 Found 1998 hits with Last Name = 'bruce' and Initial = 'i'
Found 1998 hits with Last Name = 'bruce' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
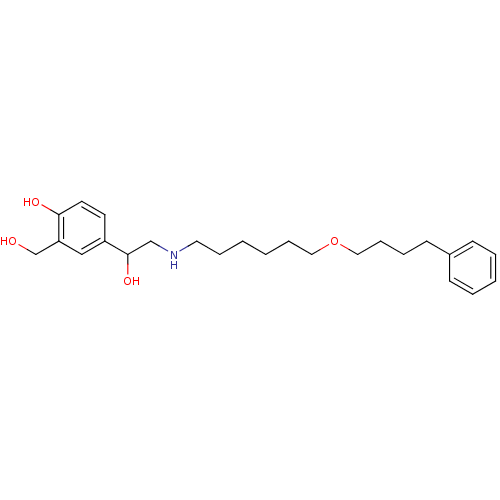
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329
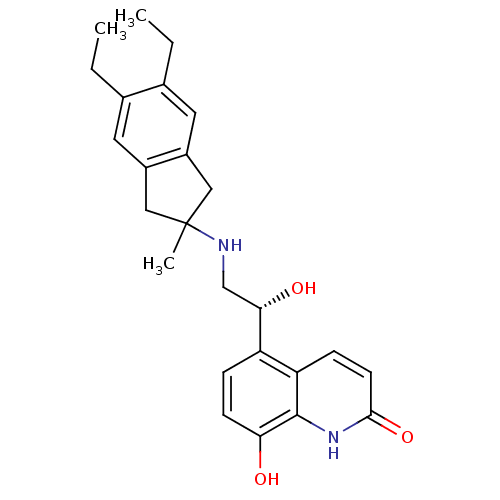
(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329

(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta1-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
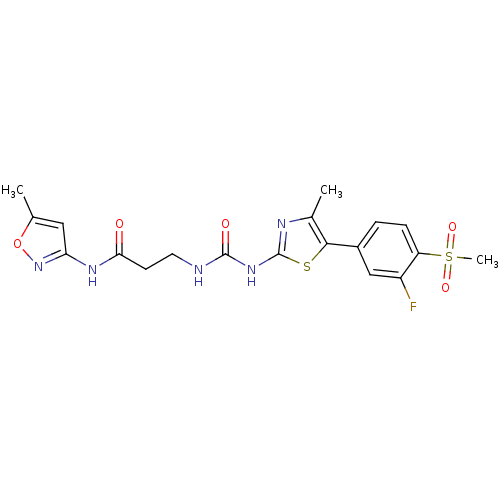
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318156
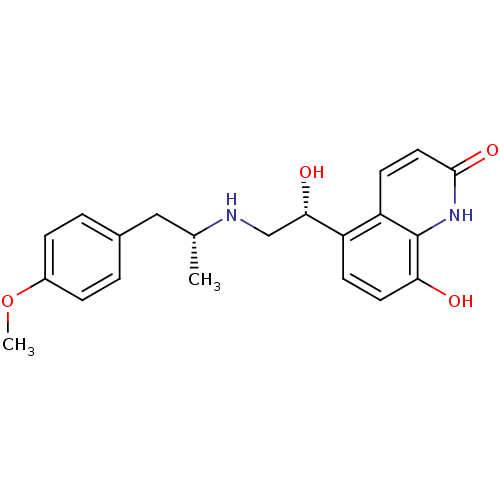
(CHEMBL1094785 | carmoterol)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)cc1 |r| Show InChI InChI=1S/C21H24N2O4/c1-13(11-14-3-5-15(27-2)6-4-14)22-12-19(25)16-7-9-18(24)21-17(16)8-10-20(26)23-21/h3-10,13,19,22,24-25H,11-12H2,1-2H3,(H,23,26)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151724
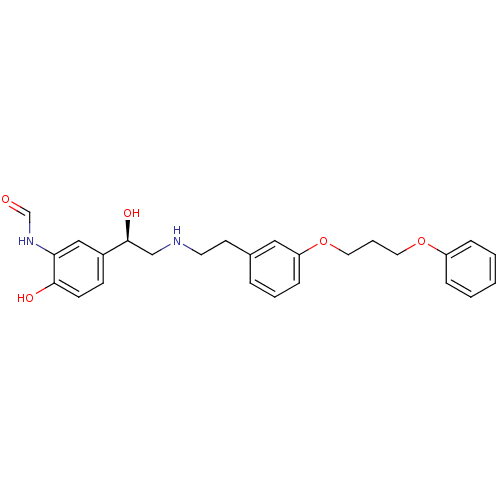
(CHEMBL183948 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[3-(...)Show SMILES