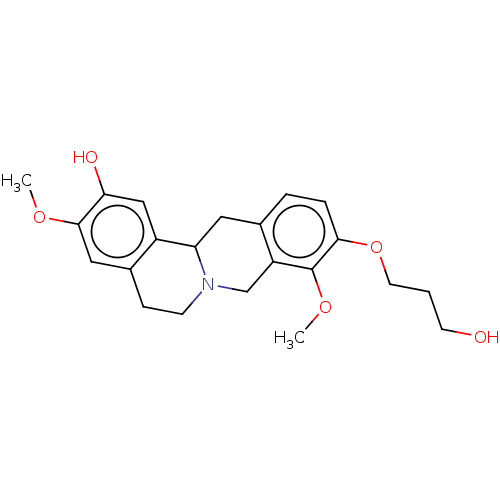 (CHEMBL3780268) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH2390 from human dopamine D1 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50544140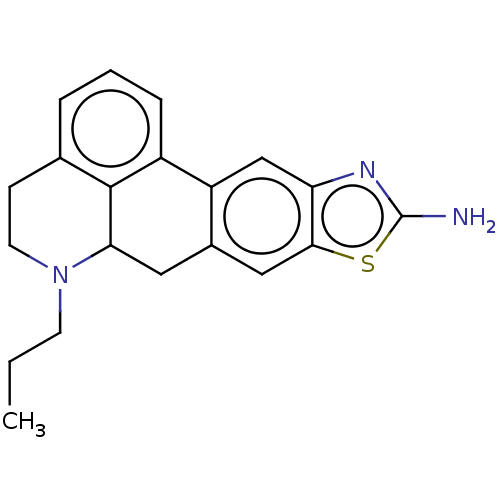 (CHEMBL4633042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-WAY100635 from human 5-HT1A receptor expresssed in stable CHO cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210754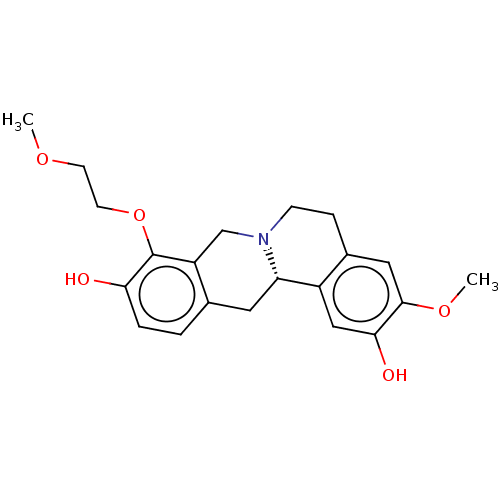 (CHEMBL3982215) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50152846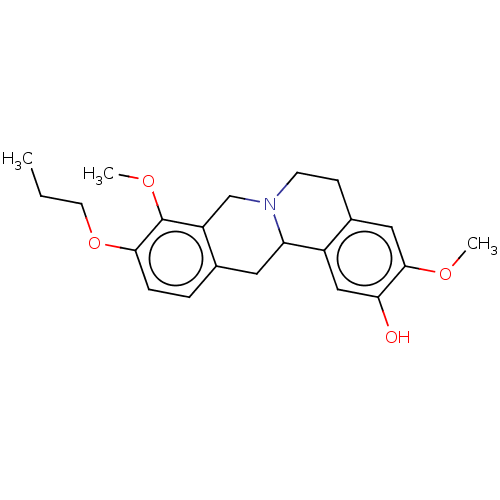 (CHEMBL3780506) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH2390 from human dopamine D1 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175529 (CHEMBL383560 | N-Hydroxy-3-[4-(pyridin-4-yloxy)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175550 (CHEMBL198377 | N-Hydroxy-4-oxo-4-[4-(pyridin-4-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175557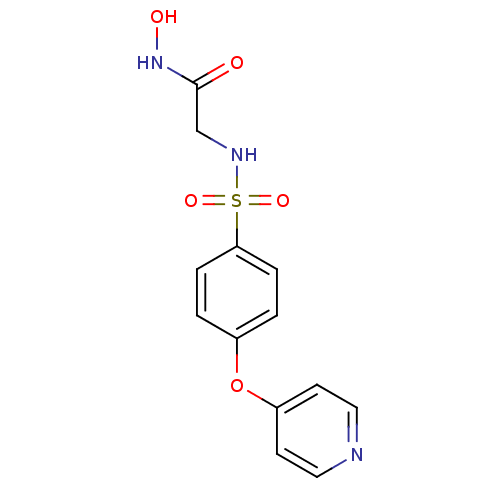 (CHEMBL194570 | N-Hydroxy-2-[4-(pyridin-4-yloxy)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50175541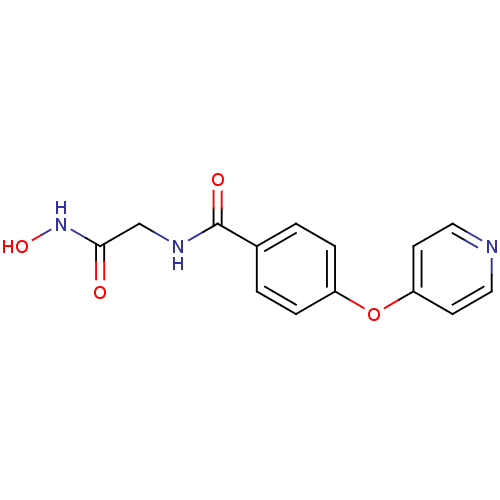 (CHEMBL438261 | N-Hydroxycarbamoylmethyl-4-(pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards (MMP-1) matrix metalloproteinase-1 | J Med Chem 48: 6585-96 (2005) Article DOI: 10.1021/jm050196j BindingDB Entry DOI: 10.7270/Q20K284B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50082556 ((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
