

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112574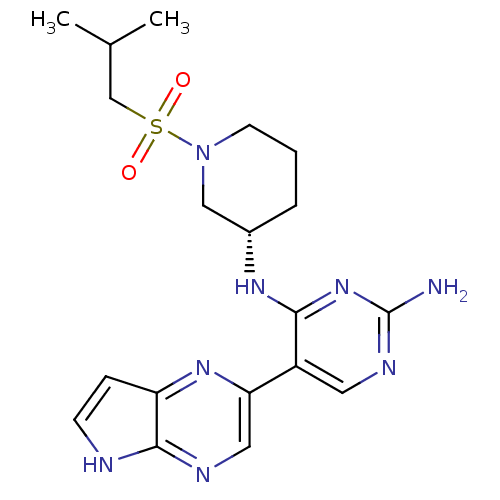 (US8618103, I-70) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000578 | n/a | 0.00115 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581548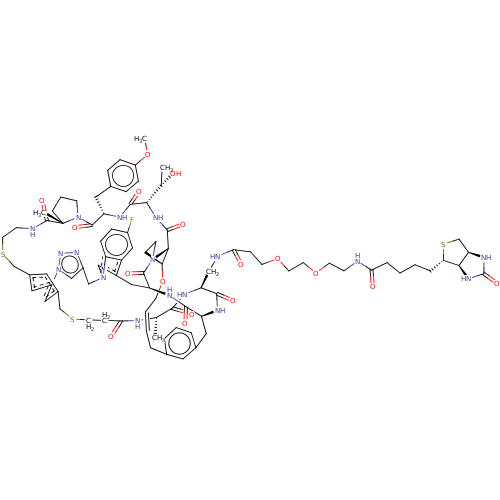 (CHEMBL5085124) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112546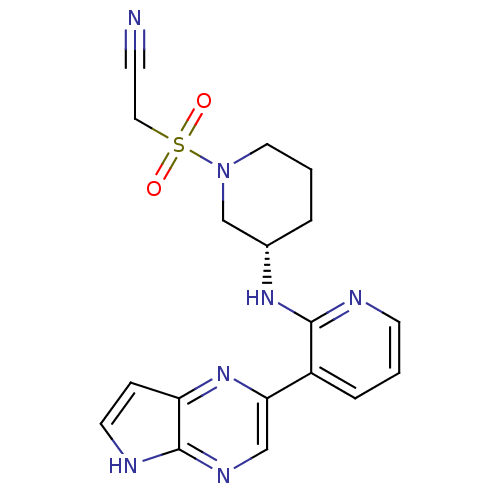 (US8618103, I-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000748 | n/a | 0.00149 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581547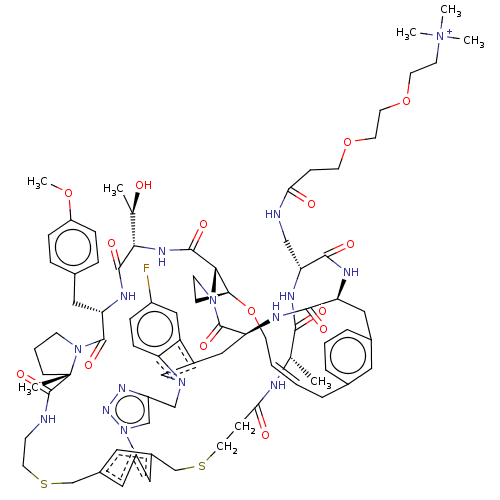 (CHEMBL5081349) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.000930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112576 (US8618103, I-72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000940 | n/a | 0.00188 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112573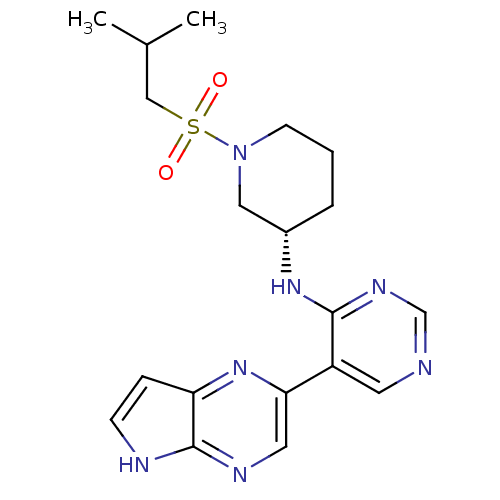 (US8618103, I-69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.000980 | n/a | 0.00196 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112601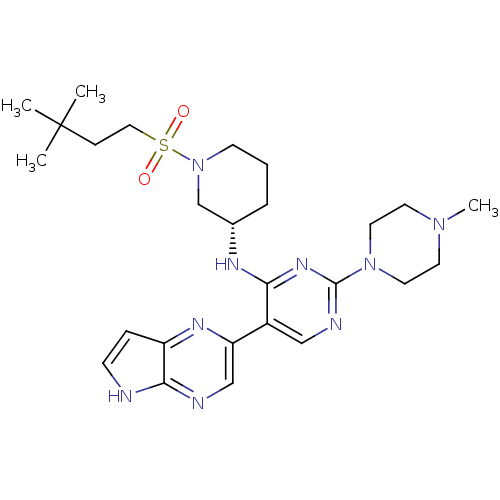 (US8618103, I-97) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00109 | n/a | 0.00218 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112536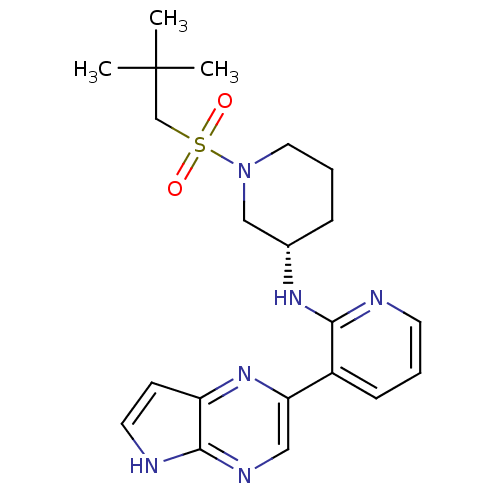 (US8618103, I-32) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00120 | n/a | 0.00240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112583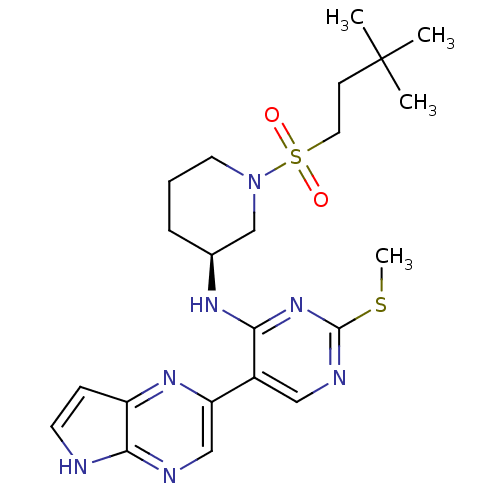 (US8618103, I-79) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00121 | n/a | 0.00242 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178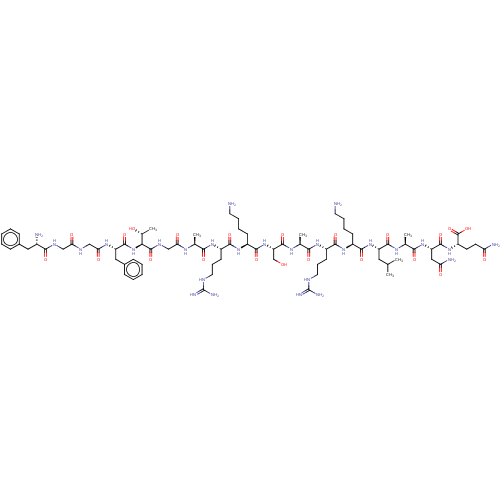 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in HEK293 cells by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402775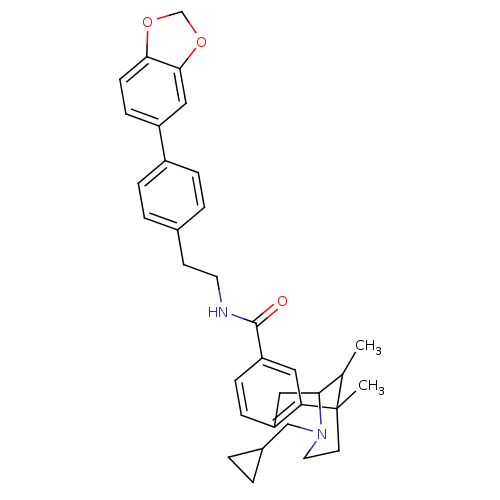 (CHEMBL2208351) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194104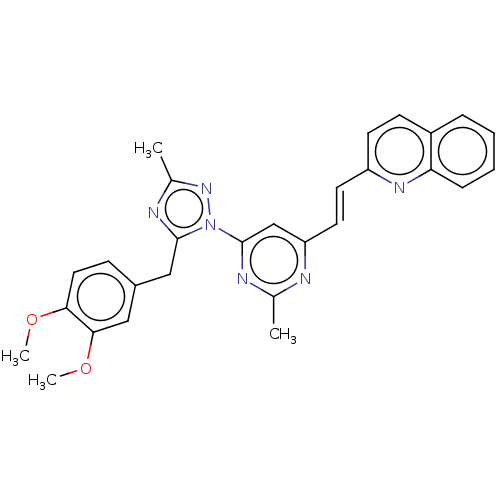 (US9200001, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194105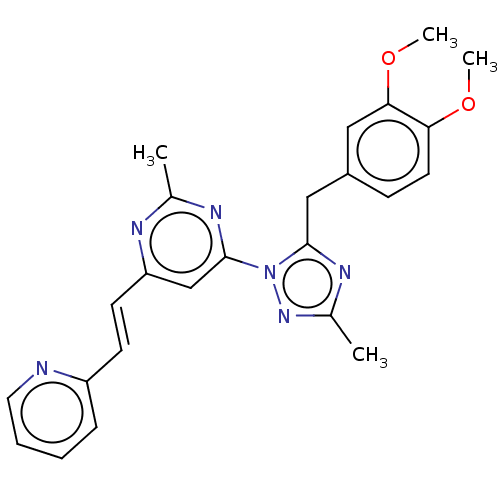 (US9200001, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112616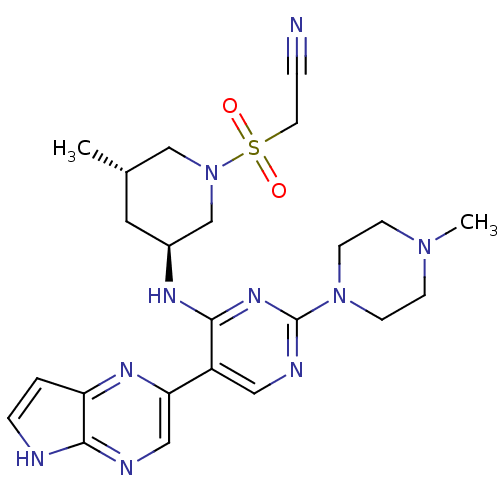 (US8618103, I-112) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00207 | n/a | 0.00415 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112568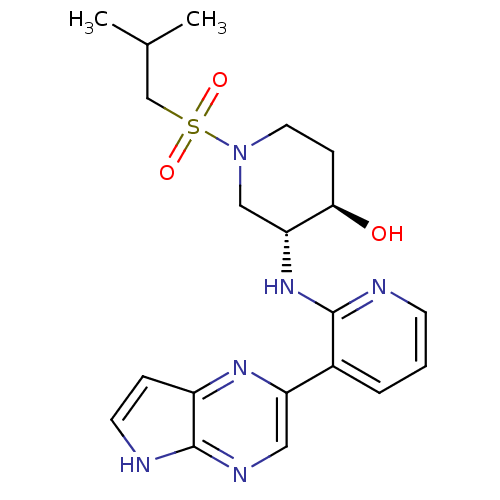 (US8618103, I-64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00210 | n/a | 0.00421 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 9 (Homo sapiens (Human)) | BDBM50581546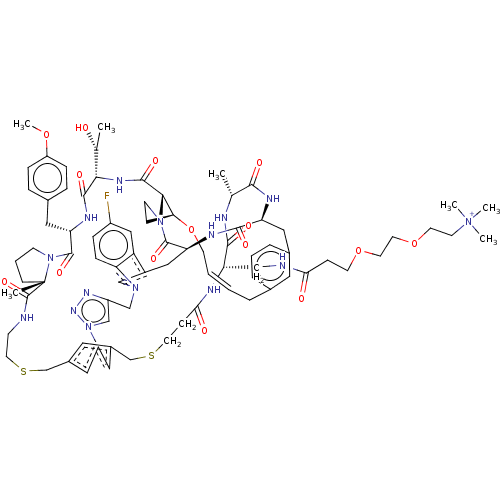 (CHEMBL5084416) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.00239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PCSK9 using Alexa Fluor 674 as substrate incubated for 2 hrs by FRET ultra assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01599 BindingDB Entry DOI: 10.7270/Q2TM7G0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112575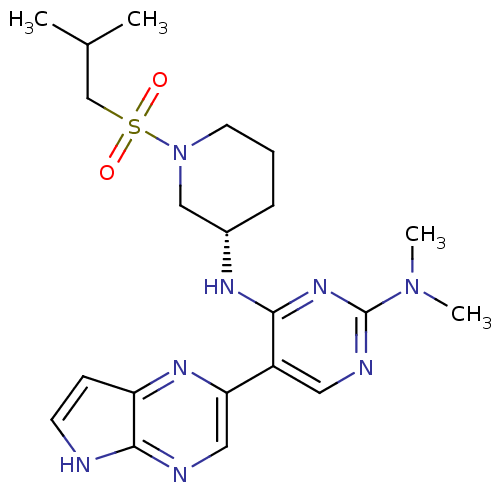 (US8618103, I-71) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00248 | n/a | 0.00496 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112622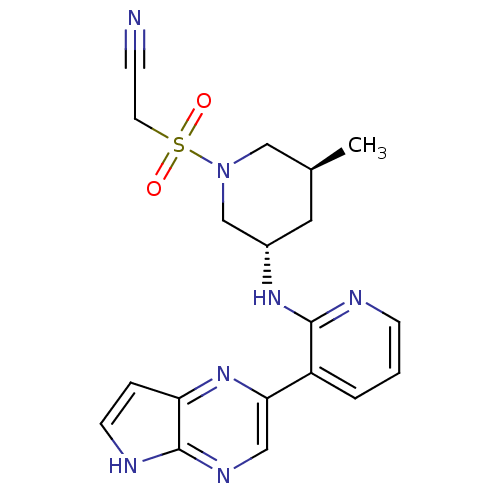 (US8618103, I-118) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00260 | n/a | 0.00521 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408679 (CHEMBL5287792) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112597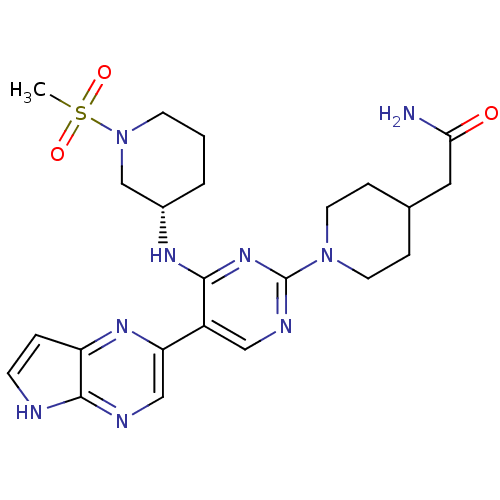 (US8618103, I-93) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00265 | n/a | 0.00530 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50007522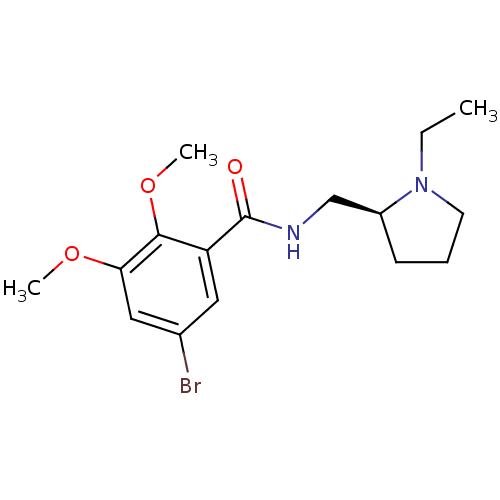 (5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet Curated by ChEMBL | Assay Description Binding affinity to dopamine D2 receptor (unknown origin) | Bioorg Med Chem 16: 6467-73 (2008) Article DOI: 10.1016/j.bmc.2008.05.039 BindingDB Entry DOI: 10.7270/Q2QN66JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards guinea pig Opioid receptor kappa 1 using radioligand [3H]U-69593 | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194113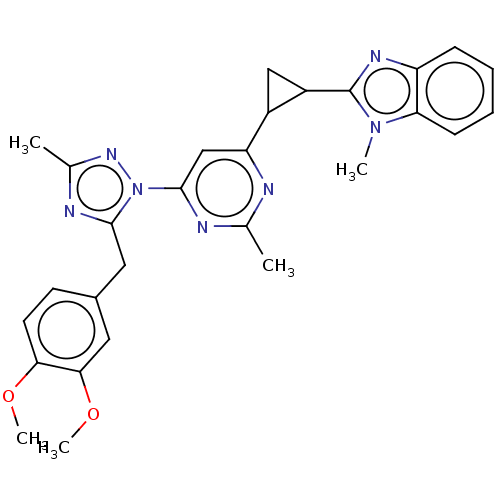 (US9200001, 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50366764 (CHEMBL1790045 | MCL-117) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112614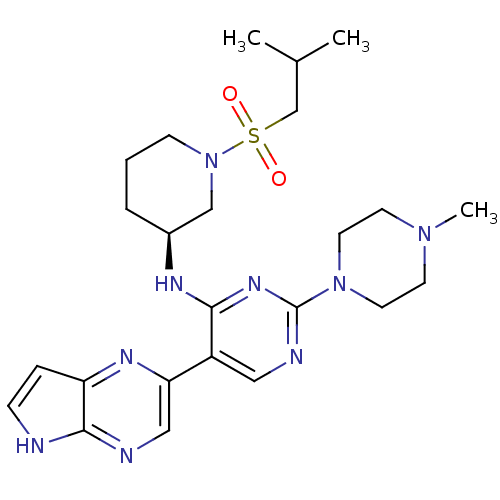 (US8618103, I-110) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00337 | n/a | 0.00675 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408509 (CHEMBL5277326) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at AT1 receptor in rat aortic rings | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112530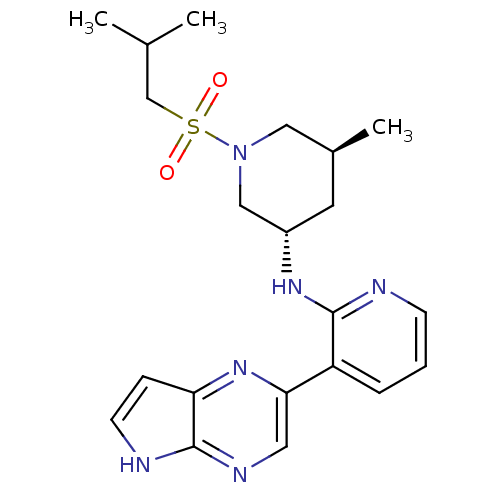 (US8618103, I-26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00344 | n/a | 0.00670 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112542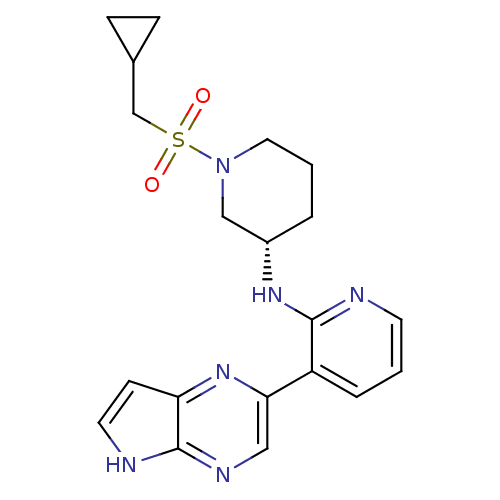 (US8618103, I-38) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00373 | n/a | 0.00747 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112569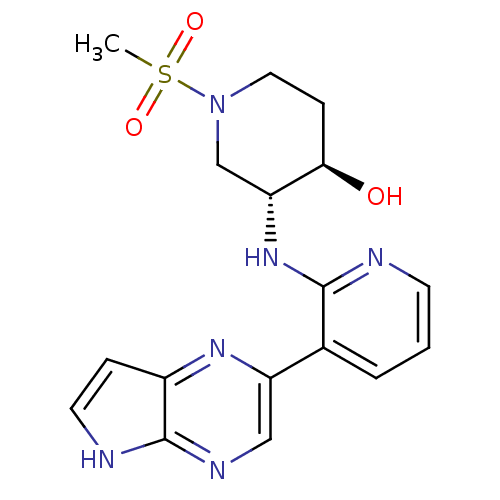 (US8618103, I-65) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00380 | n/a | 0.00762 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112567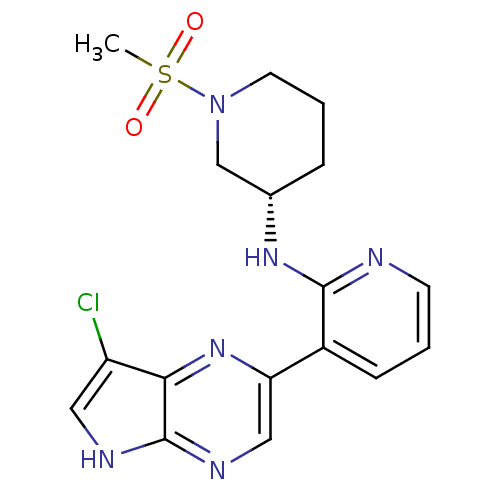 (US8618103, I-63) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00386 | n/a | 0.00773 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112529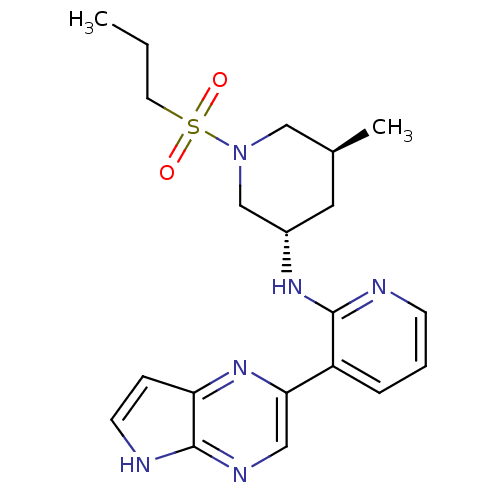 (US8618103, I-25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00398 | n/a | 0.00776 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194090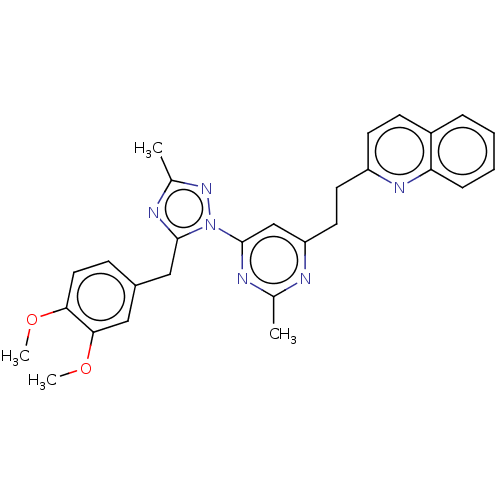 (US9200001, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112589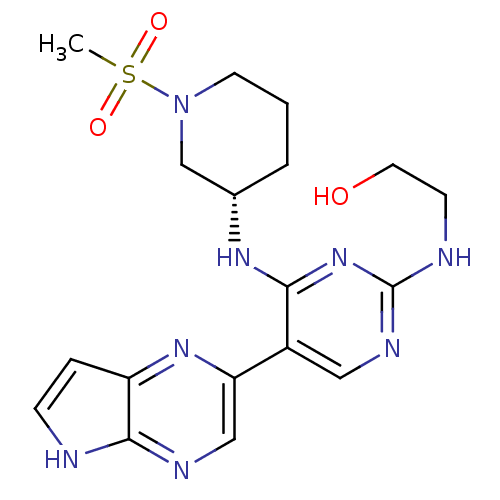 (US8618103, I-85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00402 | n/a | 0.00805 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112599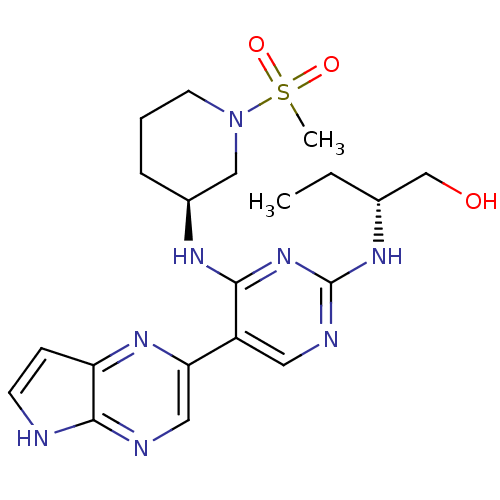 (US8618103, I-95) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00429 | n/a | 0.00858 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112506 (US8618103, I-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00429 | n/a | 0.00837 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112577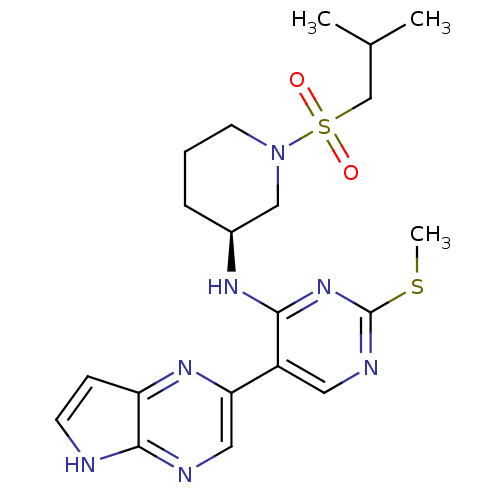 (US8618103, I-73) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00448 | n/a | 0.00897 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50451302 (CHEMBL2115245) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain membranes using [3H]DAMGO as radioligand | Bioorg Med Chem Lett 11: 2735-40 (2001) BindingDB Entry DOI: 10.7270/Q25T3M01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50214974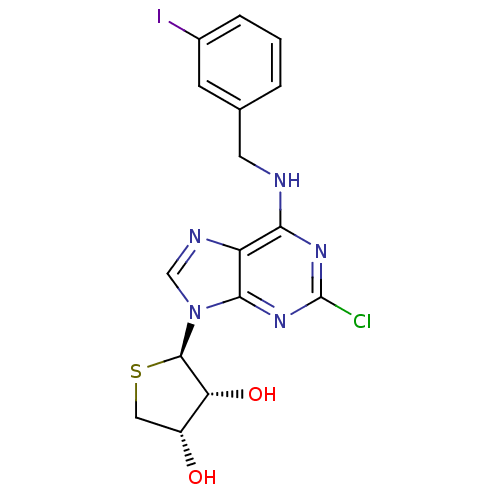 ((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402777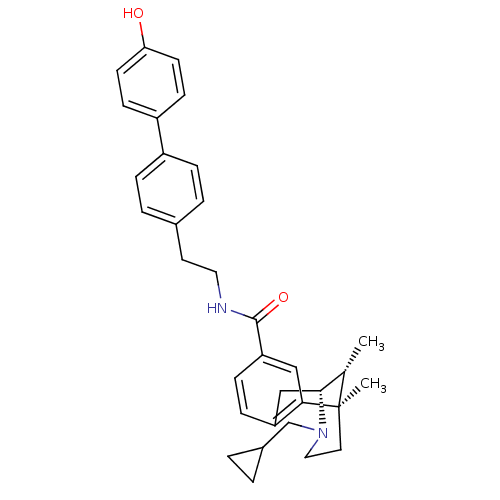 (CHEMBL2208347) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135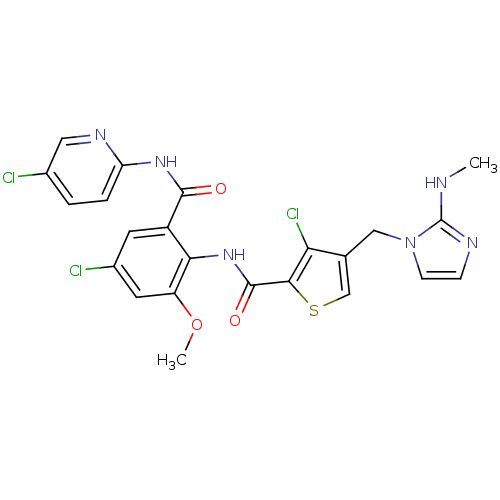 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50033531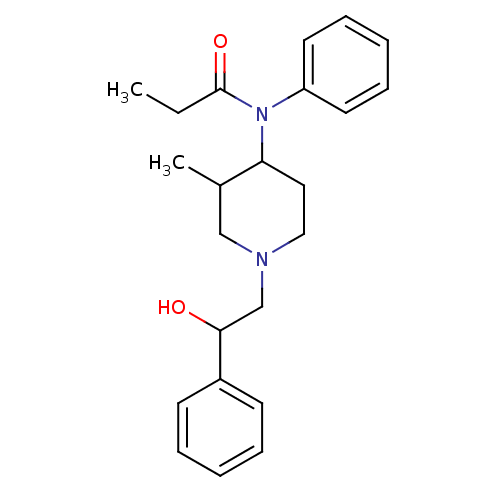 (CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. | J Med Chem 38: 3652-9 (1995) BindingDB Entry DOI: 10.7270/Q2BP01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194122 (US9200001, 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50033531 (CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. | J Med Chem 38: 3652-9 (1995) BindingDB Entry DOI: 10.7270/Q2BP01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194223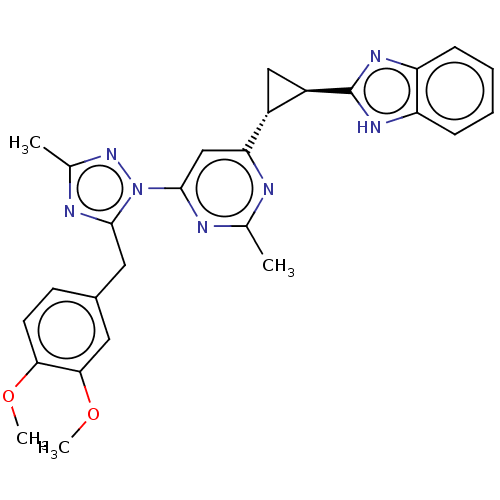 (US9200001, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112578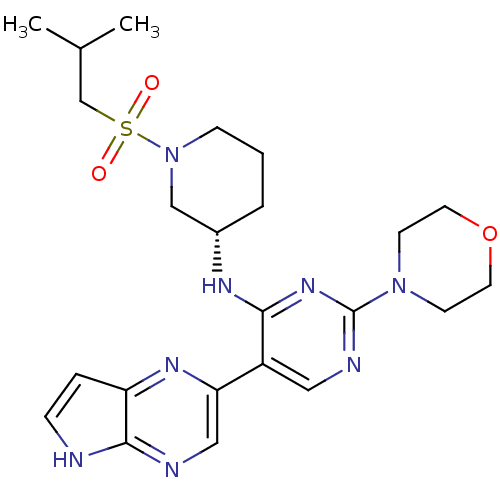 (US8618103, I-74) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00525 | n/a | 0.0105 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112590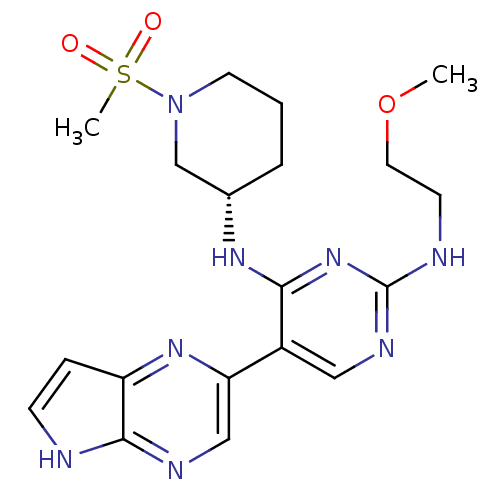 (US8618103, I-86) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00535 | n/a | 0.0107 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112509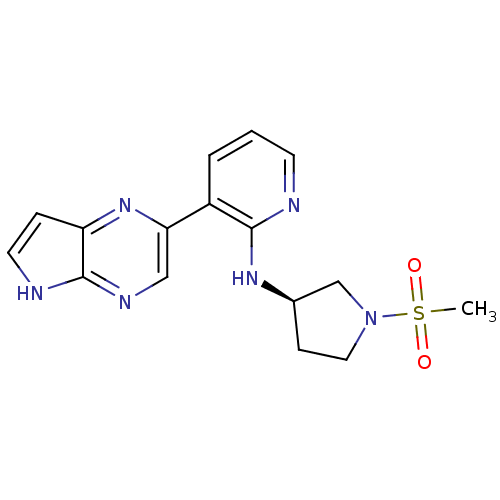 (US8618103, I-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00537 | n/a | 0.0105 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM112598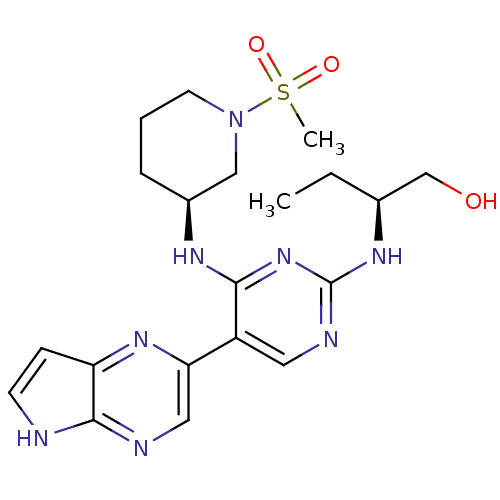 (US8618103, I-94) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00548 | n/a | 0.0110 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The kinase activity of all three JAK kinases is measured using a radioactive, end-point assay and with trace amounts of 33P-ATP. | US Patent US8618103 (2013) BindingDB Entry DOI: 10.7270/Q25H7DZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402776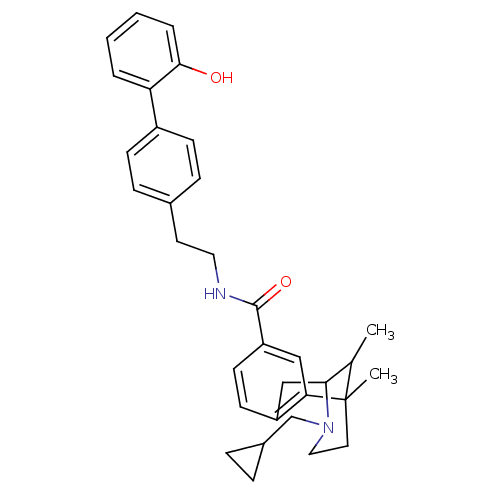 (CHEMBL2208349) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50402777 (CHEMBL2208347) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 7340-4 (2012) Article DOI: 10.1016/j.bmcl.2012.10.081 BindingDB Entry DOI: 10.7270/Q2N017Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218453 total ) | Next | Last >> |