O[C@@H](CNCCc1cccc(OCCCOc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O5/c29-19-28-24-17-21(10-11-25(24)30)26(31)18-27-13-12-20-6-4-9-23(16-20)33-15-5-14-32-22-7-2-1-3-8-22/h1-4,6-11,16-17,19,26-27,30-31H,5,12-15,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50128690
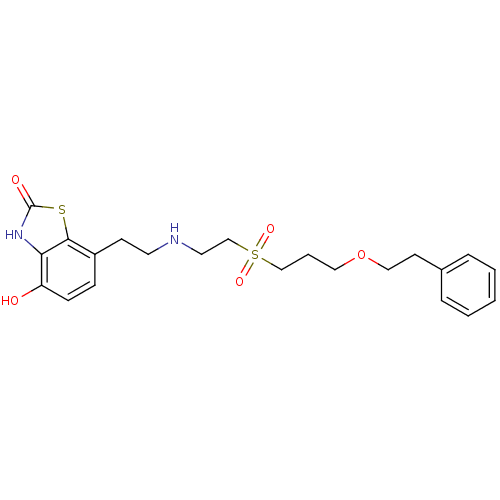
(4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfon...)Show SMILES Oc1ccc(CCNCCS(=O)(=O)CCCOCCc2ccccc2)c2sc(=O)[nH]c12 Show InChI InChI=1S/C22H28N2O5S2/c25-19-8-7-18(21-20(19)24-22(26)30-21)9-11-23-12-16-31(27,28)15-4-13-29-14-10-17-5-2-1-3-6-17/h1-3,5-8,23,25H,4,9-16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
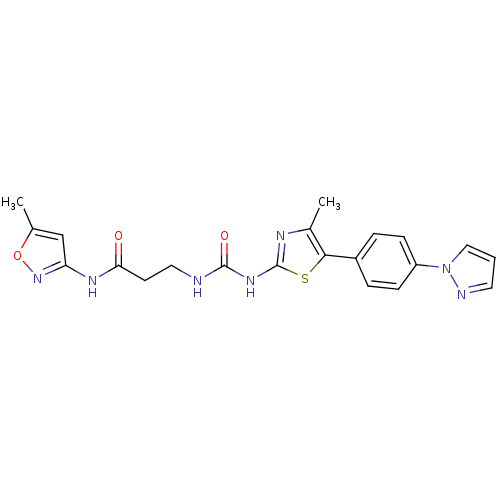
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
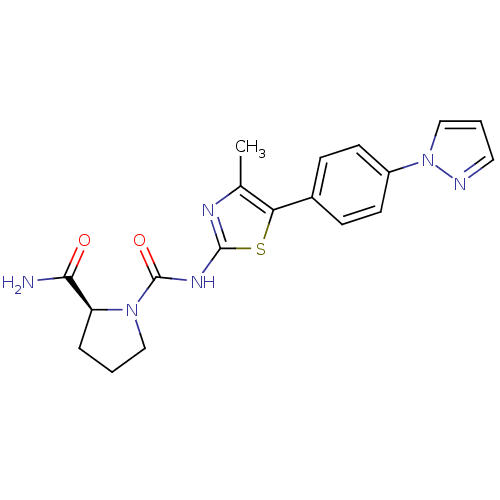
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151719
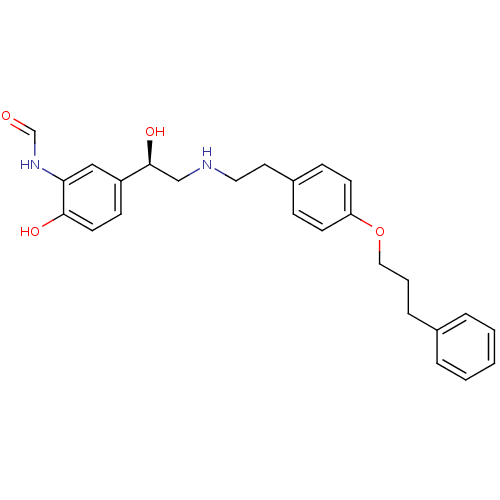
(CHEMBL363329 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[4-(...)Show SMILES O[C@@H](CNCCc1ccc(OCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O4/c29-19-28-24-17-22(10-13-25(24)30)26(31)18-27-15-14-21-8-11-23(12-9-21)32-16-4-7-20-5-2-1-3-6-20/h1-3,5-6,8-13,17,19,26-27,30-31H,4,7,14-16,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151721

