 Found 54 hits with Last Name = 'dedio' and Initial = 'j'
Found 54 hits with Last Name = 'dedio' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404
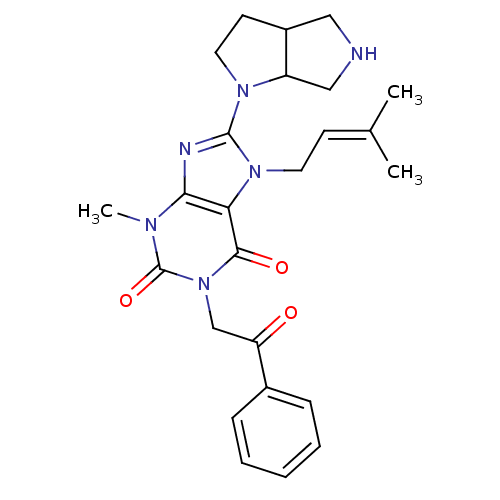
(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437403

(CHEMBL2408771)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C26H32N6O3/c1-17(2)11-13-31-22-23(28-25(31)30-14-19-10-7-12-27-20(19)15-30)29(3)26(35)32(24(22)34)16-21(33)18-8-5-4-6-9-18/h4-6,8-9,11,19-20,27H,7,10,12-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395
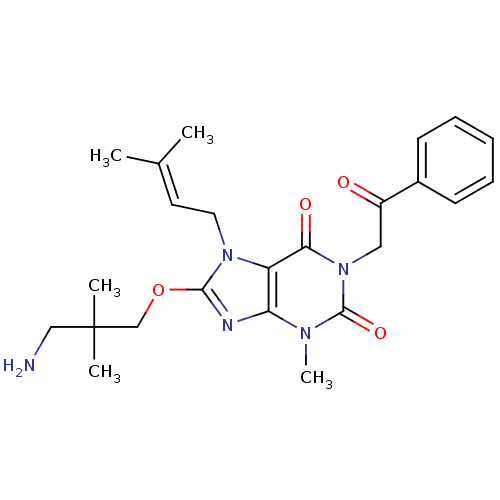
(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437395

(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437404

(CHEMBL2408655)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C25H30N6O3/c1-16(2)9-11-30-21-22(27-24(30)29-12-10-18-13-26-14-19(18)29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-9,18-19,26H,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437396

(CHEMBL2408651)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437402
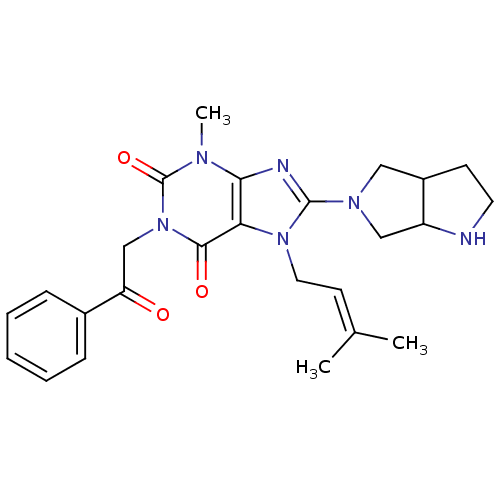
(CHEMBL2408774)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C25H30N6O3/c1-16(2)10-12-30-21-22(27-24(30)29-13-18-9-11-26-19(18)14-29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-8,10,18-19,26H,9,11-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437396

(CHEMBL2408651)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50228407

(8-((R)-3-amino-piperidin-1-yl)-3-methyl-7-(3-methy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-1 Show InChI InChI=1S/C24H30N6O3/c1-16(2)11-13-29-20-21(26-23(29)28-12-7-10-18(25)14-28)27(3)24(33)30(22(20)32)15-19(31)17-8-5-4-6-9-17/h4-6,8-9,11,18H,7,10,12-15,25H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437402

(CHEMBL2408774)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C25H30N6O3/c1-16(2)10-12-30-21-22(27-24(30)29-13-18-9-11-26-19(18)14-29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-8,10,18-19,26H,9,11-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437395

