

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309834 ((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309848 ((R)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309847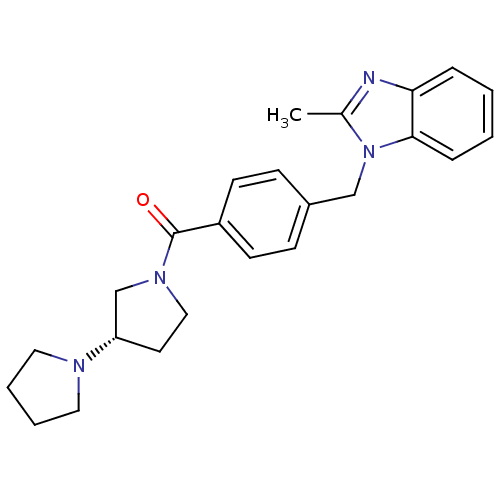 ((S)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309837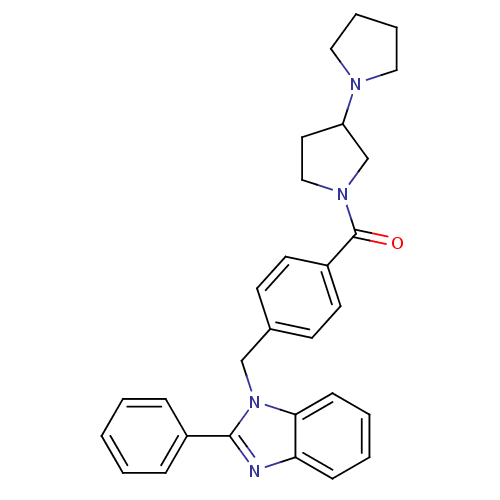 (1,3'-bipyrrolidin-1'-yl(4-((2-phenyl-1H-benzo[d]im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309845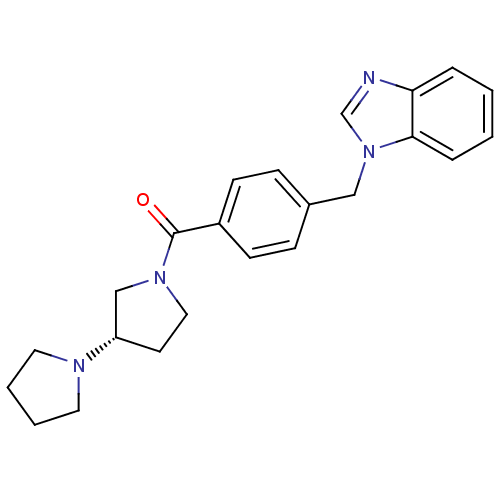 ((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309851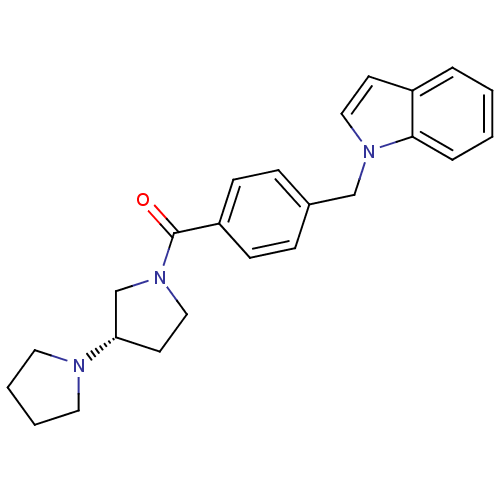 ((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-indol-1-yl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309843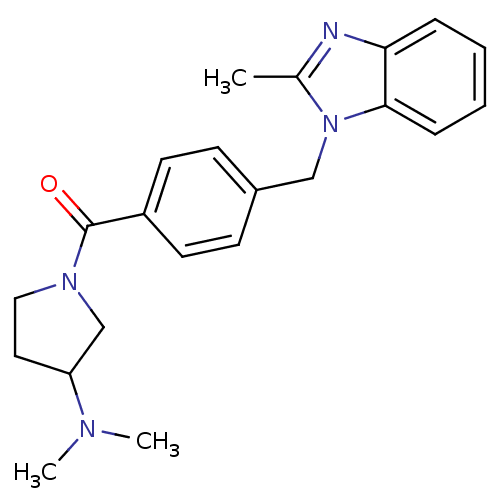 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-methyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50309834 ((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to rat histamine H3 receptor | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309844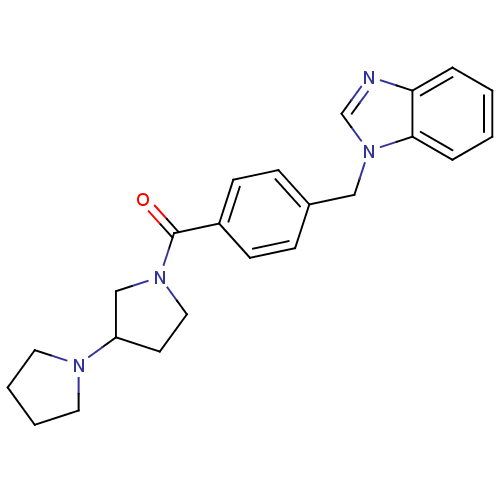 (1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309846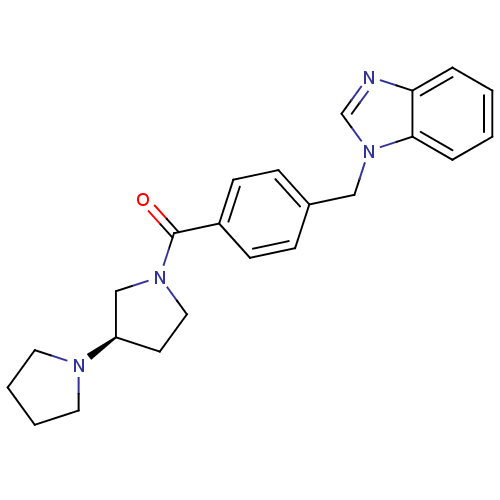 ((R)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309839 ((S)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309836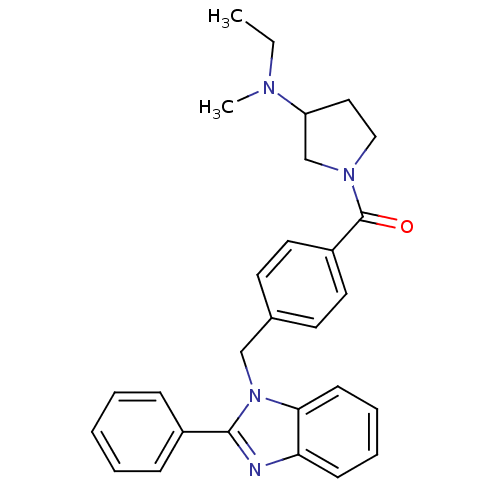 ((3-(ethyl(methyl)amino)pyrrolidin-1-yl)(4-((2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309835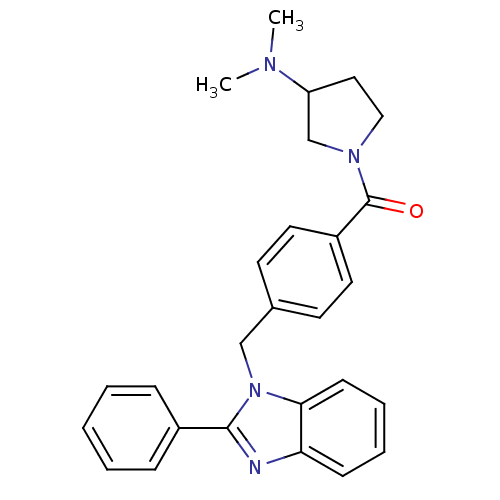 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-phenyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309853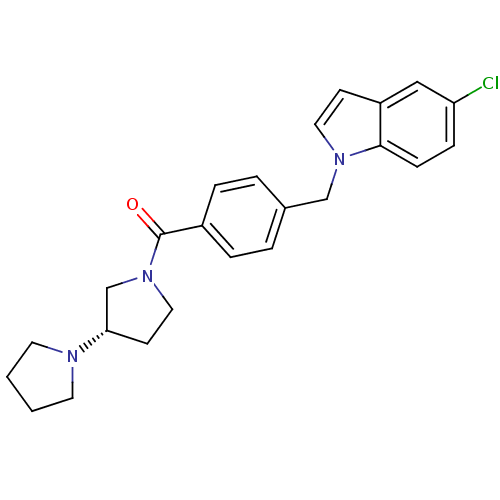 ((S)-1,3'-bipyrrolidin-1'-yl(4-((5-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309852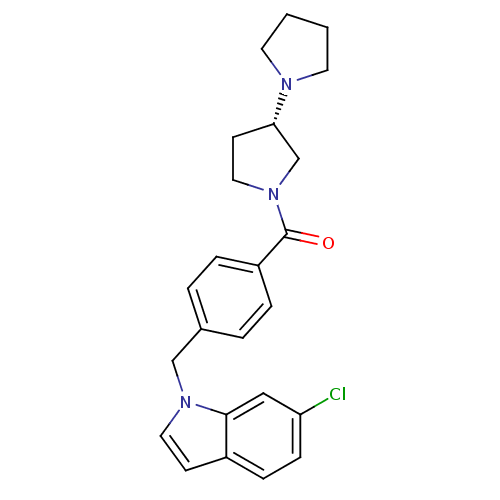 ((S)-1,3'-bipyrrolidin-1'-yl(4-((6-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309841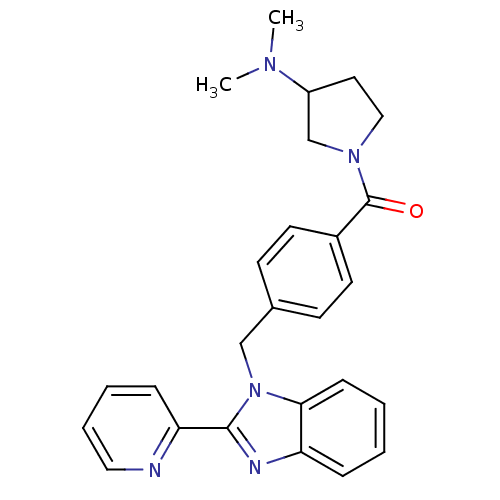 