(CHEMBL363260 | N-(2-Hydroxy-5-{(R)-1-hydroxy-2-[2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C25H28N2O4/c28-18-27-23-16-21(8-11-24(23)29)25(30)17-26-14-12-20-6-9-22(10-7-20)31-15-13-19-4-2-1-3-5-19/h1-11,16,18,25-26,29-30H,12-15,17H2,(H,27,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151718
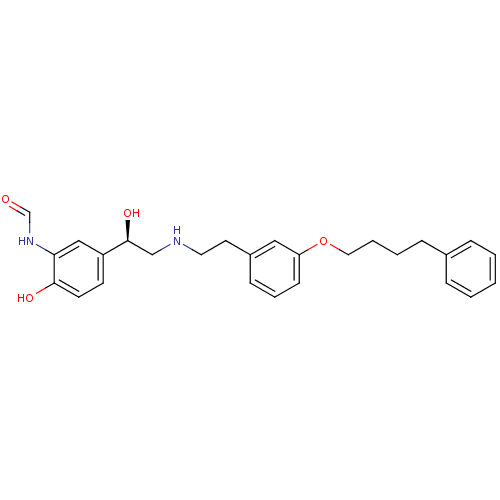
(CHEMBL440561 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1cccc(OCCCCc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(12-13-26(25)31)27(32)19-28-15-14-22-10-6-11-24(17-22)33-16-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10-13,17-18,20,27-28,31-32H,4-5,9,14-16,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151723
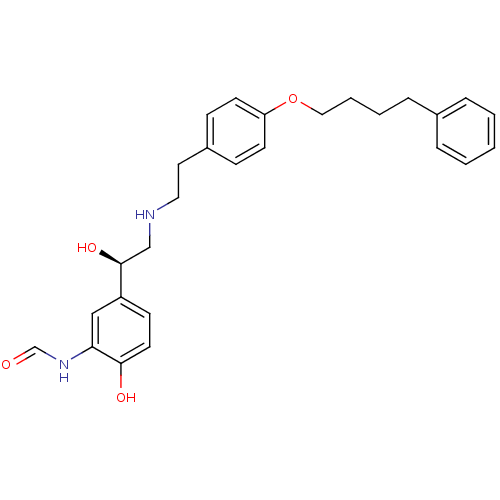
(CHEMBL185052 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(11-14-26(25)31)27(32)19-28-16-15-22-9-12-24(13-10-22)33-17-5-4-8-21-6-2-1-3-7-21/h1-3,6-7,9-14,18,20,27-28,31-32H,4-5,8,15-17,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390426
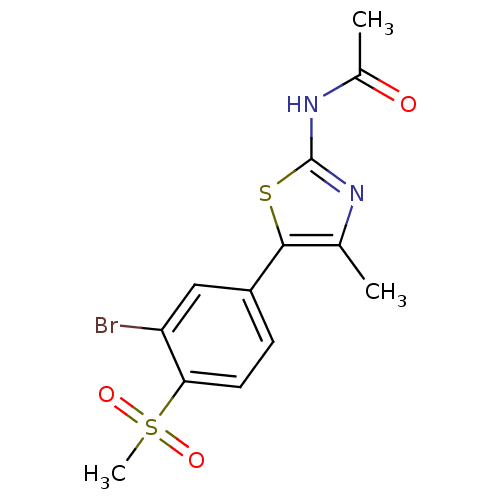
(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151717
