 Found 717 hits with Last Name = 'bertin' and Initial = 'j'
Found 717 hits with Last Name = 'bertin' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273
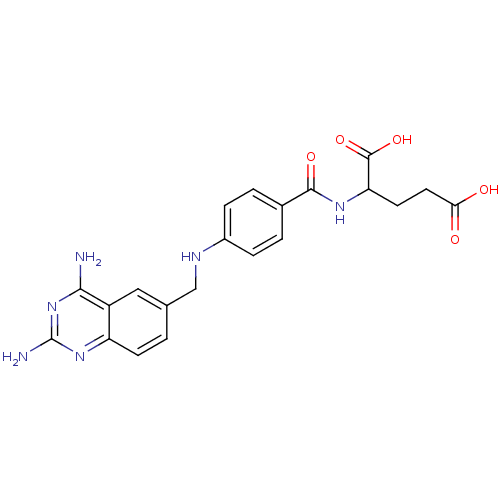
(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50026273

(2-{4-[(2,4-Diamino-quinazolin-6-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2cc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)ccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-14-9-11(1-6-15(14)26-21(23)27-18)10-24-13-4-2-12(3-5-13)19(30)25-16(20(31)32)7-8-17(28)29/h1-6,9,16,24H,7-8,10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50367055

(4-Aminofolic acid | 4-Aminopteroic acid | AMINOPTE...)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cnc2n1 |r| Show InChI InChI=1S/C19H20N8O5/c20-15-14-16(27-19(21)26-15)23-8-11(24-14)7-22-10-3-1-9(2-4-10)17(30)25-12(18(31)32)5-6-13(28)29/h1-4,8,12,22H,5-7H2,(H,25,30)(H,28,29)(H,31,32)(H4,20,21,23,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495
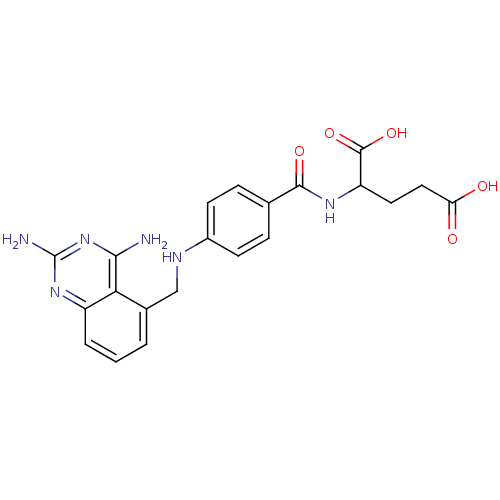
(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159507
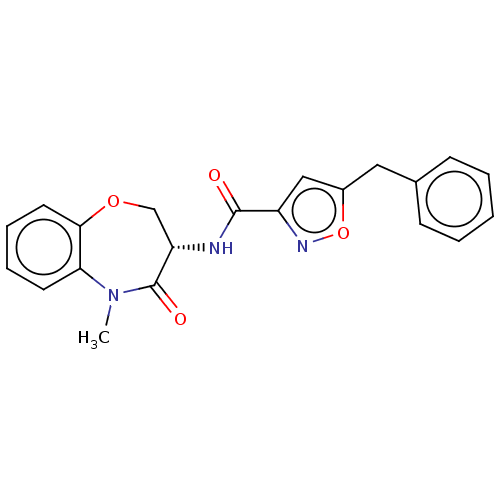
(CHEMBL3785703)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-18-9-5-6-10-19(18)27-13-17(21(24)26)22-20(25)16-12-15(28-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484
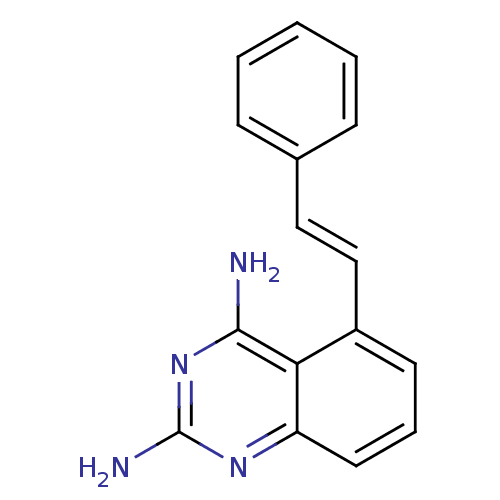
(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036495