(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437398
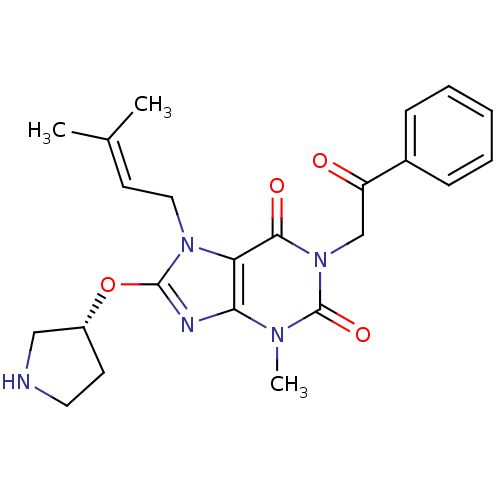
(CHEMBL2408646)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437401

(CHEMBL2408642)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6])c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C18H26N6O2/c1-11(2)5-7-24-14-15(21(3)18(26)22(4)16(14)25)20-17(24)23-8-6-12-9-19-10-13(12)23/h5,12-13,19H,6-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437395

(CHEMBL2408638)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#6]-[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H31N5O4/c1-16(2)11-12-28-19-20(26-22(28)33-15-24(3,4)14-25)27(5)23(32)29(21(19)31)13-18(30)17-9-7-6-8-10-17/h6-11H,12-15,25H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437401

(CHEMBL2408642)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6])c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C18H26N6O2/c1-11(2)5-7-24-14-15(21(3)18(26)22(4)16(14)25)20-17(24)23-8-6-12-9-19-10-13(12)23/h5,12-13,19H,6-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437399
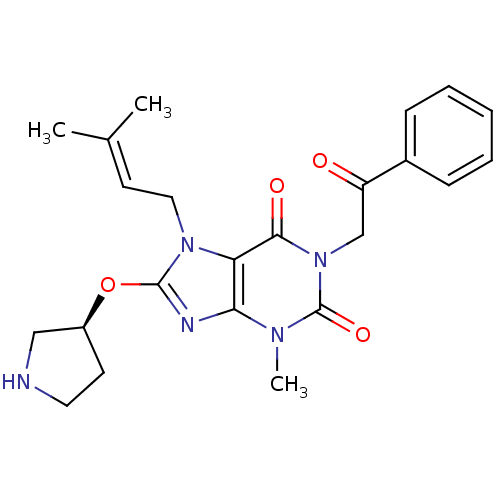
(CHEMBL2408644)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437398

(CHEMBL2408646)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437401

(CHEMBL2408642)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6])c(=O)c12)-[#7]-1-[#6]-[#6]-[#6]-2-[#6]-[#7]-[#6]-[#6]-1-2 Show InChI InChI=1S/C18H26N6O2/c1-11(2)5-7-24-14-15(21(3)18(26)22(4)16(14)25)20-17(24)23-8-6-12-9-19-10-13(12)23/h5,12-13,19H,6-10H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437400

(CHEMBL2408643)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-2-[#6]-[#6]-[#7]-[#6]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-14-28-20-21(26-23(28)33-18-9-12-25-13-10-18)27(3)24(32)29(22(20)31)15-19(30)17-7-5-4-6-8-17/h4-8,11,18,25H,9-10,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437396

(CHEMBL2408651)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437399

(CHEMBL2408644)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437394

(CHEMBL2408639)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C23H29N5O4/c1-15(2)11-12-27-18-19(25-21(27)32-14-23(3,4)24)26(5)22(31)28(20(18)30)13-17(29)16-9-7-6-8-10-16/h6-11H,12-14,24H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150261

(US8980938, 8)Show SMILES CC(C)(NC(=O)c1ccc2ccsc2c1OCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C22H23NO4S/c1-22(2,21(25)26)23-20(24)17-11-10-16-12-14-28-19(16)18(17)27-13-6-9-15-7-4-3-5-8-15/h3-5,7-8,10-12,14H,6,9,13H2,1-2H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437402