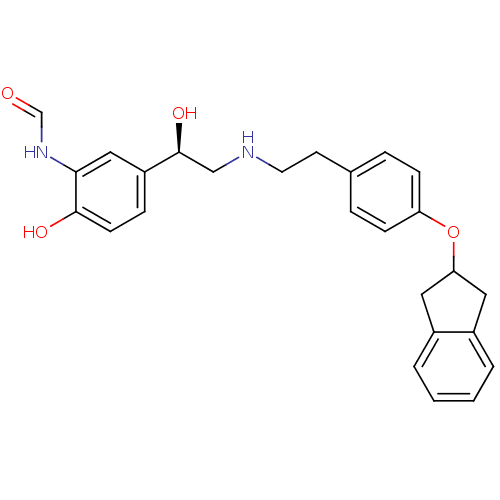
(CHEMBL184538 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[4-(...)Show SMILES O[C@@H](CNCCc1ccc(OC2Cc3ccccc3C2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H28N2O4/c29-17-28-24-15-21(7-10-25(24)30)26(31)16-27-12-11-18-5-8-22(9-6-18)32-23-13-19-3-1-2-4-20(19)14-23/h1-10,15,17,23,26-27,30-31H,11-14,16H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159
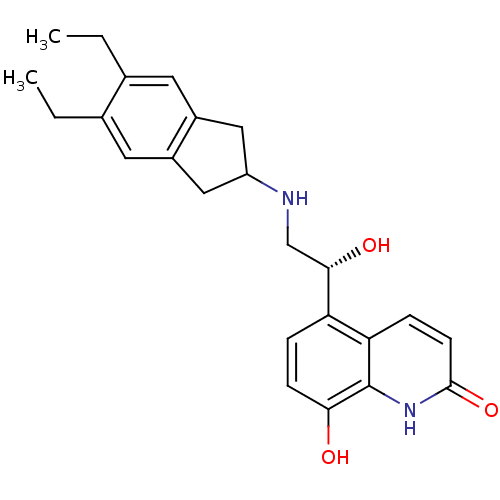
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421327
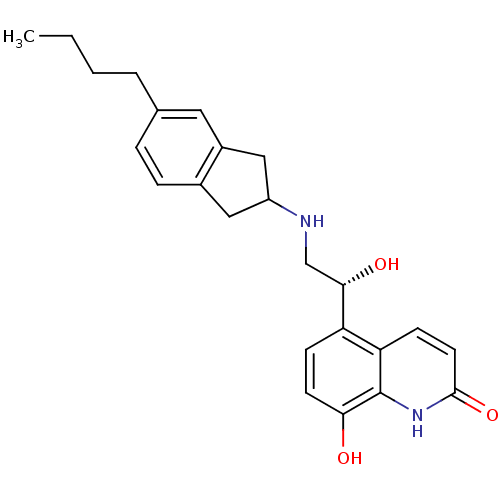
(CHEMBL2088199)Show SMILES CCCCc1ccc2CC(Cc2c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-2-3-4-15-5-6-16-12-18(13-17(16)11-15)25-14-22(28)19-7-9-21(27)24-20(19)8-10-23(29)26-24/h5-11,18,22,25,27-28H,2-4,12-14H2,1H3,(H,26,29)/t18?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390419
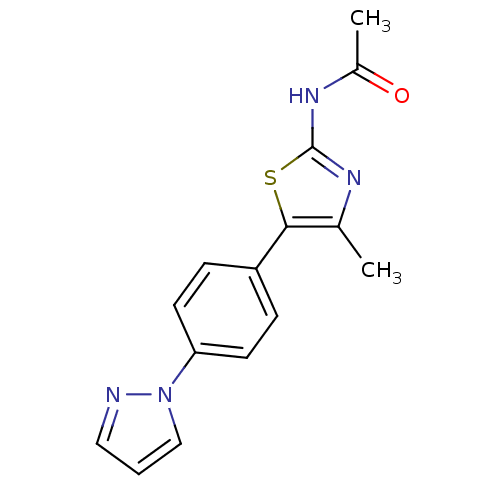
(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390423
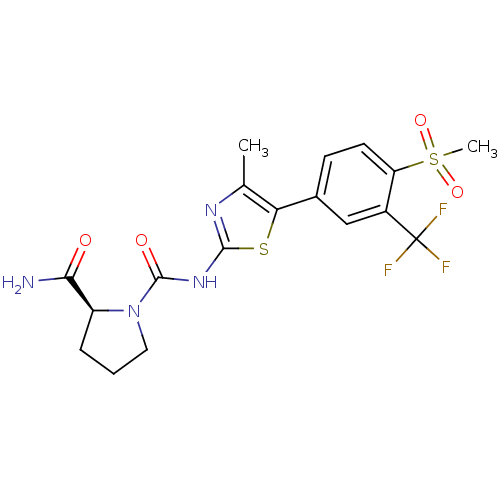
(CHEMBL2071337)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C18H19F3N4O4S2/c1-9-14(10-5-6-13(31(2,28)29)11(8-10)18(19,20)21)30-16(23-9)24-17(27)25-7-3-4-12(25)15(22)26/h5-6,8,12H,3-4,7H2,1-2H3,(H2,22,26)(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390425