(2-{4-[(2,4-Diamino-quinazolin-5-ylmethyl)-amino]-b...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cccc2n1 Show InChI InChI=1S/C21H22N6O5/c22-18-17-12(2-1-3-14(17)26-21(23)27-18)10-24-13-6-4-11(5-7-13)19(30)25-15(20(31)32)8-9-16(28)29/h1-7,15,24H,8-10H2,(H,25,30)(H,28,29)(H,31,32)(H4,22,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483
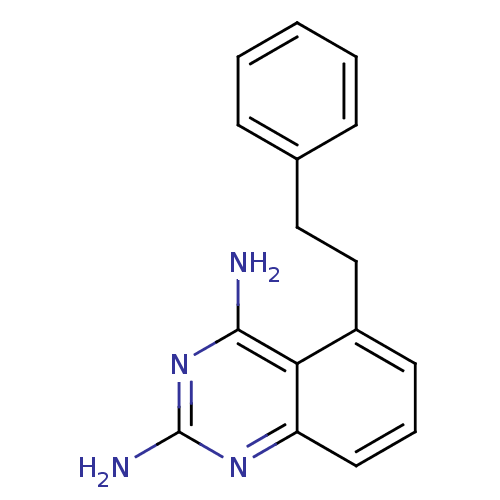
(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036491
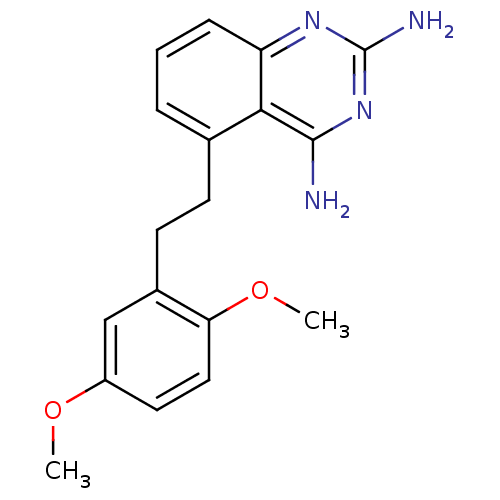
(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-quinazoline-2,4...)Show InChI InChI=1S/C18H20N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,6-7H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036486
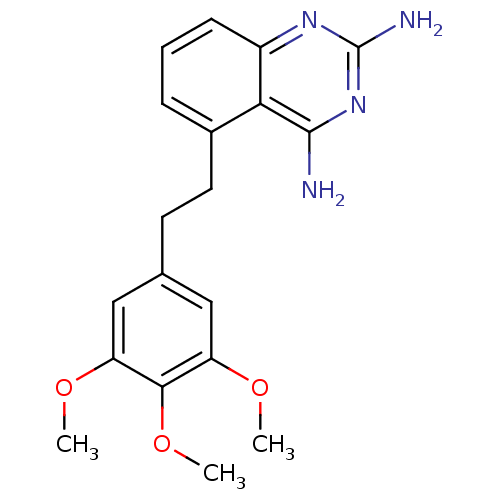
(5-[2-(3,4,5-Trimethoxy-phenyl)-ethyl]-quinazoline-...)Show InChI InChI=1S/C19H22N4O3/c1-24-14-9-11(10-15(25-2)17(14)26-3)7-8-12-5-4-6-13-16(12)18(20)23-19(21)22-13/h4-6,9-10H,7-8H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036484

(5-((E)-Styryl)-quinazoline-2,4-diamine | CHEMBL164...)Show InChI InChI=1S/C16H14N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-10H,(H4,17,18,19,20)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036483

(5-Phenethyl-quinazoline-2,4-diamine | CHEMBL341703)Show InChI InChI=1S/C16H16N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,9-10H2,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036488
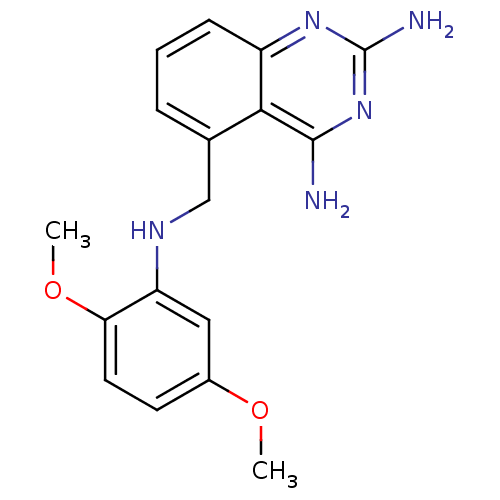
(5-[(2,5-Dimethoxy-phenylamino)-methyl]-quinazoline...)Show InChI InChI=1S/C17H19N5O2/c1-23-11-6-7-14(24-2)13(8-11)20-9-10-4-3-5-12-15(10)16(18)22-17(19)21-12/h3-8,20H,9H2,1-2H3,(H4,18,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036491