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-(pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309842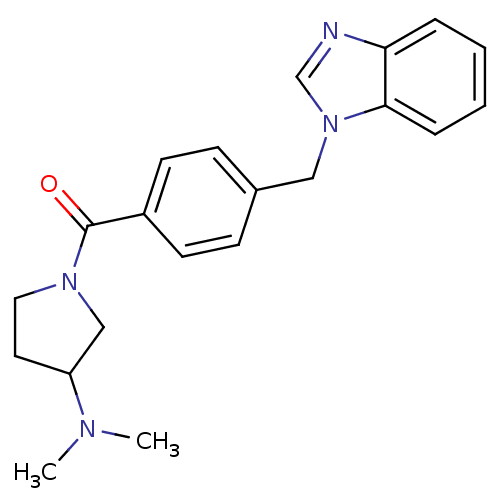 ((4-((1H-benzo[d]imidazol-1-yl)methyl)phenyl)(3-(di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309849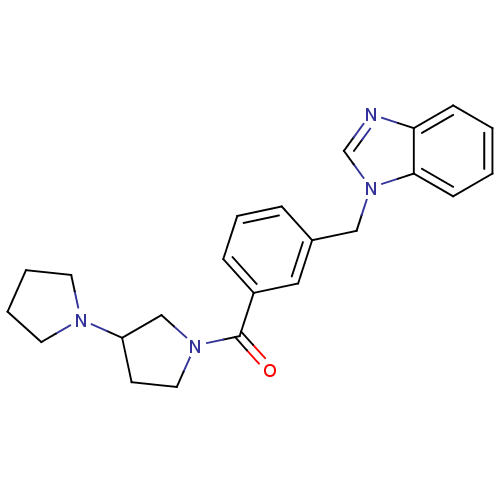 (1,3'-bipyrrolidin-1'-yl(3-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309840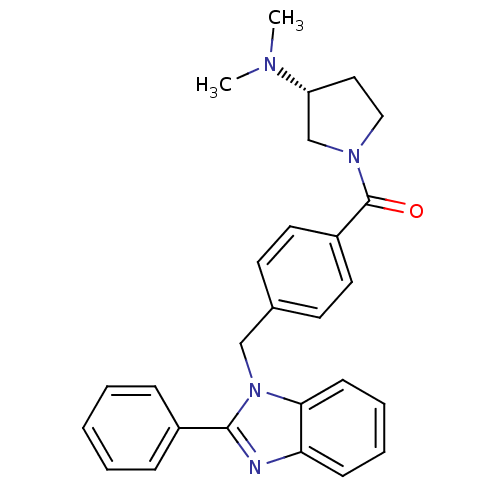 ((R)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309838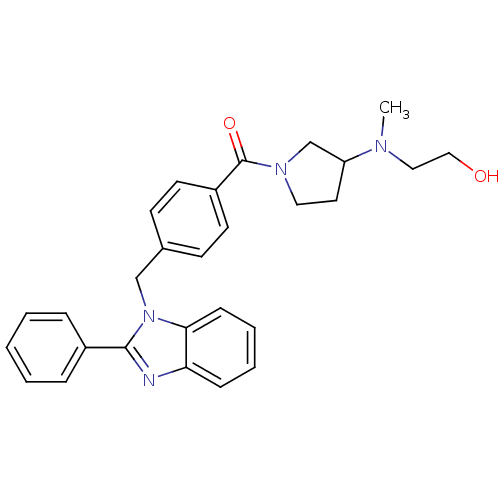 ((3-((2-hydroxyethyl)(methyl)amino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309850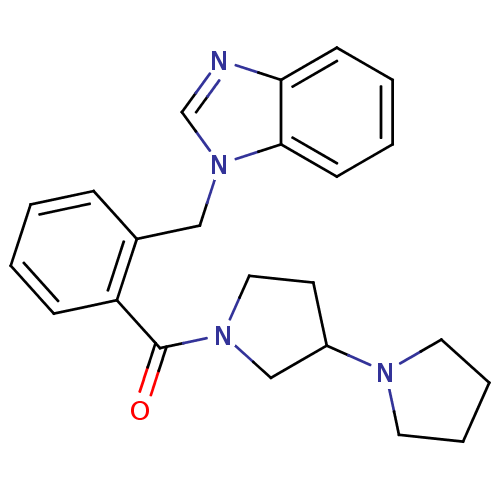 (1,3'-bipyrrolidin-1'-yl(2-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317169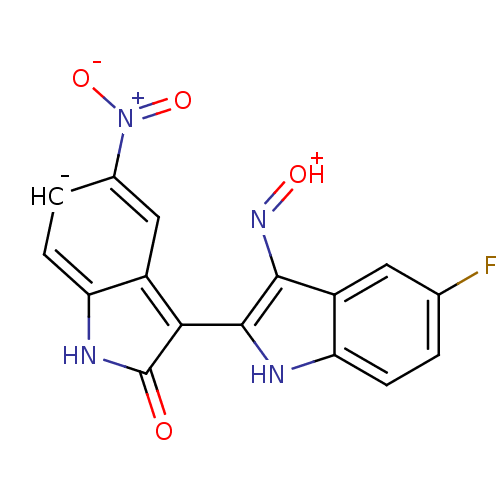 ((2'Z,3'E)-5-Nitro-5'-fluoro-indirubin-3'-oxime | C...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317171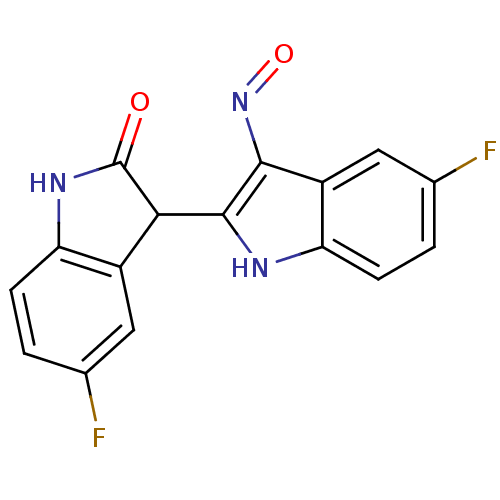 ((2'Z,3'E)-5-Fluoro-5'-fluoro-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317161 ((2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317163 ((2'Z,3'E)-5-Fluoro-5'-hydroxy-indirubin-3'-oxime |...