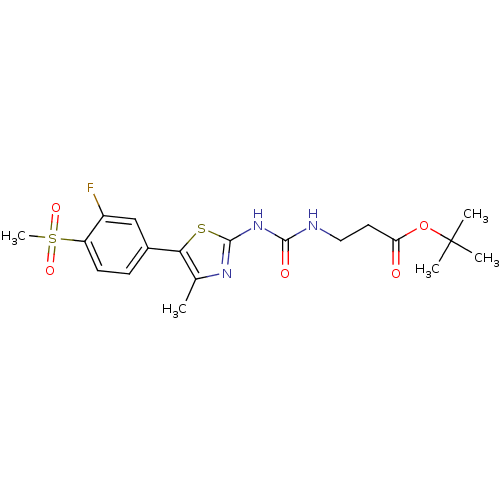
(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151720
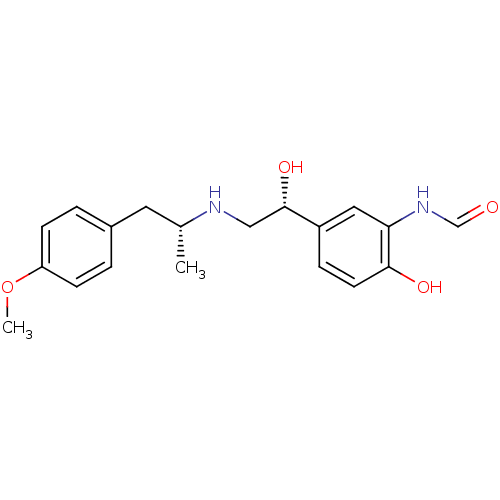
(ARFORMOTEROL TARTRATE | CHEMBL1363 | CHEMBL605993 ...)Show SMILES COc1ccc(C[C@@H](C)NC[C@H](O)c2ccc(O)c(NC=O)c2)cc1 |r| Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390418
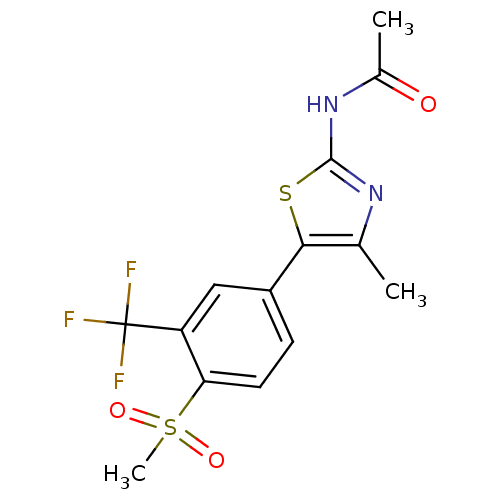
(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151722

(CHEMBL185262 | N-(2-Hydroxy-5-{1-hydroxy-2-[2-(4-p...)Show SMILES O[C@@H](CNCCc1ccc(Oc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C23H24N2O4/c26-16-25-21-14-18(8-11-22(21)27)23(28)15-24-13-12-17-6-9-20(10-7-17)29-19-4-2-1-3-5-19/h1-11,14,16,23-24,27-28H,12-13,15H2,(H,25,26)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390415
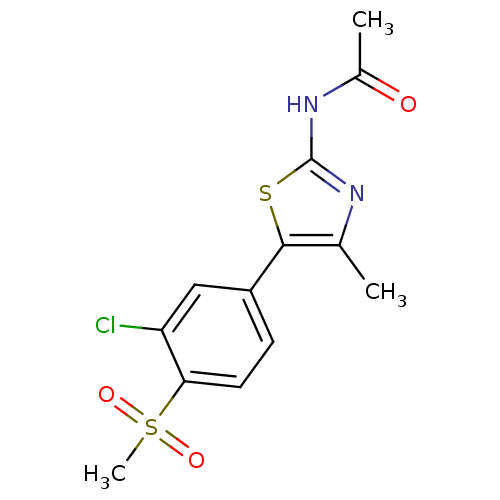
(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390411
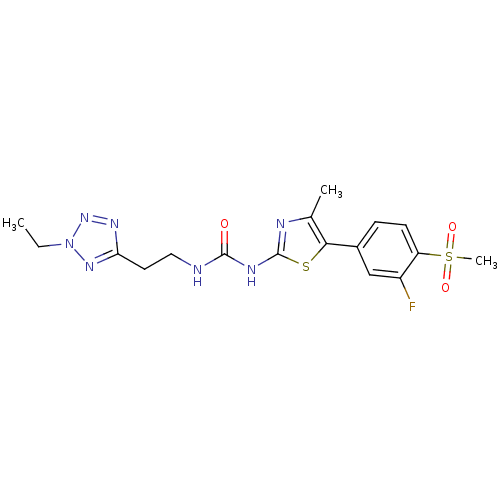