(5-[2-(2,5-Dimethoxy-phenyl)-ethyl]-quinazoline-2,4...)Show InChI InChI=1S/C18H20N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,6-7H2,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036487
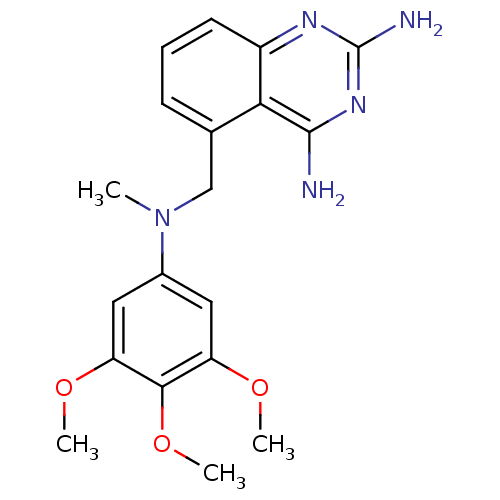
(5-{[Methyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1cccc2nc(N)nc(N)c12 Show InChI InChI=1S/C19H23N5O3/c1-24(12-8-14(25-2)17(27-4)15(9-12)26-3)10-11-6-5-7-13-16(11)18(20)23-19(21)22-13/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036486

(5-[2-(3,4,5-Trimethoxy-phenyl)-ethyl]-quinazoline-...)Show InChI InChI=1S/C19H22N4O3/c1-24-14-9-11(10-15(25-2)17(14)26-3)7-8-12-5-4-6-13-16(12)18(20)23-19(21)22-13/h4-6,9-10H,7-8H2,1-3H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036493
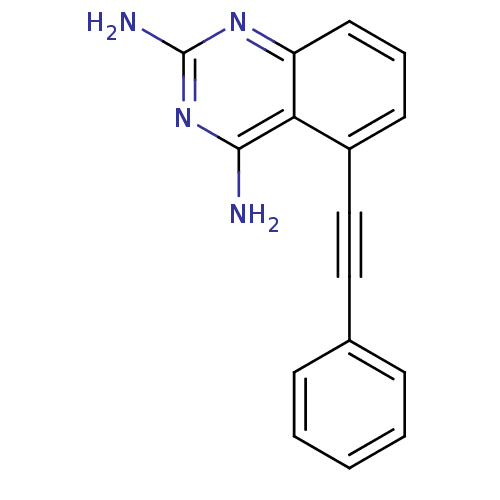
(5-Phenylethynyl-quinazoline-2,4-diamine | CHEMBL35...)Show InChI InChI=1S/C16H12N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
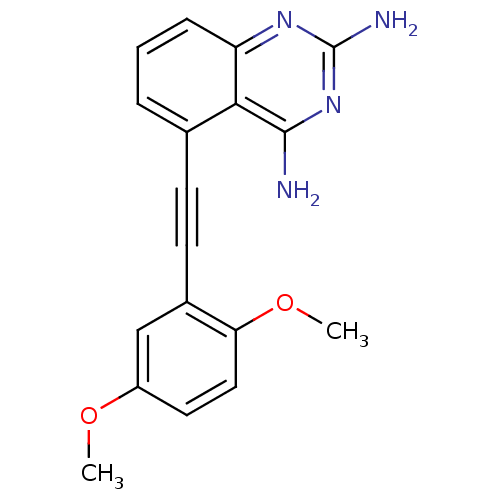
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036489
(5-(2,5-Dimethoxy-phenylethynyl)-quinazoline-2,4-di...)Show InChI InChI=1S/C18H16N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036488

