

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50089269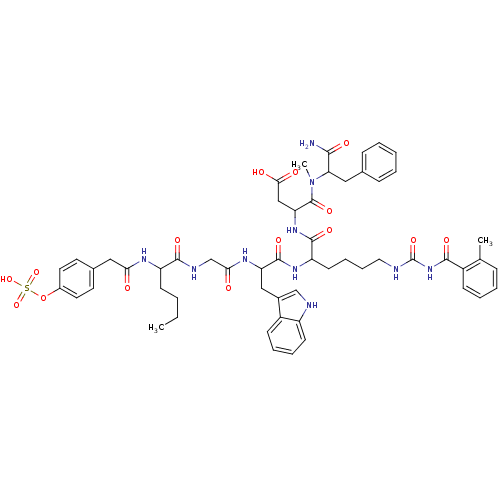 (CHEMBL276192 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828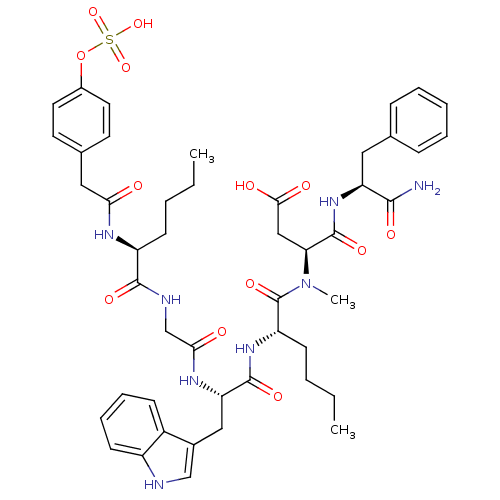 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061828 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061829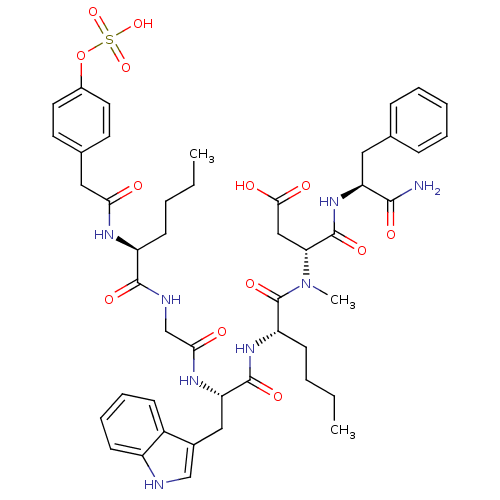 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-({(S)-2-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089268 (CHEMBL267861 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061832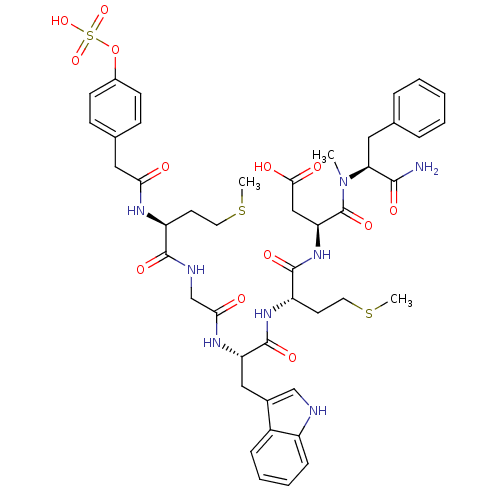 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061833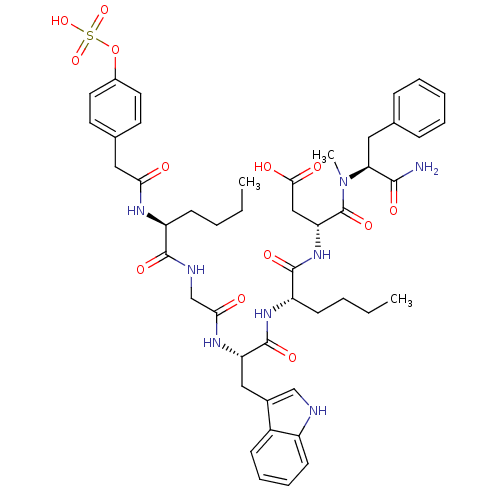 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369326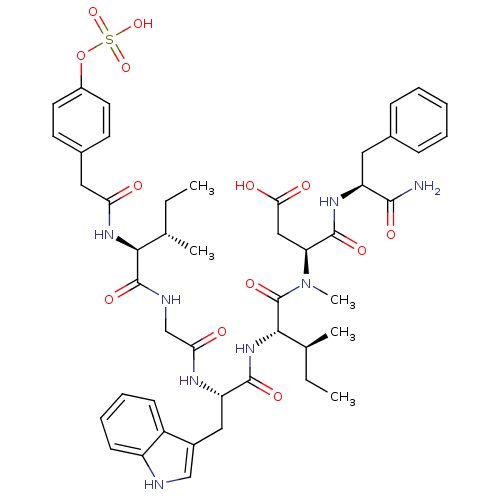 (CHEMBL1791002) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50369325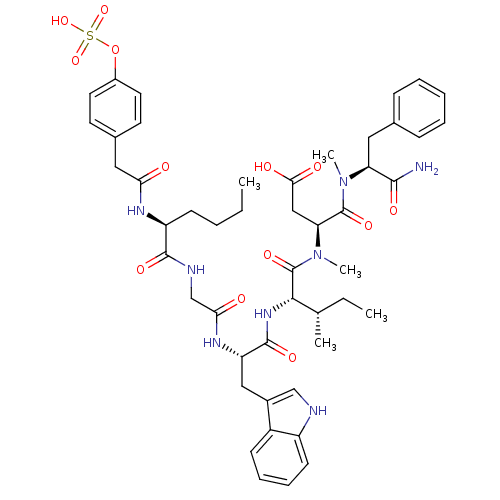 (CHEMBL1791005) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50241119 (7-(4-Biphenyl-3-ylmethyl-piperazin-1-yl)-3H-benzoo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089276 (CHEMBL430608 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061834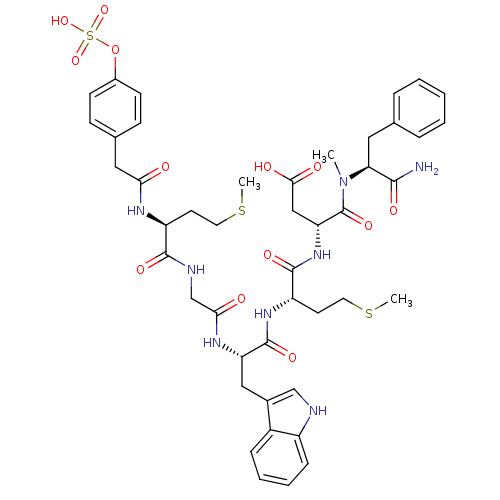 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061832 ((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089275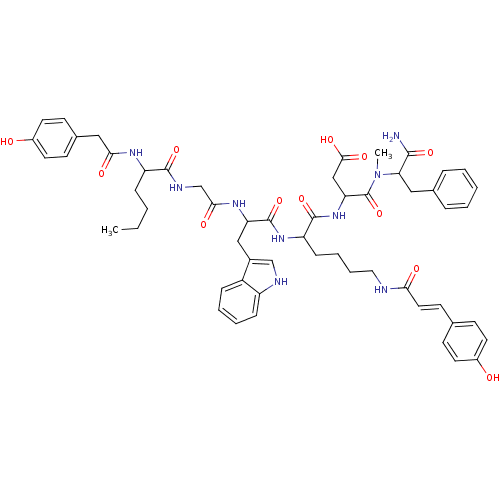 (CHEMBL384303 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089277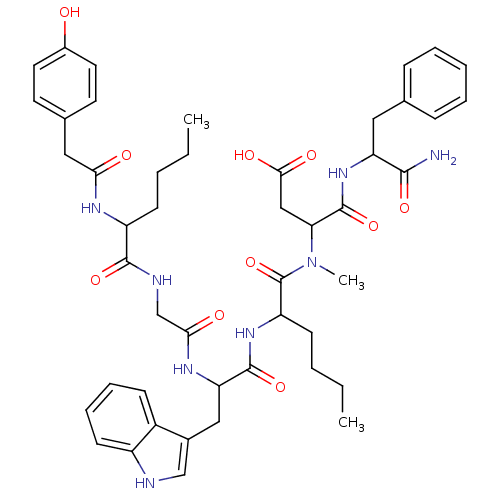 (CHEMBL311187 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-({...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426674 (CHEMBL2326472) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50061830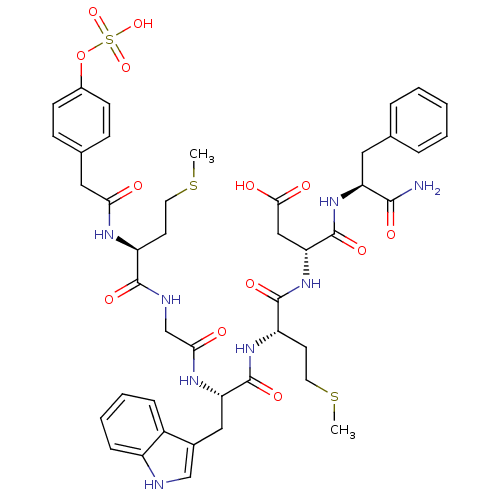 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissue | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089272 (CHEMBL312359 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426661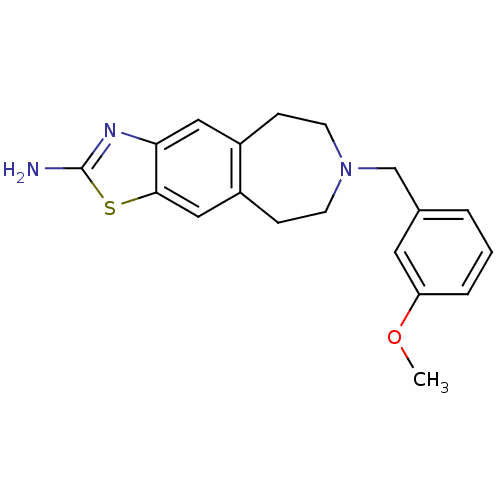 (CHEMBL2326480) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089275 (CHEMBL384303 