De Novo Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-1 | J Med Chem 47: 2761-7 (2004) Article DOI: 10.1021/jm031061l BindingDB Entry DOI: 10.7270/Q2ZG6T0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D2 receptor by PDSP assay | Bioorg Med Chem 24: 2060-71 (2016) Article DOI: 10.1016/j.bmc.2016.03.037 BindingDB Entry DOI: 10.7270/Q2SX6G3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50210763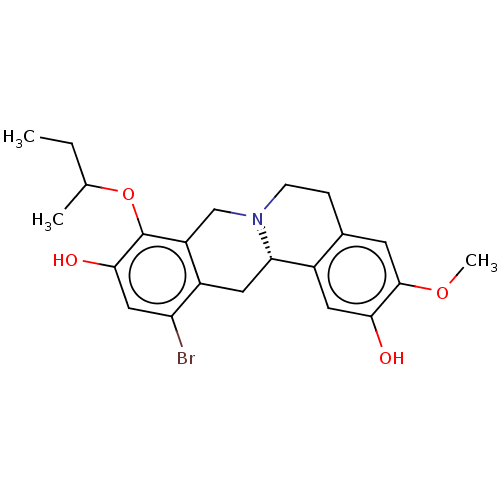 (CHEMBL3894329) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human D1 receptor expressed in HEKT cell membranes after 90 mins by microbeta scintillation counting method | Eur J Med Chem 125: 255-268 (2017) Article DOI: 10.1016/j.ejmech.2016.09.036 BindingDB Entry DOI: 10.7270/Q2KW5J1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50544137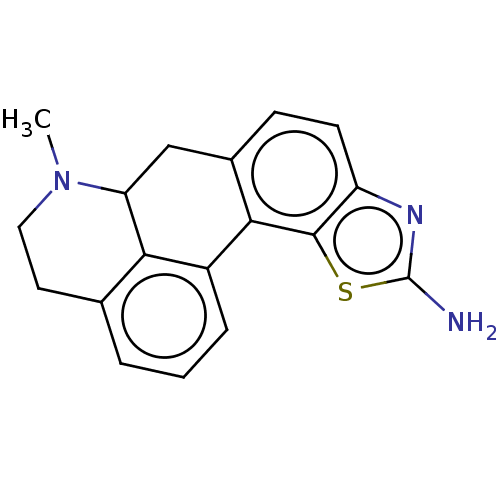 (CHEMBL4641502) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT7A receptor expresssed in stable HEK cell membrane incubated for 90 mins by microbeta counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115578 BindingDB Entry DOI: 10.7270/Q22B92KJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 371 total ) | Next | Last >> |