(5-[(2,5-Dimethoxy-phenylamino)-methyl]-quinazoline...)Show InChI InChI=1S/C17H19N5O2/c1-23-11-6-7-14(24-2)13(8-11)20-9-10-4-3-5-12-15(10)16(18)22-17(19)21-12/h3-8,20H,9H2,1-2H3,(H4,18,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036492
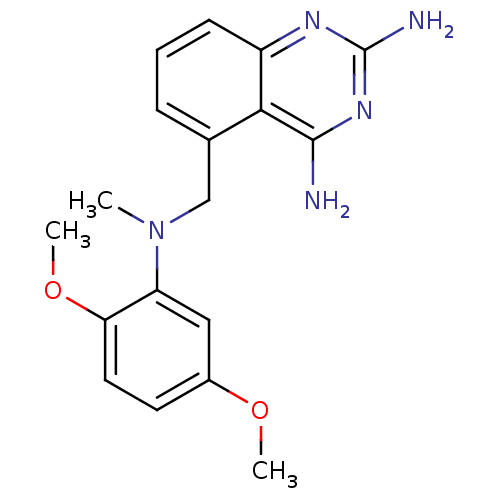
(5-{[(2,5-Dimethoxy-phenyl)-methyl-amino]-methyl}-q...)Show InChI InChI=1S/C18H21N5O2/c1-23(14-9-12(24-2)7-8-15(14)25-3)10-11-5-4-6-13-16(11)17(19)22-18(20)21-13/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036485
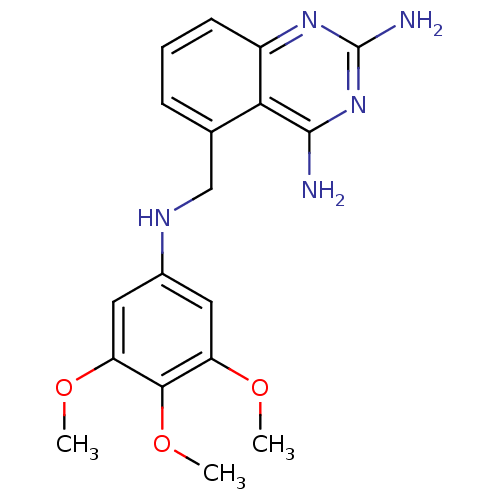
(5-[(3,4,5-Trimethoxy-phenylamino)-methyl]-quinazol...)Show InChI InChI=1S/C18H21N5O3/c1-24-13-7-11(8-14(25-2)16(13)26-3)21-9-10-5-4-6-12-15(10)17(19)23-18(20)22-12/h4-8,21H,9H2,1-3H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductase |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036492

(5-{[(2,5-Dimethoxy-phenyl)-methyl-amino]-methyl}-q...)Show InChI InChI=1S/C18H21N5O2/c1-23(14-9-12(24-2)7-8-15(14)25-3)10-11-5-4-6-13-16(11)17(19)22-18(20)21-13/h4-9H,10H2,1-3H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036489

(5-(2,5-Dimethoxy-phenylethynyl)-quinazoline-2,4-di...)Show InChI InChI=1S/C18H16N4O2/c1-23-13-8-9-15(24-2)12(10-13)7-6-11-4-3-5-14-16(11)17(19)22-18(20)21-14/h3-5,8-10H,1-2H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036485

(5-[(3,4,5-Trimethoxy-phenylamino)-methyl]-quinazol...)Show InChI InChI=1S/C18H21N5O3/c1-24-13-7-11(8-14(25-2)16(13)26-3)21-9-10-5-4-6-12-15(10)17(19)23-18(20)22-12/h4-8,21H,9H2,1-3H3,(H4,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036493

(5-Phenylethynyl-quinazoline-2,4-diamine | CHEMBL35...)Show InChI InChI=1S/C16H12N4/c17-15-14-12(10-9-11-5-2-1-3-6-11)7-4-8-13(14)19-16(18)20-15/h1-8H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory activity against Wild-type human DHFR |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50036487