(CHEMBL2071342)Show SMILES CCn1nnc(CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)n1 Show InChI InChI=1S/C17H20FN7O3S2/c1-4-25-23-14(22-24-25)7-8-19-16(26)21-17-20-10(2)15(29-17)11-5-6-13(12(18)9-11)30(3,27)28/h5-6,9H,4,7-8H2,1-3H3,(H2,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421325

(CHEMBL2088197)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2NC(=O)CCc12 |r| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5,7,9-10,18,22,25,27-28H,3-4,6,8,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
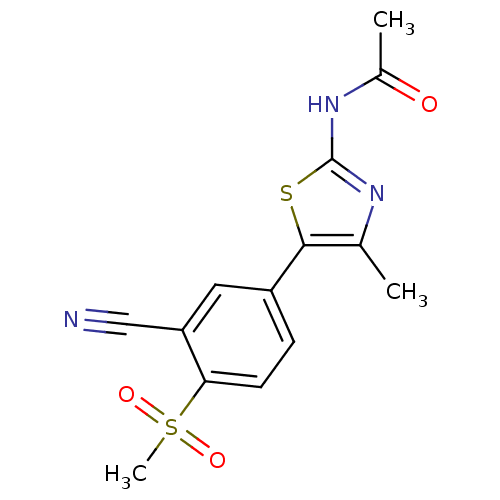
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390417
(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
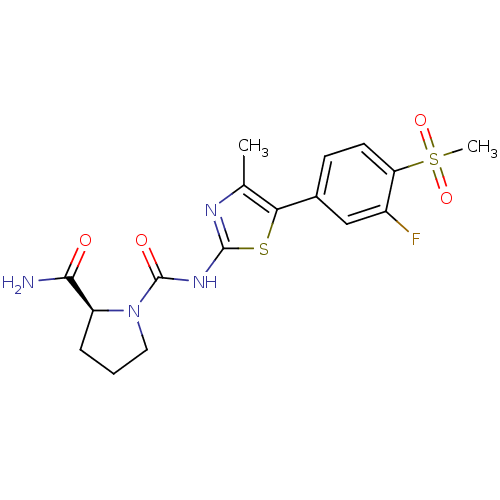
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390422
(CHEMBL2071336)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C17H19FN4O4S2/c1-9-14(10-5-6-13(11(18)8-10)28(2,25)26)27-16(20-9)21-17(24)22-7-3-4-12(22)15(19)23/h5-6,8,12H,3-4,7H2,1-2H3,(H2,19,23)(H,20,21,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159

(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421330
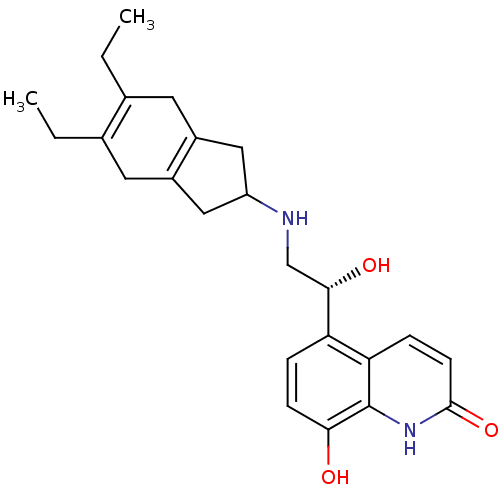