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317162 ((2'Z,3'E)-5-Chloro-5'-hydroxy-indirubin-3'-oxime |...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317177 (5-nitroindirubin-3'-oxime | CHEMBL369303) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317170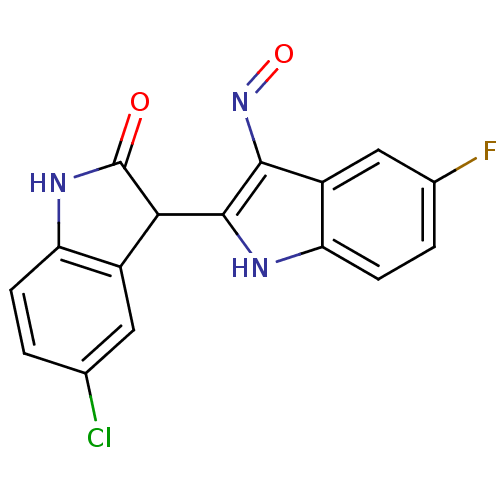 ((2'Z,3'E)-5-Chloro-5'-fluoro-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317164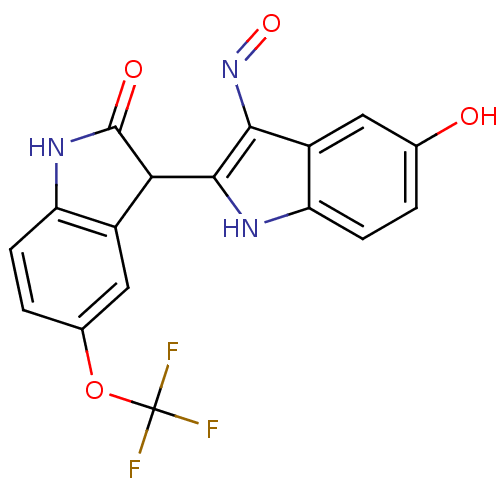 ((2'Z,3'E)-5-Trifluoromethoxy-5'-hydroxy-indirubin-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317173 ((2'Z,3'E)-5-Nitro-5'-methyl-indirubin-3'-oxime | C...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317167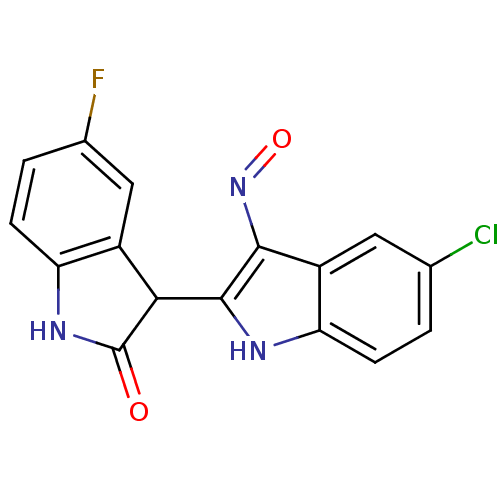 ((2'Z,3'E)-5-Fluoro-5'-chloro-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317166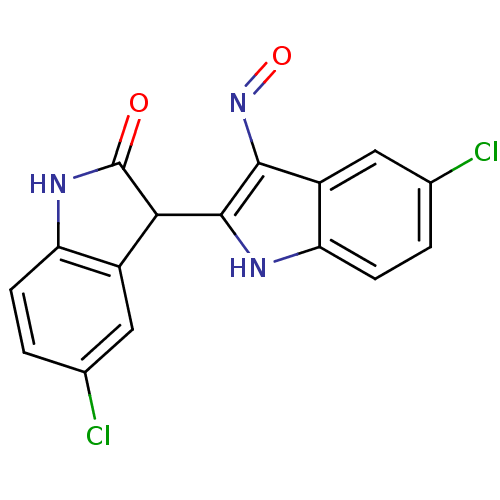 ((2'Z,3'E)-5-Chloro-5'-chloro-indirubin-3'-oxime | ...