(5-{[Methyl-(3,4,5-trimethoxy-phenyl)-amino]-methyl...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1cccc2nc(N)nc(N)c12 Show InChI InChI=1S/C19H23N5O3/c1-24(12-8-14(25-2)17(27-4)15(9-12)26-3)10-11-6-5-7-13-16(11)18(20)23-19(21)22-13/h5-9H,10H2,1-4H3,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibitory compound against Wild-type human DHFR. |
J Med Chem 38: 745-52 (1995)
BindingDB Entry DOI: 10.7270/Q2JQ11PK |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233227
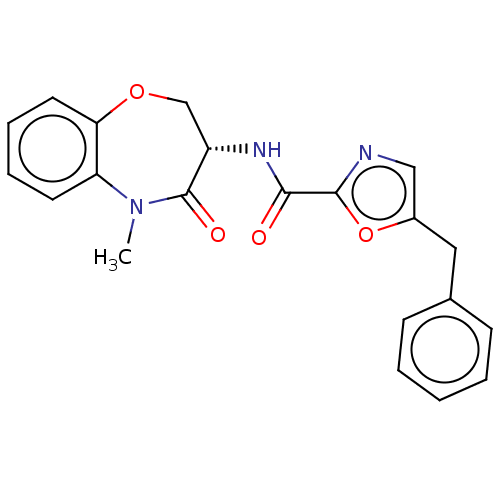
(CHEMBL4067372)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2ncc(Cc3ccccc3)o2)C1=O |r| Show InChI InChI=1S/C21H19N3O4/c1-24-17-9-5-6-10-18(17)27-13-16(21(24)26)23-19(25)20-22-12-15(28-20)11-14-7-3-2-4-8-14/h2-10,12,16H,11,13H2,1H3,(H,23,25)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159697
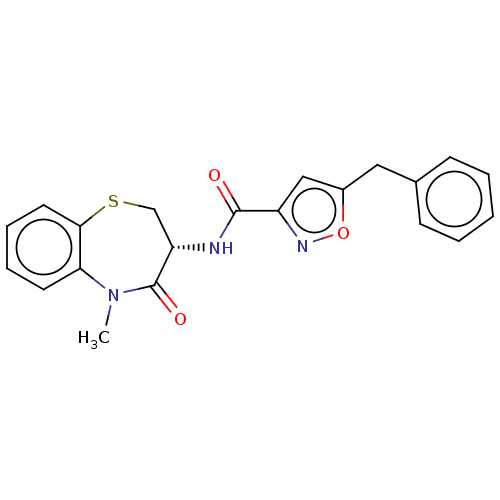
(CHEMBL3785745)Show SMILES CN1c2ccccc2SC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C21H19N3O3S/c1-24-18-9-5-6-10-19(18)28-13-17(21(24)26)22-20(25)16-12-15(27-23-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,25)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233256
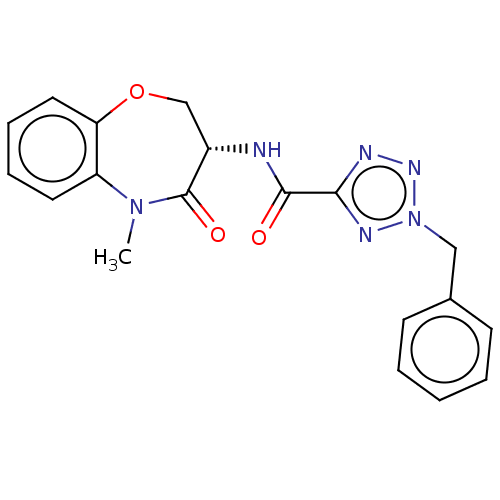
(CHEMBL4093220)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2nnn(Cc3ccccc3)n2)C1=O |r| Show InChI InChI=1S/C19H18N6O3/c1-24-15-9-5-6-10-16(15)28-12-14(19(24)27)20-18(26)17-21-23-25(22-17)11-13-7-3-2-4-8-13/h2-10,14H,11-12H2,1H3,(H,20,26)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159696
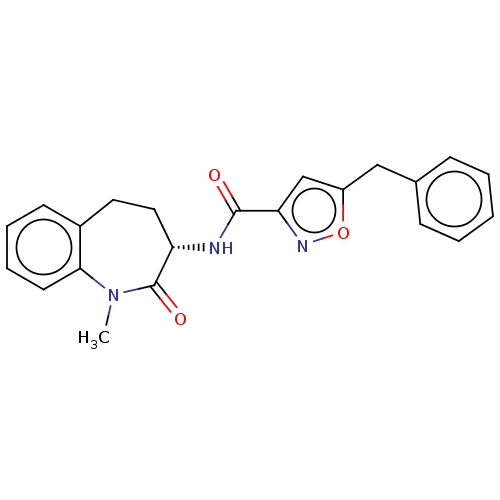
(CHEMBL3786997)Show SMILES CN1c2ccccc2CC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C22H21N3O3/c1-25-20-10-6-5-9-16(20)11-12-18(22(25)27)23-21(26)19-14-17(28-24-19)13-15-7-3-2-4-8-15/h2-10,14,18H,11-13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159696