(CHEMBL2088202)Show SMILES CCC1=C(CC)CC2=C(CC(C2)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)C1 |r,c:2,t:7| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-8,18,22,25,27-28H,3-4,9-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390410
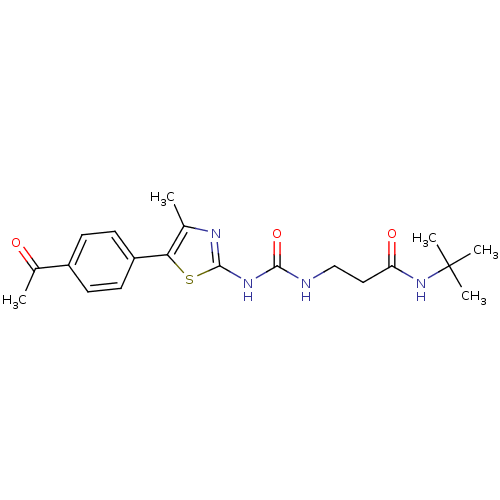
(CHEMBL2069328)Show SMILES CC(=O)c1ccc(cc1)-c1sc(NC(=O)NCCC(=O)NC(C)(C)C)nc1C Show InChI InChI=1S/C20H26N4O3S/c1-12-17(15-8-6-14(7-9-15)13(2)25)28-19(22-12)23-18(27)21-11-10-16(26)24-20(3,4)5/h6-9H,10-11H2,1-5H3,(H,24,26)(H2,21,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50390425

(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110beta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390413
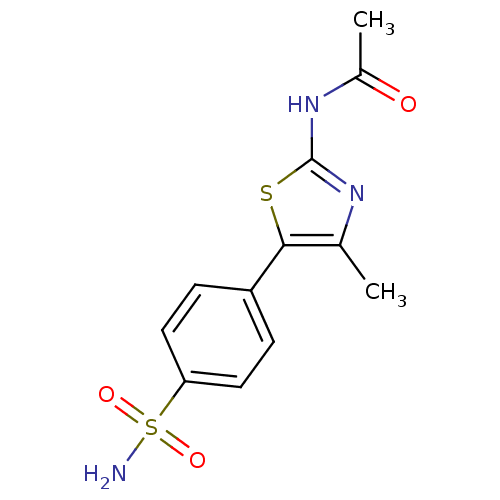
(CHEMBL2071326)Show InChI InChI=1S/C12H13N3O3S2/c1-7-11(19-12(14-7)15-8(2)16)9-3-5-10(6-4-9)20(13,17)18/h3-6H,1-2H3,(H2,13,17,18)(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390424

(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390413

(CHEMBL2071326)Show InChI InChI=1S/C12H13N3O3S2/c1-7-11(19-12(14-7)15-8(2)16)9-3-5-10(6-4-9)20(13,17)18/h3-6H,1-2H3,(H2,13,17,18)(H,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318157
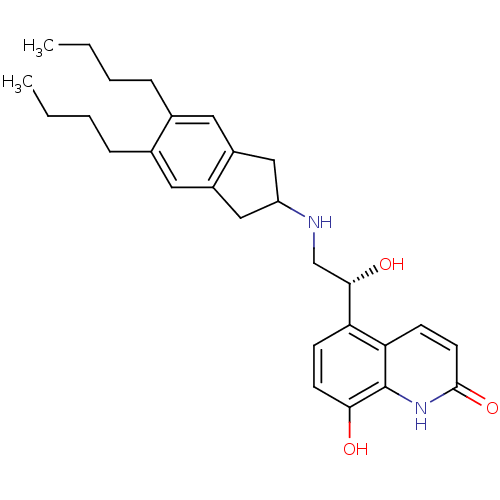
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-butylindan-...)Show SMILES CCCCc1cc2CC(Cc2cc1CCCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C28H36N2O3/c1-3-5-7-18-13-20-15-22(16-21(20)14-19(18)8-6-4-2)29-17-26(32)23-9-11-25(31)28-24(23)10-12-27(33)30-28/h9-14,22,26,29,31-32H,3-8,15-17H2,1-2H3,(H,30,33)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318158
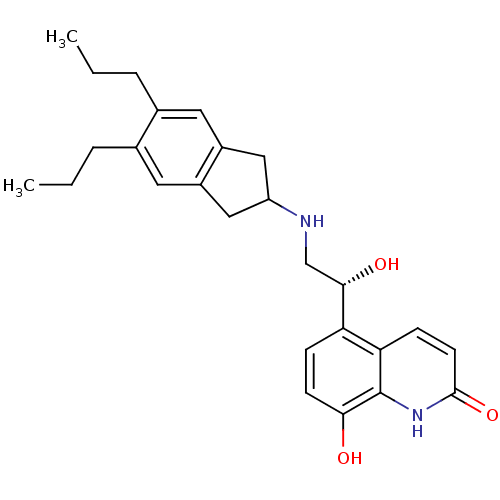
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-di-n-propylindan...)Show SMILES CCCc1cc2CC(Cc2cc1CCC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C26H32N2O3/c1-3-5-16-11-18-13-20(14-19(18)12-17(16)6-4-2)27-15-24(30)21-7-9-23(29)26-22(21)8-10-25(31)28-26/h7-12,20,24,27,29-30H,3-6,13-15H2,1-2H3,(H,28,31)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]cyanopindolol from human recombinant beta2 adrenergic receptor expressed in CHO cells by filtration assay |
J Med Chem 53: 3675-84 (2010)
Article DOI: 10.1021/jm100068m
BindingDB Entry DOI: 10.7270/Q27H1JRT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390424

(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data