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453246 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-5,7-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM50317161 ((2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK1/cyclin B after 40 mins by liquid scintillation counting | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317165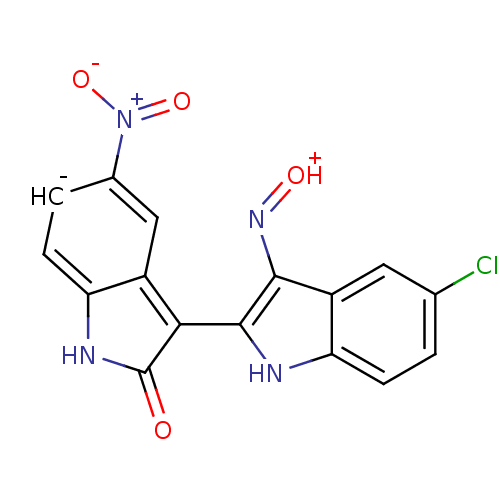 ((2'Z,3'E)-5-Nitro-5'-chloro-indirubin-3'-oxime | C...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453265 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-7,8-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453264 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-7,8-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM453265 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-7,8-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM453246 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-5,7-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453228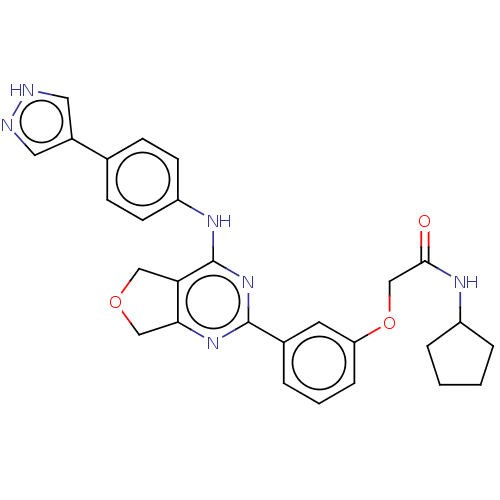 (US10729691, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM453264 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-7,8-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50514308 (CHEMBL4448899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM50538375 (CHEMBL4633651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317172 ((2'Z,3'E)-5-Trifluoromethoxy-5'-fluoro-indirubin-3...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453227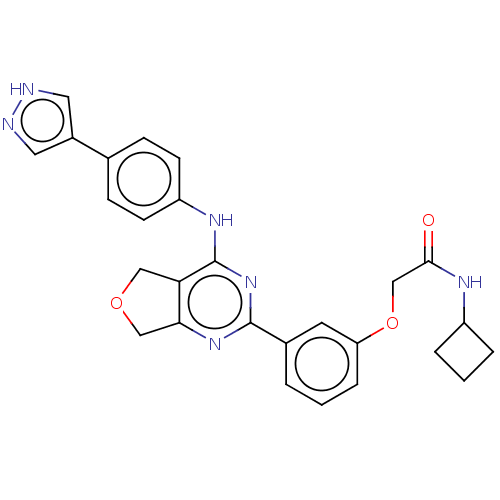 (US10729691, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50538383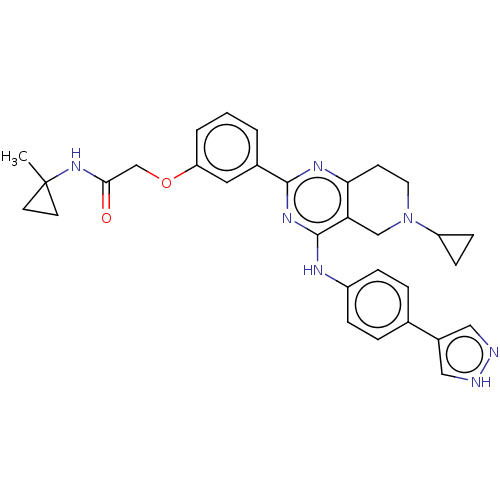 (CHEMBL4645036) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM50317168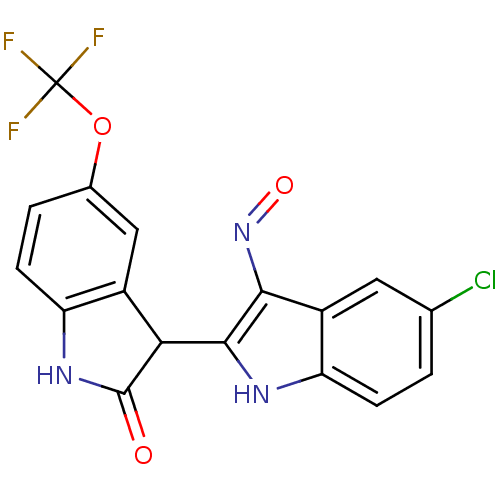 ((2'Z,3'E)-5-Trifluoromethoxy-5'-chloro-indirubin-3...