(CHEMBL3786997)Show SMILES CN1c2ccccc2CC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C22H21N3O3/c1-25-20-10-6-5-9-16(20)11-12-18(22(25)27)23-21(26)19-14-17(28-24-19)13-15-7-3-2-4-8-15/h2-10,14,18H,11-13H2,1H3,(H,23,26)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233248
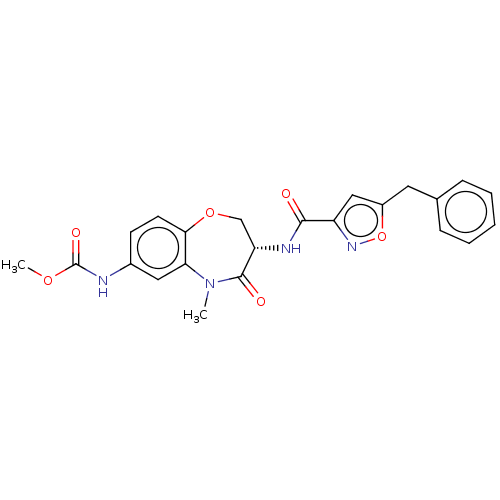
(CHEMBL4096224)Show SMILES COC(=O)Nc1ccc2OC[C@H](NC(=O)c3cc(Cc4ccccc4)on3)C(=O)N(C)c2c1 |r| Show InChI InChI=1S/C23H22N4O6/c1-27-19-11-15(24-23(30)31-2)8-9-20(19)32-13-18(22(27)29)25-21(28)17-12-16(33-26-17)10-14-6-4-3-5-7-14/h3-9,11-12,18H,10,13H2,1-2H3,(H,24,30)(H,25,28)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233244
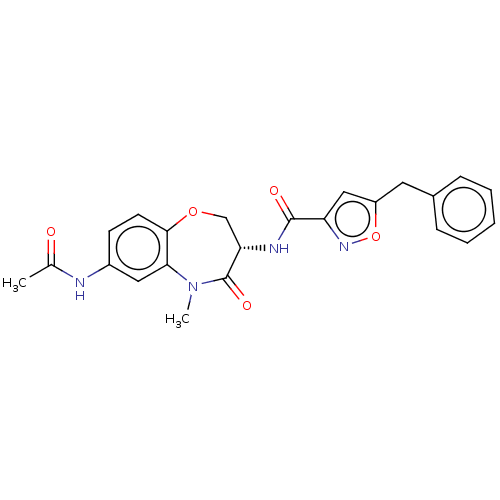
(CHEMBL4068954)Show SMILES CN1c2cc(NC(C)=O)ccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O |r| Show InChI InChI=1S/C23H22N4O5/c1-14(28)24-16-8-9-21-20(11-16)27(2)23(30)19(13-31-21)25-22(29)18-12-17(32-26-18)10-15-6-4-3-5-7-15/h3-9,11-12,19H,10,13H2,1-2H3,(H,24,28)(H,25,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50502339
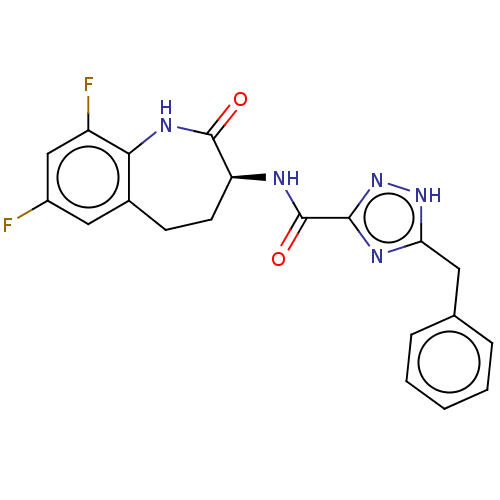
(CHEMBL4452233)Show SMILES Fc1cc(F)c2NC(=O)[C@H](CCc2c1)NC(=O)c1n[nH]c(Cc2ccccc2)n1 |r| Show InChI InChI=1S/C20H17F2N5O2/c21-13-9-12-6-7-15(19(28)25-17(12)14(22)10-13)23-20(29)18-24-16(26-27-18)8-11-4-2-1-3-5-11/h1-5,9-10,15H,6-8H2,(H,23,29)(H,25,28)(H,24,26,27)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta mRNA overexpression measured at 21 ... |
ACS Med Chem Lett 10: 857-862 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00108
BindingDB Entry DOI: 10.7270/Q2VT1WB8 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50502339