(CHEMBL2408774)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12)-[#7]-1-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-2-[#6]-1 Show InChI InChI=1S/C25H30N6O3/c1-16(2)10-12-30-21-22(27-24(30)29-13-18-9-11-26-19(18)14-29)28(3)25(34)31(23(21)33)15-20(32)17-7-5-4-6-8-17/h4-8,10,18-19,26H,9,11-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 645 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437397

(CHEMBL2408649)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 705 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437399

(CHEMBL2408644)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 715 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150262
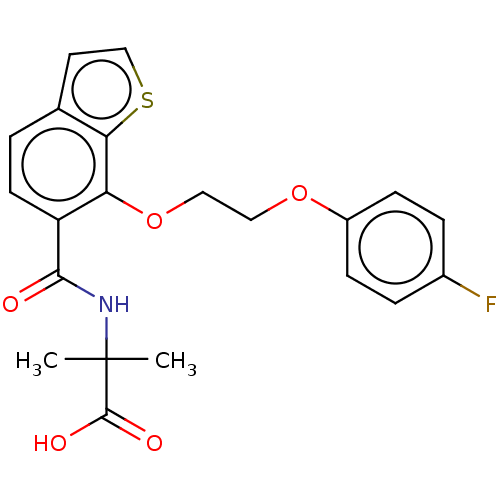
(US8980938, 11)Show SMILES CC(C)(NC(=O)c1ccc2ccsc2c1OCCOc1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C21H20FNO5S/c1-21(2,20(25)26)23-19(24)16-8-3-13-9-12-29-18(13)17(16)28-11-10-27-15-6-4-14(22)5-7-15/h3-9,12H,10-11H2,1-2H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437394

(CHEMBL2408639)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C23H29N5O4/c1-15(2)11-12-27-18-19(25-21(27)32-14-23(3,4)24)26(5)22(31)28(20(18)30)13-17(29)16-9-7-6-8-10-16/h6-11H,12-14,24H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437398

(CHEMBL2408646)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6@@H]-2-[#6]-[#6]-[#7]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C23H27N5O4/c1-15(2)10-12-27-19-20(25-22(27)32-17-9-11-24-13-17)26(3)23(31)28(21(19)30)14-18(29)16-7-5-4-6-8-16/h4-8,10,17,24H,9,11-14H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM100343
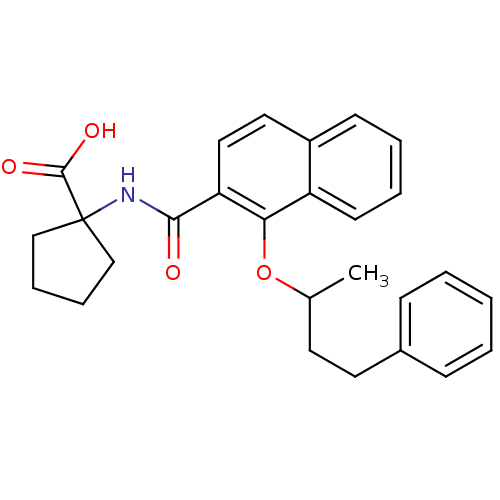
(US8501981, 52)Show SMILES CC(CCc1ccccc1)Oc1c(ccc2ccccc12)C(=O)NC1(CCCC1)C(O)=O Show InChI InChI=1S/C27H29NO4/c1-19(13-14-20-9-3-2-4-10-20)32-24-22-12-6-5-11-21(22)15-16-23(24)25(29)28-27(26(30)31)17-7-8-18-27/h2-6,9-12,15-16,19H,7-8,13-14,17-18H2,1H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
CXCR2 inhibition using calcium fluorescence assay (FLIPR). |
US Patent US8501981 (2013)
BindingDB Entry DOI: 10.7270/Q24Q7SMB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437400

(CHEMBL2408643)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-2-[#6]-[#6]-[#7]-[#6]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-14-28-20-21(26-23(28)33-18-9-12-25-13-10-18)27(3)24(32)29(22(20)31)15-19(30)17-7-5-4-6-8-17/h4-8,11,18,25H,9-10,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150258
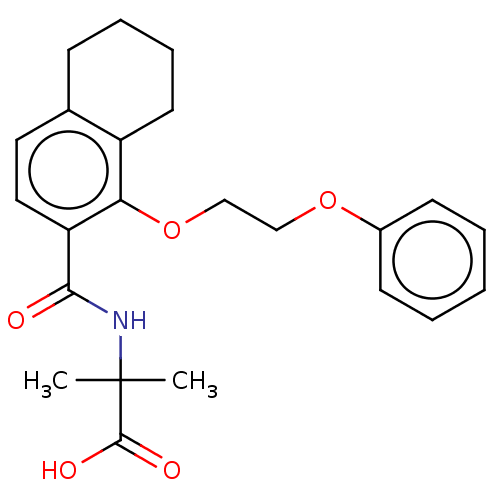
(US8980938, 3)Show SMILES CC(C)(NC(=O)c1ccc2CCCCc2c1OCCOc1ccccc1)C(O)=O Show InChI InChI=1S/C23H27NO5/c1-23(2,22(26)27)24-21(25)19-13-12-16-8-6-7-11-18(16)20(19)29-15-14-28-17-9-4-3-5-10-17/h3-5,9-10,12-13H,6-8,11,14-15H2,1-2H3,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437400

(CHEMBL2408643)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-2-[#6]-[#6]-[#7]-[#6]-[#6]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-14-28-20-21(26-23(28)33-18-9-12-25-13-10-18)27(3)24(32)29(22(20)31)15-19(30)17-7-5-4-6-8-17/h4-8,11,18,25H,9-10,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM100345
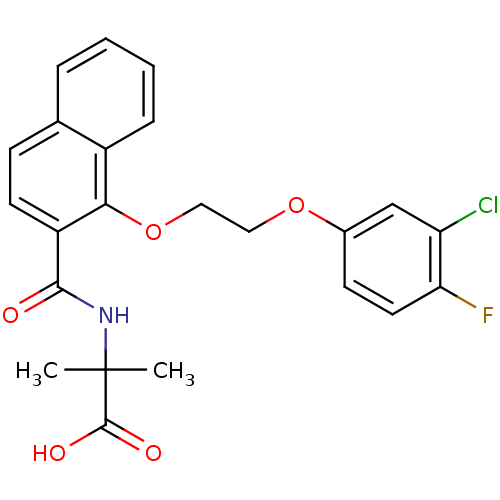
(US8501981, 73)Show SMILES CC(C)(NC(=O)c1ccc2ccccc2c1OCCOc1ccc(F)c(Cl)c1)C(O)=O Show InChI InChI=1S/C23H21ClFNO5/c1-23(2,22(28)29)26-21(27)17-9-7-14-5-3-4-6-16(14)20(17)31-12-11-30-15-8-10-19(25)18(24)13-15/h3-10,13H,11-12H2,1-2H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
CXCR2 inhibition using calcium fluorescence assay (FLIPR). |
US Patent US8501981 (2013)
BindingDB Entry DOI: 10.7270/Q24Q7SMB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150257
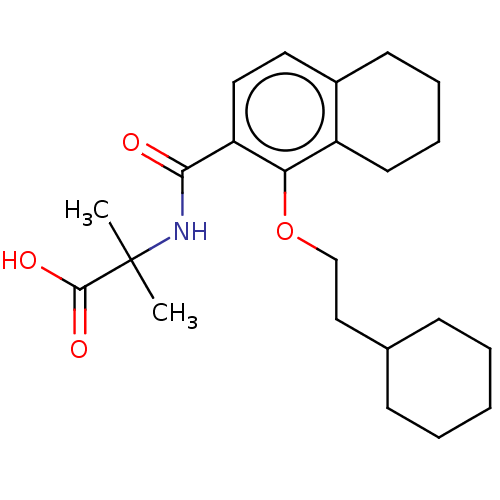
(US8980938, 2)Show SMILES CC(C)(NC(=O)c1ccc2CCCCc2c1OCCC1CCCCC1)C(O)=O Show InChI InChI=1S/C23H33NO4/c1-23(2,22(26)27)24-21(25)19-13-12-17-10-6-7-11-18(17)20(19)28-15-14-16-8-4-3-5-9-16/h12-13,16H,3-11,14-15H2,1-2H3,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM100347
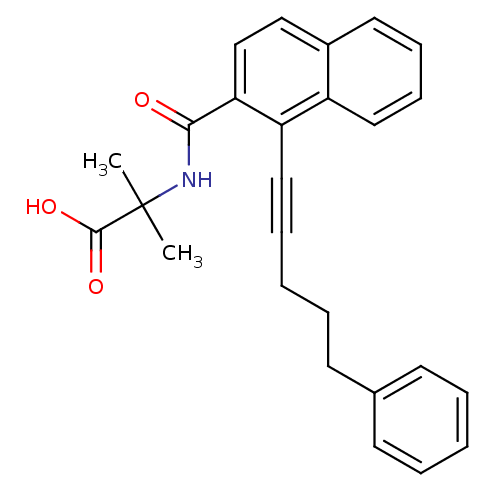
(US8501981, 166)Show SMILES CC(C)(NC(=O)c1ccc2ccccc2c1C#CCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C26H25NO3/c1-26(2,25(29)30)27-24(28)23-18-17-20-14-9-10-15-21(20)22(23)16-8-4-7-13-19-11-5-3-6-12-19/h3,5-6,9-12,14-15,17-18H,4,7,13H2,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
CXCR2 inhibition using calcium fluorescence assay (FLIPR). |
US Patent US8501981 (2013)
BindingDB Entry DOI: 10.7270/Q24Q7SMB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150263
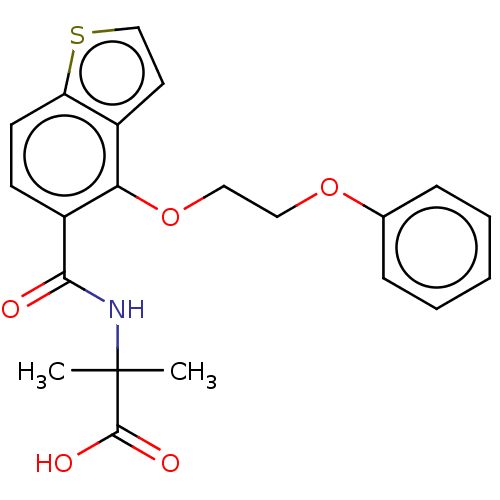
(US8980938, 12)Show SMILES CC(C)(NC(=O)c1ccc2sccc2c1OCCOc1ccccc1)C(O)=O Show InChI InChI=1S/C21H21NO5S/c1-21(2,20(24)25)22-19(23)16-8-9-17-15(10-13-28-17)18(16)27-12-11-26-14-6-4-3-5-7-14/h3-10,13H,11-12H2,1-2H3,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM100342
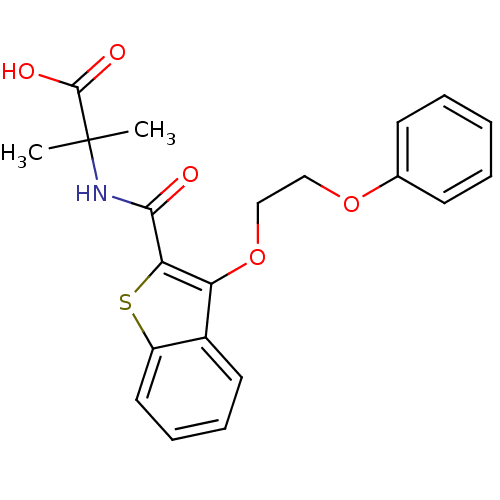
(US8501981, 46)Show SMILES CC(C)(NC(=O)c1sc2ccccc2c1OCCOc1ccccc1)C(O)=O Show InChI InChI=1S/C21H21NO5S/c1-21(2,20(24)25)22-19(23)18-17(15-10-6-7-11-16(15)28-18)27-13-12-26-14-8-4-3-5-9-14/h3-11H,12-13H2,1-2H3,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
CXCR2 inhibition using calcium fluorescence assay (FLIPR). |
US Patent US8501981 (2013)
BindingDB Entry DOI: 10.7270/Q24Q7SMB |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150259

(US8980938, 5)Show SMILES CC(C)(NC(=O)c1ccc2CCCCc2c1OCCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C25H31NO4/c1-25(2,24(28)29)26-23(27)21-16-15-19-13-6-7-14-20(19)22(21)30-17-9-8-12-18-10-4-3-5-11-18/h3-5,10-11,15-16H,6-9,12-14,17H2,1-2H3,(H,26,27)(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM150260

(US8980938, 7)Show SMILES CC(C)(NC(=O)c1ccc2CCCCc2c1OCCCOc1ccccc1)C(O)=O Show InChI InChI=1S/C24H29NO5/c1-24(2,23(27)28)25-22(26)20-14-13-17-9-6-7-12-19(17)21(20)30-16-8-15-29-18-10-4-3-5-11-18/h3-5,10-11,13-14H,6-9,12,15-16H2,1-2H3,(H,25,26)(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Sanofi
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). A l... |
US Patent US8980938 (2015)
BindingDB Entry DOI: 10.7270/Q2C53JMK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM103368
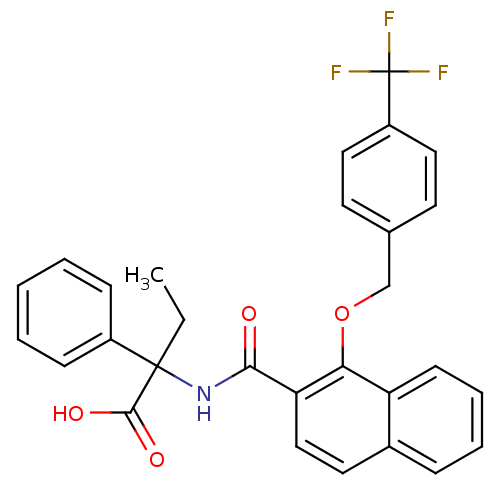
(US8552033, 10)Show SMILES CCC(NC(=O)c1ccc2ccccc2c1OCc1ccc(cc1)C(F)(F)F)(C(O)=O)c1ccccc1 Show InChI InChI=1S/C29H24F3NO4/c1-2-28(27(35)36,21-9-4-3-5-10-21)33-26(34)24-17-14-20-8-6-7-11-23(20)25(24)37-18-19-12-15-22(16-13-19)29(30,31)32/h3-17H,2,18H2,1H3,(H,33,34)(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI
US Patent
| Assay Description
The assay is based on the detection of intracellular calcium changes detected by the selective, calcium-chelating dye, Fluo-4 (Molecular Probes). |
US Patent US8552033 (2013)
BindingDB Entry DOI: 10.7270/Q2NC5ZTJ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50437397

(CHEMBL2408649)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]-[#6@H]-2-[#6]-[#6]-[#6]-[#7]-2)nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C24H29N5O4/c1-16(2)11-13-28-20-21(26-23(28)33-15-18-10-7-12-25-18)27(3)24(32)29(22(20)31)14-19(30)17-8-5-4-6-9-17/h4-6,8-9,11,18,25H,7,10,12-15H2,1-3H3/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of porcine DPP4 |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50437394

(CHEMBL2408639)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#8]-[#6]C([#6])([#6])[#7])nc2n(-[#6])c(=O)n(-[#6]-[#6](=O)-c3ccccc3)c(=O)c12 Show InChI InChI=1S/C23H29N5O4/c1-15(2)11-12-27-18-19(25-21(27)32-14-23(3,4)24)26(5)22(31)28(20(18)30)13-17(29)16-9-7-6-8-10-16/h6-11H,12-14,24H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate by fluorescence assay |
ACS Med Chem Lett 4: 768-72 (2013)
Article DOI: 10.1021/ml400171b
BindingDB Entry DOI: 10.7270/Q2HQ41BX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data