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426679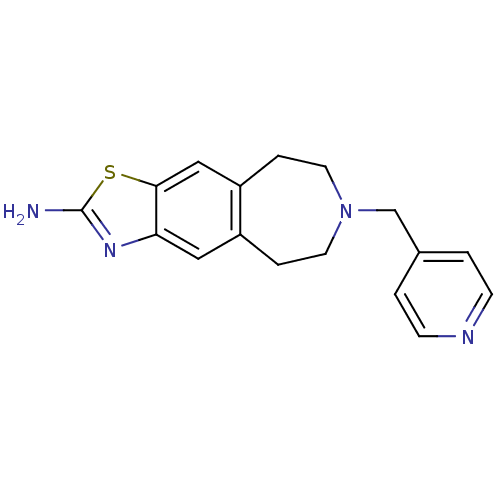 (CHEMBL2326485) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426659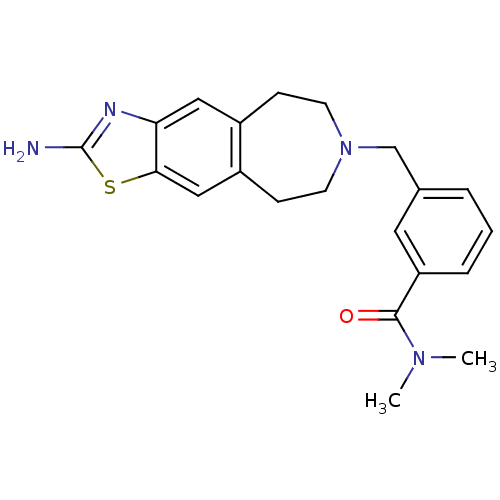 (CHEMBL2326482) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426660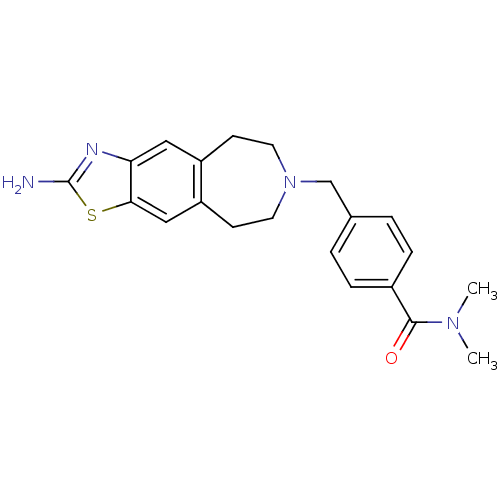 (CHEMBL2326481) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426663 (CHEMBL2326478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50089270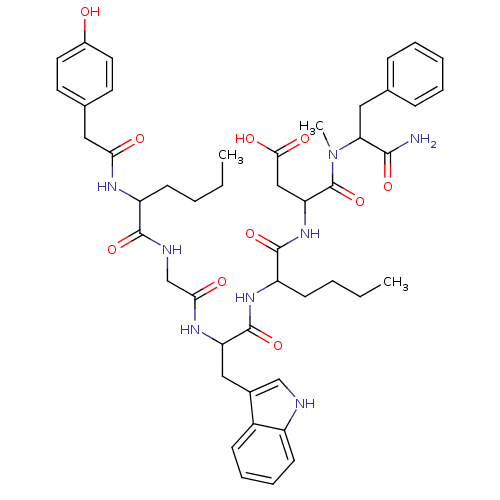 (CHEMBL308622 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissue | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426664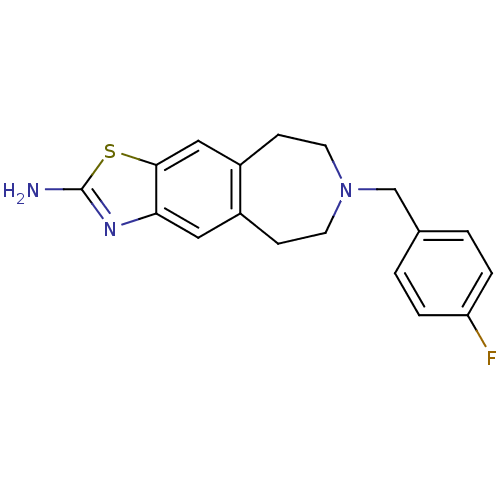 (CHEMBL2326477) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426665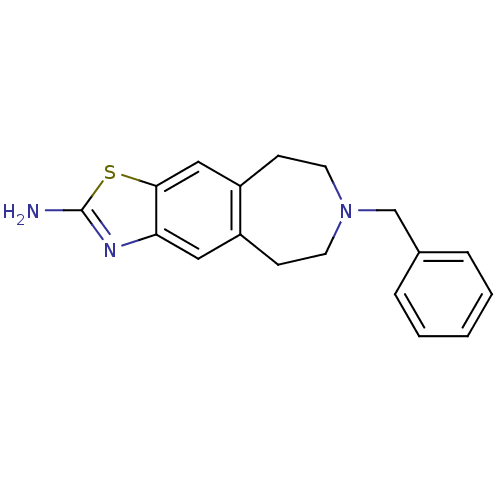 (CHEMBL2326476) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061834 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089276 (CHEMBL430608 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426662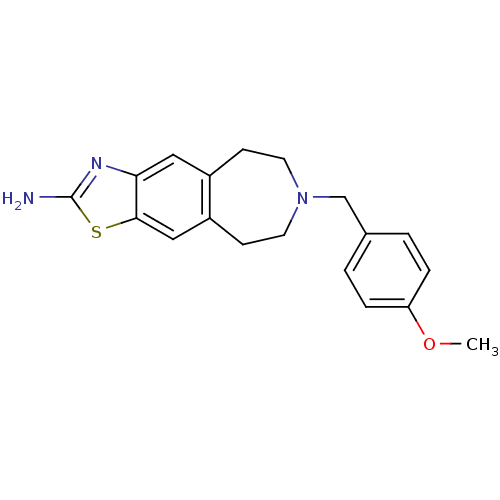 (CHEMBL2326479) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089269 (CHEMBL276192 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089272 (CHEMBL312359 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061830 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426682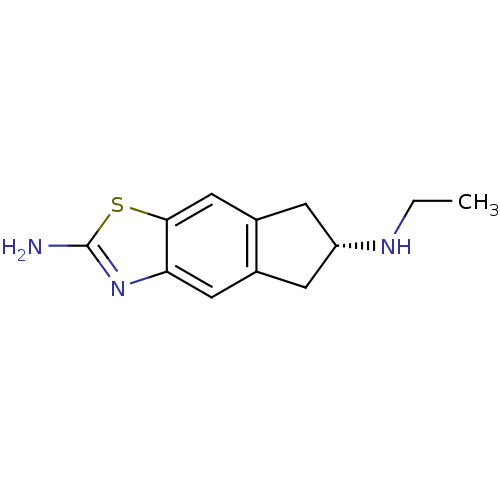 (CHEMBL2326486) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426680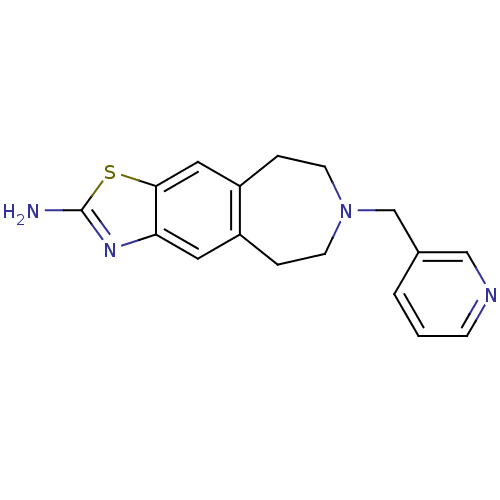 (CHEMBL2326484) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089268 (CHEMBL267861 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061828 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061828 (CHEMBL76248 | CID44356929 | N-(1-Carbamoyl-2-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426678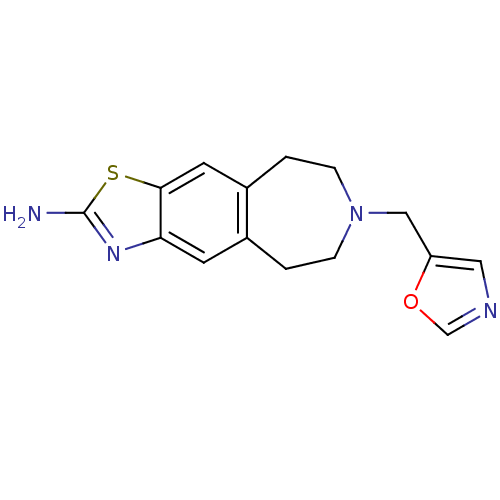 (CHEMBL2326468) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426673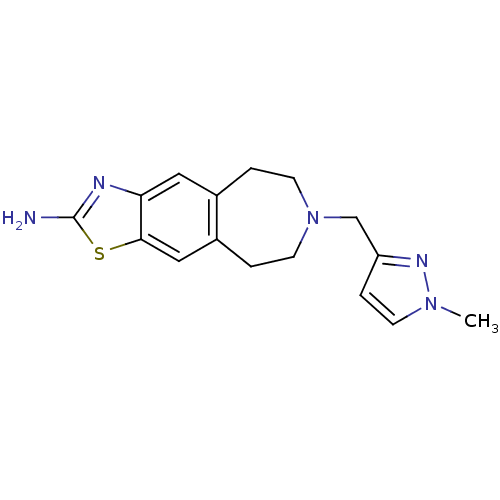 (CHEMBL2326473) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50061833 ((R)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester Curated by ChEMBL | Assay Description Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes | J Med Chem 40: 4302-7 (1998) Article DOI: 10.1021/jm970477u BindingDB Entry DOI: 10.7270/Q2B858TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426677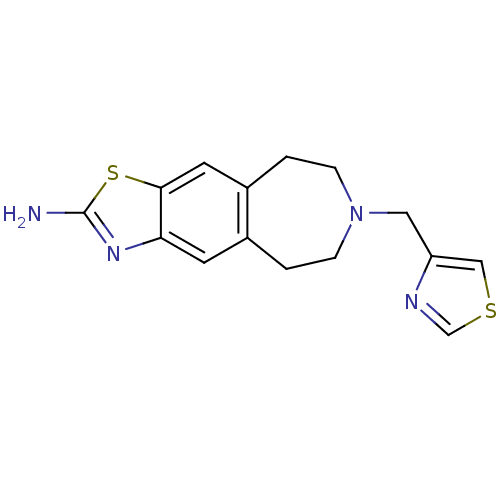 (CHEMBL2326469) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426676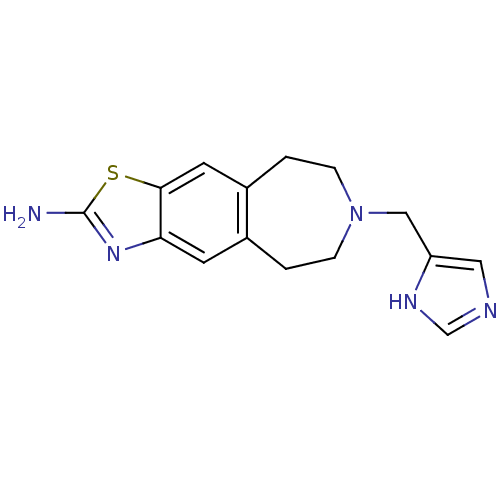 (CHEMBL2326470) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50426667 (CHEMBL2326474) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from human D2 dopamine receptor expressed in CHO cell membranes | Bioorg Med Chem Lett 23: 543-7 (2012) Article DOI: 10.1016/j.bmcl.2012.11.023 BindingDB Entry DOI: 10.7270/Q2639R2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50089277 (CHEMBL311187 | N-(1-Carbamoyl-2-phenyl-ethyl)-3-({...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand | J Med Chem 43: 2350-5 (2000) BindingDB Entry DOI: 10.7270/Q2FX7B4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 114 total ) | Next | Last >> |