(CHEMBL4452233)Show SMILES Fc1cc(F)c2NC(=O)[C@H](CCc2c1)NC(=O)c1n[nH]c(Cc2ccccc2)n1 |r| Show InChI InChI=1S/C20H17F2N5O2/c21-13-9-12-6-7-15(19(28)25-17(12)14(22)10-13)23-20(29)18-24-16(26-27-18)8-11-4-2-1-3-5-11/h1-5,9-10,15H,6-8H2,(H,23,29)(H,25,28)(H,24,26,27)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta cytokine overproduction measured at... |
ACS Med Chem Lett 10: 857-862 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00108
BindingDB Entry DOI: 10.7270/Q2VT1WB8 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50159693

(CHEMBL3785482)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)[nH]n2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-25-18-9-5-6-10-19(18)28-13-17(21(25)27)22-20(26)16-12-15(23-24-16)11-14-7-3-2-4-8-14/h2-10,12,17H,11,13H2,1H3,(H,22,26)(H,23,24)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assay |
J Med Chem 59: 2163-78 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01898
BindingDB Entry DOI: 10.7270/Q26H4K97 |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233228
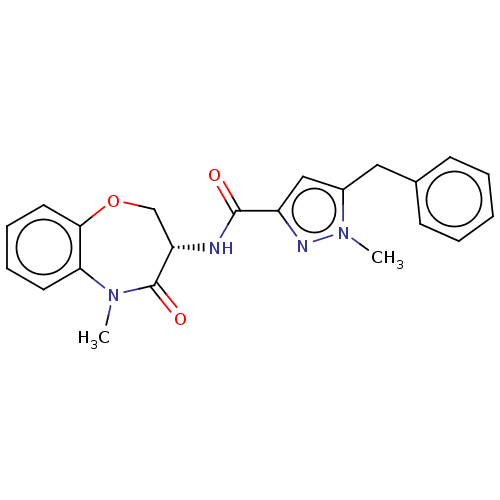
(CHEMBL4060644)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)n(C)n2)C1=O |r| Show InChI InChI=1S/C22H22N4O3/c1-25-19-10-6-7-11-20(19)29-14-18(22(25)28)23-21(27)17-13-16(26(2)24-17)12-15-8-4-3-5-9-15/h3-11,13,18H,12,14H2,1-2H3,(H,23,27)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
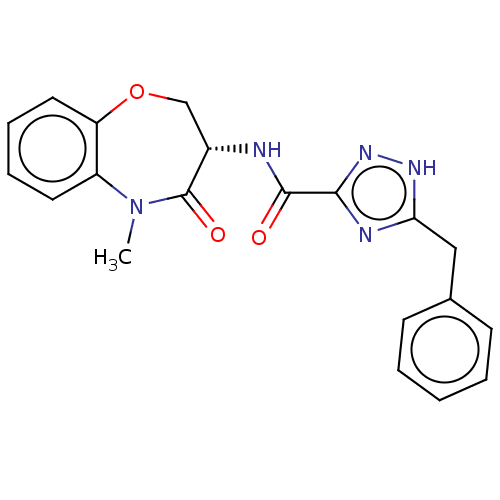
(Homo sapiens (Human)) | BDBM50233225
(CHEMBL4071864)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2n[nH]c(Cc3ccccc3)n2)C1=O |r| Show InChI InChI=1S/C20H19N5O3/c1-25-15-9-5-6-10-16(15)28-12-14(20(25)27)21-19(26)18-22-17(23-24-18)11-13-7-3-2-4-8-13/h2-10,14H,11-12H2,1H3,(H,21,26)(H,22,23,24)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-induced necroptosis at 21 hrs post-st... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
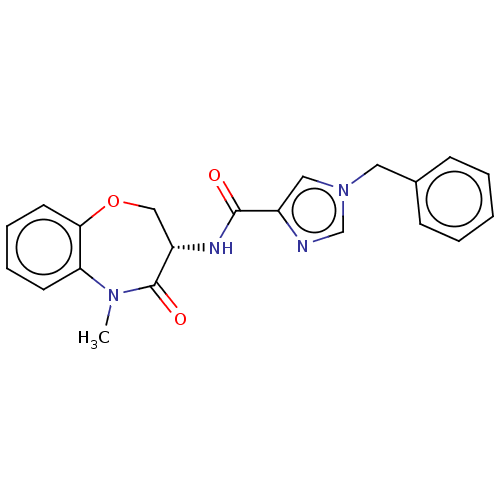
(Homo sapiens (Human)) | BDBM50233255
(CHEMBL4069318)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2cn(Cc3ccccc3)cn2)C1=O |r| Show InChI InChI=1S/C21H20N4O3/c1-24-18-9-5-6-10-19(18)28-13-17(21(24)27)23-20(26)16-12-25(14-22-16)11-15-7-3-2-4-8-15/h2-10,12,14,17H,11,13H2,1H3,(H,23,26)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50233225

(CHEMBL4071864)Show SMILES CN1c2ccccc2OC[C@H](NC(=O)c2n[nH]c(Cc3ccccc3)n2)C1=O |r| Show InChI InChI=1S/C20H19N5O3/c1-25-15-9-5-6-10-16(15)28-12-14(20(25)27)21-19(26)18-22-17(23-24-18)11-13-7-3-2-4-8-13/h2-10,14H,11-12H2,1H3,(H,21,26)(H,22,23,24)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-MIP-1beta production at 21 hrs post-s... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
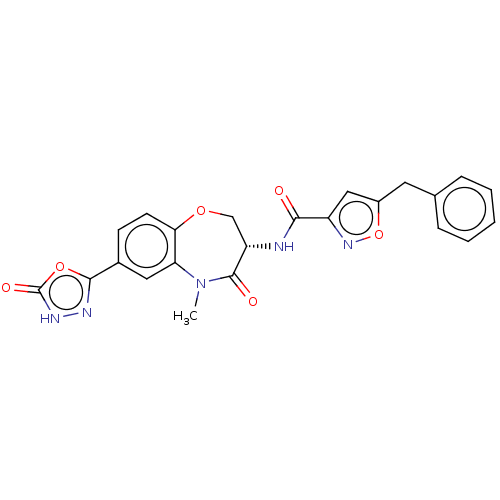
(Homo sapiens (Human)) | BDBM50233226
(CHEMBL4089558)Show SMILES CN1c2cc(ccc2OC[C@H](NC(=O)c2cc(Cc3ccccc3)on2)C1=O)-c1n[nH]c(=O)o1 |r| Show InChI InChI=1S/C23H19N5O6/c1-28-18-10-14(21-25-26-23(31)33-21)7-8-19(18)32-12-17(22(28)30)24-20(29)16-11-15(34-27-16)9-13-5-3-2-4-6-13/h2-8,10-11,17H,9,12H2,1H3,(H,24,29)(H,26,31)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Queen Mary University of London
Curated by ChEMBL
| Assay Description
Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a... |
J Med Chem 60: 1247-1261 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01751
BindingDB Entry DOI: 10.7270/Q2SQ92NR |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50513015
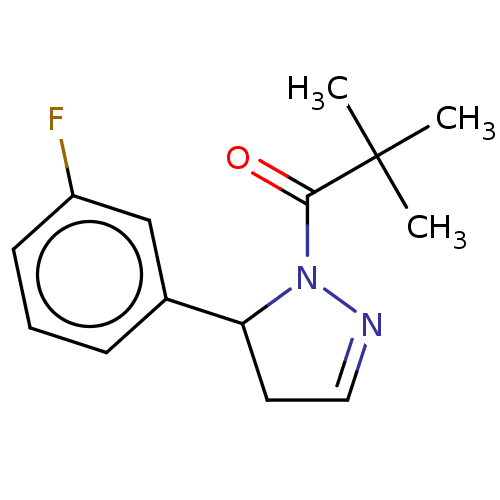
(CHEMBL4537171)Show InChI InChI=1S/C14H17FN2O/c1-14(2,3)13(18)17-12(7-8-16-17)10-5-4-6-11(15)9-10/h4-6,8-9,12H,7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... |
J Med Chem 62: 5096-5110 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00318
BindingDB Entry DOI: 10.7270/Q24J0JFT |
More data for this
Ligand-Target Pair | |
Receptor-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50502339

(CHEMBL4452233)Show SMILES Fc1cc(F)c2NC(=O)[C@H](CCc2c1)NC(=O)c1n[nH]c(Cc2ccccc2)n1 |r| Show InChI InChI=1S/C20H17F2N5O2/c21-13-9-12-6-7-15(19(28)25-17(12)14(22)10-13)23-20(29)18-24-16(26-27-18)8-11-4-2-1-3-5-11/h1-5,9-10,15H,6-8H2,(H,23,29)(H,25,28)(H,24,26,27)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated necroptosis by measuring LDH release measured ... |
ACS Med Chem Lett 10: 857-862 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00108
BindingDB Entry DOI: 10.7270/Q2VT1WB8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data