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin E | J Med Chem 53: 3696-706 (2010) Article DOI: 10.1021/jm100080z BindingDB Entry DOI: 10.7270/Q2X92BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 3 (Homo sapiens (Human)) | BDBM453217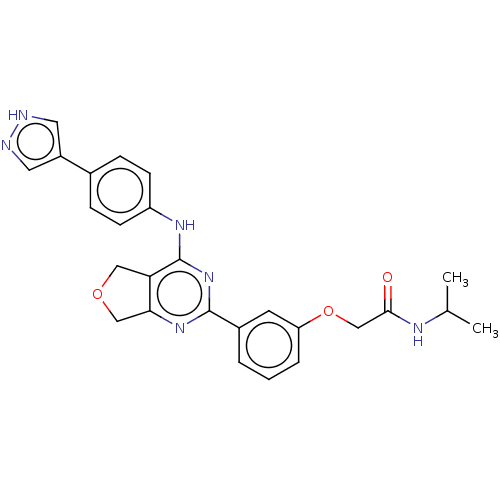 (2-(3-(4-((4-(1H-pyrazol-4-yl)phenyl)amino)-5,7-dih...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT3 in SLC2A1-deficient human DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM453228 (US10729691, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 2, facilitated glucose transporter member 1 (Homo sapiens (Human)) | BDBM50538382 (CHEMBL4649311) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Kadmon Corporation, LLC. Curated by ChEMBL | Assay Description Inhibition of GLUT1 in human wild-type DLD1 cells assessed as reduction in ATP level measured after 90 mins by Celltiter-glo luminescent assay | J Med Chem 63: 5201-5211 (2020) Article DOI: 10.1021/acs.jmedchem.9b02153 BindingDB Entry DOI: 10.7270/Q2GH9NGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |