 Found 479 hits with Last Name = 'gohda' and Initial = 'k'
Found 479 hits with Last Name = 'gohda' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasminogen
(Homo sapiens (Human)) | BDBM50138836
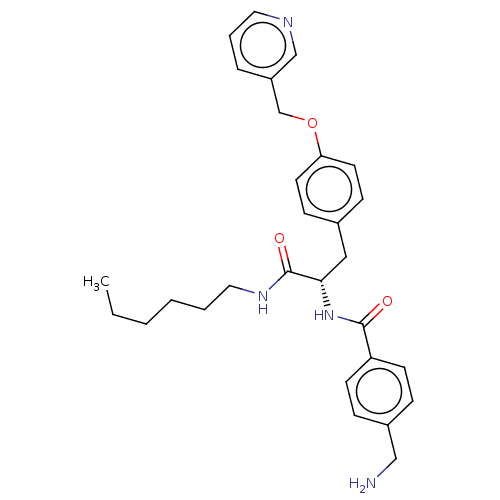
(CHEMBL3754193)Show SMILES CCCCCCNC(=O)[C@H](Cc1ccc(OCc2cccnc2)cc1)NC(=O)c1ccc(CN)cc1 |r| Show InChI InChI=1S/C29H36N4O3/c1-2-3-4-5-17-32-29(35)27(33-28(34)25-12-8-23(19-30)9-13-25)18-22-10-14-26(15-11-22)36-21-24-7-6-16-31-20-24/h6-16,20,27H,2-5,17-19,21,30H2,1H3,(H,32,35)(H,33,34)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin assessed as inhibition of amidolysis in presence of fibrinogen |
Bioorg Med Chem 24: 545-53 (2016)
Article DOI: 10.1016/j.bmc.2015.12.009
BindingDB Entry DOI: 10.7270/Q29888VQ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50428067

(CL-65336 | Cyklokapron | Lysteda | TRANEXAMIC ACID...)Show SMILES NC[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:5.8,wD:2.1,(1.2,4.21,;-.14,3.44,;-.14,1.9,;-1.47,1.13,;-1.47,-.41,;-.14,-1.18,;1.2,-.41,;1.2,1.13,;-.14,-2.72,;-1.47,-3.49,;1.2,-3.49,)| Show InChI InChI=1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| DrugBank
MMDB
PDB
Article
PubMed
| 4.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin assessed as inhibition of amidolysis in presence of fibrinogen |
Bioorg Med Chem 24: 545-53 (2016)
Article DOI: 10.1016/j.bmc.2015.12.009
BindingDB Entry DOI: 10.7270/Q29888VQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510491
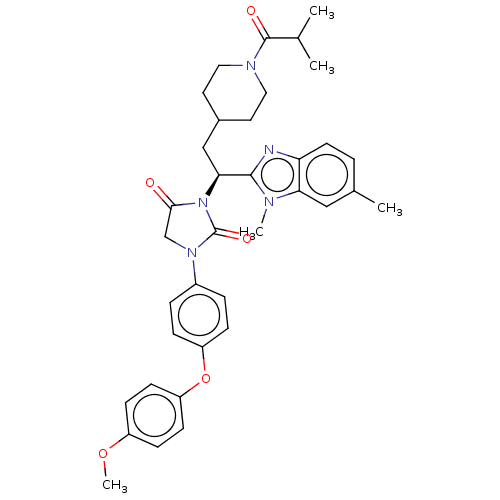
(CHEMBL4552760)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(C)cc4n3C)C2=O)cc1 |r| Show InChI InChI=1S/C36H41N5O5/c1-23(2)35(43)39-18-16-25(17-19-39)21-32(34-37-30-15-6-24(3)20-31(30)38(34)4)41-33(42)22-40(36(41)44)26-7-9-28(10-8-26)46-29-13-11-27(45-5)12-14-29/h6-15,20,23,25,32H,16-19,21-22H2,1-5H3/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50552997

(CHEMBL4797745)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(F)cc4n3C)C2=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286734

(CHEMBL4172988)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccccc3n2C)CC1 |r| Show InChI InChI=1S/C34H37N5O4/c1-23(2)33(41)37-19-17-24(18-20-37)21-30(32-35-28-11-7-8-12-29(28)36(32)3)39-31(40)22-38(34(39)42)25-13-15-27(16-14-25)43-26-9-5-4-6-10-26/h4-16,23-24,30H,17-22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510491

(CHEMBL4552760)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(C)cc4n3C)C2=O)cc1 |r| Show InChI InChI=1S/C36H41N5O5/c1-23(2)35(43)39-18-16-25(17-19-39)21-32(34-37-30-15-6-24(3)20-31(30)38(34)4)41-33(42)22-40(36(41)44)26-7-9-28(10-8-26)46-29-13-11-27(45-5)12-14-29/h6-15,20,23,25,32H,16-19,21-22H2,1-5H3/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286760

(CHEMBL4170032)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccc(C)cc3n2C)CC1 |r| Show InChI InChI=1S/C35H39N5O4/c1-23(2)34(42)38-18-16-25(17-19-38)21-31(33-36-29-15-10-24(3)20-30(29)37(33)4)40-32(41)22-39(35(40)43)26-11-13-28(14-12-26)44-27-8-6-5-7-9-27/h5-15,20,23,25,31H,16-19,21-22H2,1-4H3/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286760

(CHEMBL4170032)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccc(C)cc3n2C)CC1 |r| Show InChI InChI=1S/C35H39N5O4/c1-23(2)34(42)38-18-16-25(17-19-38)21-31(33-36-29-15-10-24(3)20-30(29)37(33)4)40-32(41)22-39(35(40)43)26-11-13-28(14-12-26)44-27-8-6-5-7-9-27/h5-15,20,23,25,31H,16-19,21-22H2,1-4H3/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510487

(CHEMBL4569266)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3cc(C)ccc3n2C)CC1 |r| Show InChI InChI=1S/C35H39N5O4/c1-23(2)34(42)38-18-16-25(17-19-38)21-31(33-36-29-20-24(3)10-15-30(29)37(33)4)40-32(41)22-39(35(40)43)26-11-13-28(14-12-26)44-27-8-6-5-7-9-27/h5-15,20,23,25,31H,16-19,21-22H2,1-4H3/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286761

(CHEMBL4169187)Show SMILES CC(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccccc3n2C)CC1 |r| Show InChI InChI=1S/C32H33N5O4/c1-22(38)35-18-16-23(17-19-35)20-29(31-33-27-10-6-7-11-28(27)34(31)2)37-30(39)21-36(32(37)40)24-12-14-26(15-13-24)41-25-8-4-3-5-9-25/h3-15,23,29H,16-21H2,1-2H3/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286736
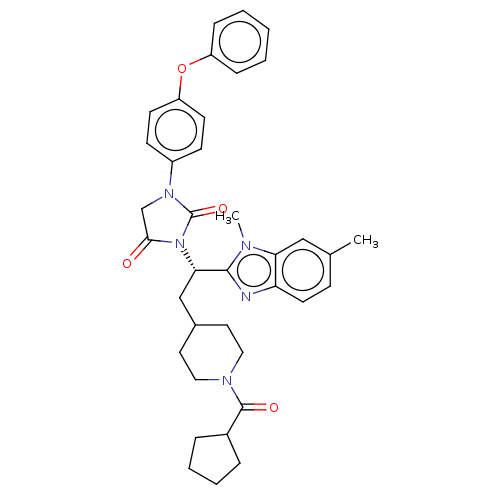
(CHEMBL4161262)Show SMILES Cc1ccc2nc([C@H](CC3CCN(CC3)C(=O)C3CCCC3)N3C(=O)CN(C3=O)c3ccc(Oc4ccccc4)cc3)n(C)c2c1 |r| Show InChI InChI=1S/C37H41N5O4/c1-25-12-17-31-32(22-25)39(2)35(38-31)33(23-26-18-20-40(21-19-26)36(44)27-8-6-7-9-27)42-34(43)24-41(37(42)45)28-13-15-30(16-14-28)46-29-10-4-3-5-11-29/h3-5,10-17,22,26-27,33H,6-9,18-21,23-24H2,1-2H3/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510495

(CHEMBL4453417)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccc(C)cc3n2C)CC1 |r| Show InChI InChI=1S/C35H38FN5O4/c1-22(2)34(43)39-17-15-24(16-18-39)20-31(33-37-29-14-5-23(3)19-30(29)38(33)4)41-32(42)21-40(35(41)44)26-8-12-28(13-9-26)45-27-10-6-25(36)7-11-27/h5-14,19,22,24,31H,15-18,20-21H2,1-4H3/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50553000

(CHEMBL4783205)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccc(F)cc3n2C2CC2)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286763

(CHEMBL4169596)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccc(Cl)cc3n2C)CC1 |r| Show InChI InChI=1S/C34H36ClN5O4/c1-22(2)33(42)38-17-15-23(16-18-38)19-30(32-36-28-14-9-24(35)20-29(28)37(32)3)40-31(41)21-39(34(40)43)25-10-12-27(13-11-25)44-26-7-5-4-6-8-26/h4-14,20,22-23,30H,15-19,21H2,1-3H3/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510495

(CHEMBL4453417)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccc(C)cc3n2C)CC1 |r| Show InChI InChI=1S/C35H38FN5O4/c1-22(2)34(43)39-17-15-24(16-18-39)20-31(33-37-29-14-5-23(3)19-30(29)38(33)4)41-32(42)21-40(35(41)44)26-8-12-28(13-9-26)45-27-10-6-25(36)7-11-27/h5-14,19,22,24,31H,15-18,20-21H2,1-4H3/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286733
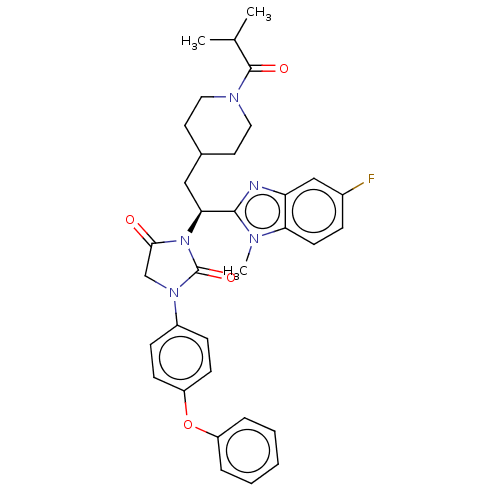
(CHEMBL4162312)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3cc(F)ccc3n2C)CC1 |r| Show InChI InChI=1S/C34H36FN5O4/c1-22(2)33(42)38-17-15-23(16-18-38)19-30(32-36-28-20-24(35)9-14-29(28)37(32)3)40-31(41)21-39(34(40)43)25-10-12-27(13-11-25)44-26-7-5-4-6-8-26/h4-14,20,22-23,30H,15-19,21H2,1-3H3/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50552999

(CHEMBL4749439)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccc(C)cc3n2C2CC2)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50552998

(CHEMBL4783777)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccc(F)cc3n2C)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50552996
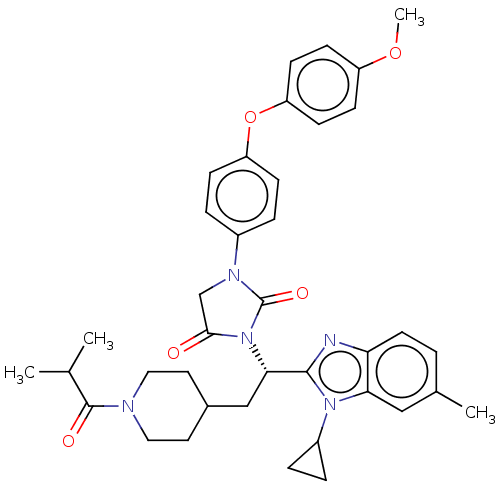
(CHEMBL4785930)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(C)cc4n3C3CC3)C2=O)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286762

(CHEMBL4159402)Show SMILES CC(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3ccc(C)cc3n2C)CC1 |r| Show InChI InChI=1S/C33H35N5O4/c1-22-9-14-28-29(19-22)35(3)32(34-28)30(20-24-15-17-36(18-16-24)23(2)39)38-31(40)21-37(33(38)41)25-10-12-27(13-11-25)42-26-7-5-4-6-8-26/h4-14,19,24,30H,15-18,20-21H2,1-3H3/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223914
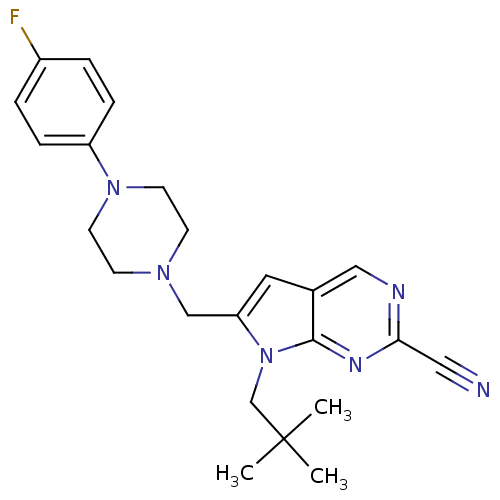
(6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...)Show SMILES CC(C)(C)Cn1c(CN2CCN(CC2)c2ccc(F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C23H27FN6/c1-23(2,3)16-30-20(12-17-14-26-21(13-25)27-22(17)30)15-28-8-10-29(11-9-28)19-6-4-18(24)5-7-19/h4-7,12,14H,8-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223939
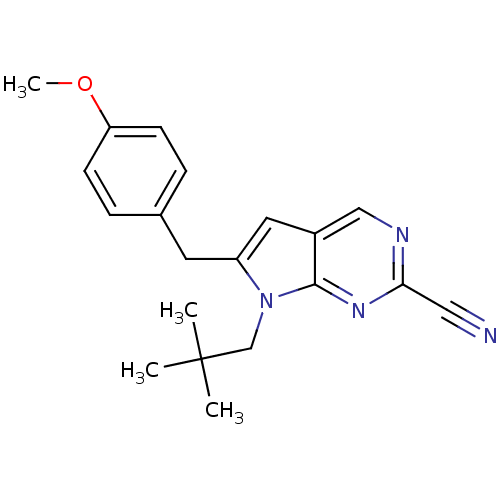
(6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H22N4O/c1-20(2,3)13-24-16(9-14-5-7-17(25-4)8-6-14)10-15-12-22-18(11-21)23-19(15)24/h5-8,10,12H,9,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50286764

(CHEMBL4176369)Show SMILES CC(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)c2nc3cc(F)ccc3n2C)CC1 |r| Show InChI InChI=1S/C32H32FN5O4/c1-21(39)36-16-14-22(15-17-36)18-29(31-34-27-19-23(33)8-13-28(27)35(31)2)38-30(40)20-37(32(38)41)24-9-11-26(12-10-24)42-25-6-4-3-5-7-25/h3-13,19,22,29H,14-18,20H2,1-2H3/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... |
ACS Med Chem Lett 9: 78-83 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00363
BindingDB Entry DOI: 10.7270/Q2GQ7199 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223921
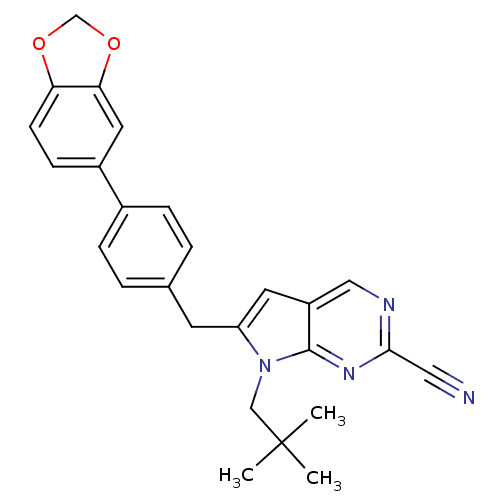
(6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(cc2)-c2ccc3OCOc3c2)cc2cnc(nc12)C#N Show InChI InChI=1S/C26H24N4O2/c1-26(2,3)15-30-21(11-20-14-28-24(13-27)29-25(20)30)10-17-4-6-18(7-5-17)19-8-9-22-23(12-19)32-16-31-22/h4-9,11-12,14H,10,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223935
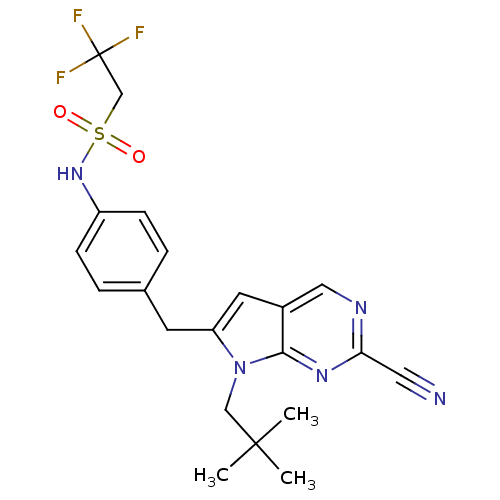
(CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(NS(=O)(=O)CC(F)(F)F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C21H22F3N5O2S/c1-20(2,3)12-29-17(9-15-11-26-18(10-25)27-19(15)29)8-14-4-6-16(7-5-14)28-32(30,31)13-21(22,23)24/h4-7,9,11,28H,8,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223915

(6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...)Show InChI InChI=1S/C19H20N4/c1-19(2,3)13-23-16(9-14-7-5-4-6-8-14)10-15-12-21-17(11-20)22-18(15)23/h4-8,10,12H,9,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223925
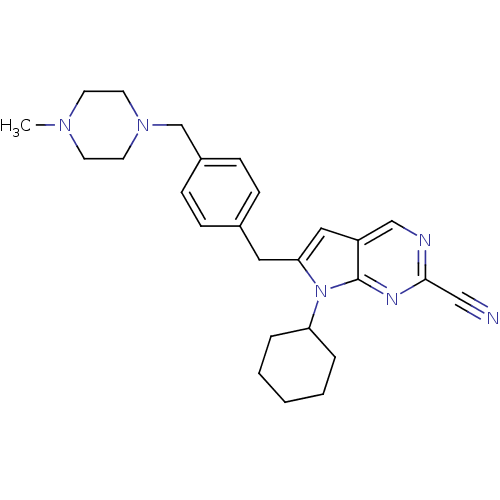
(6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...)Show SMILES CN1CCN(Cc2ccc(Cc3cc4cnc(nc4n3C3CCCCC3)C#N)cc2)CC1 Show InChI InChI=1S/C26H32N6/c1-30-11-13-31(14-12-30)19-21-9-7-20(8-10-21)15-24-16-22-18-28-25(17-27)29-26(22)32(24)23-5-3-2-4-6-23/h7-10,16,18,23H,2-6,11-15,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223919
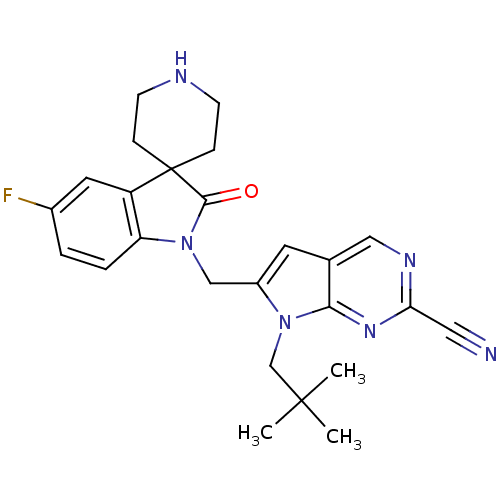
(7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)C3(CCNCC3)c3cc(F)ccc23)cc2cnc(nc12)C#N Show InChI InChI=1S/C25H27FN6O/c1-24(2,3)15-32-18(10-16-13-29-21(12-27)30-22(16)32)14-31-20-5-4-17(26)11-19(20)25(23(31)33)6-8-28-9-7-25/h4-5,10-11,13,28H,6-9,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50553001

(CHEMBL4778665)Show SMILES CC(C)C(=O)N1CCC(C[C@H](N2C(=O)CN(C2=O)c2ccc(Oc3ccc(F)cc3)cc2)c2nc3ccccc3n2C2CC2)CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity against recombinant human FXR transfected in human HuH-7 cells co-transfected with FRE-luciferase assessed as reduction in CDCA-i... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223910

(6-(4-chlorobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H19ClN4/c21-16-8-6-14(7-9-16)10-18-11-15-13-23-19(12-22)24-20(15)25(18)17-4-2-1-3-5-17/h6-9,11,13,17H,1-5,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510488

(CHEMBL4519419)Show SMILES CC(C)Cn1c(nc2ccc(C)cc12)[C@H](CC1CCN(CC1)C(=O)C(C)C)N1C(=O)CN(C1=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C38H45N5O4/c1-25(2)23-42-33-21-27(5)11-16-32(33)39-36(42)34(22-28-17-19-40(20-18-28)37(45)26(3)4)43-35(44)24-41(38(43)46)29-12-14-31(15-13-29)47-30-9-7-6-8-10-30/h6-16,21,25-26,28,34H,17-20,22-24H2,1-5H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223911

(7-neopentyl-6-((pyridin-4-yloxy)methyl)-7H-pyrrolo...)Show InChI InChI=1S/C18H19N5O/c1-18(2,3)12-23-14(11-24-15-4-6-20-7-5-15)8-13-10-21-16(9-19)22-17(13)23/h4-8,10H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376511

(CHEMBL261511)Show InChI InChI=1S/C22H25N5/c1-22(2,3)16-25-21-18(14-24-20(13-23)26-21)9-6-11-27-12-10-17-7-4-5-8-19(17)15-27/h4-5,7-8,14H,10-12,15-16H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223920
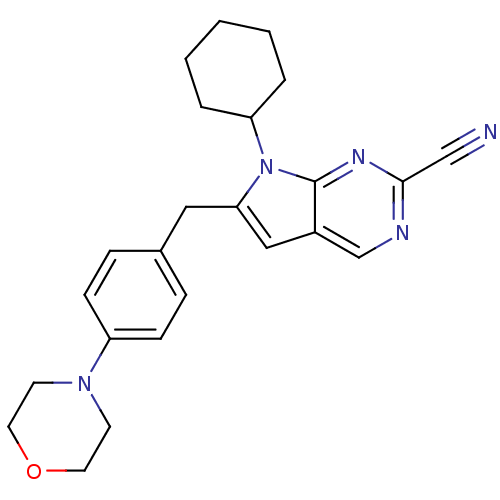
(6-(4-morpholinobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3...)Show SMILES N#Cc1ncc2cc(Cc3ccc(cc3)N3CCOCC3)n(C3CCCCC3)c2n1 Show InChI InChI=1S/C24H27N5O/c25-16-23-26-17-19-15-22(29(24(19)27-23)21-4-2-1-3-5-21)14-18-6-8-20(9-7-18)28-10-12-30-13-11-28/h6-9,15,17,21H,1-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376500
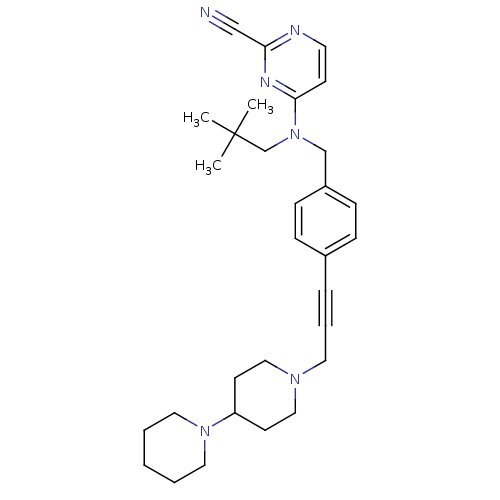
(CHEMBL261700)Show SMILES CC(C)(C)CN(Cc1ccc(cc1)C#CCN1CCC(CC1)N1CCCCC1)c1ccnc(n1)C#N Show InChI InChI=1S/C30H40N6/c1-30(2,3)24-36(29-13-16-32-28(22-31)33-29)23-26-11-9-25(10-12-26)8-7-17-34-20-14-27(15-21-34)35-18-5-4-6-19-35/h9-13,16,27H,4-6,14-15,17-21,23-24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376507
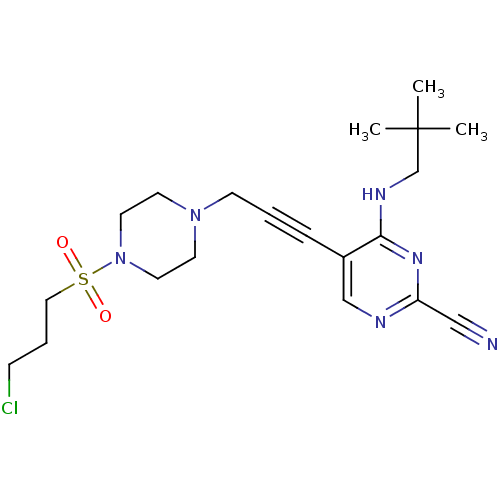
(CHEMBL429147)Show SMILES CC(C)(C)CNc1nc(ncc1C#CCN1CCN(CC1)S(=O)(=O)CCCCl)C#N Show InChI InChI=1S/C20H29ClN6O2S/c1-20(2,3)16-24-19-17(15-23-18(14-22)25-19)6-4-8-26-9-11-27(12-10-26)30(28,29)13-5-7-21/h15H,5,7-13,16H2,1-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376503
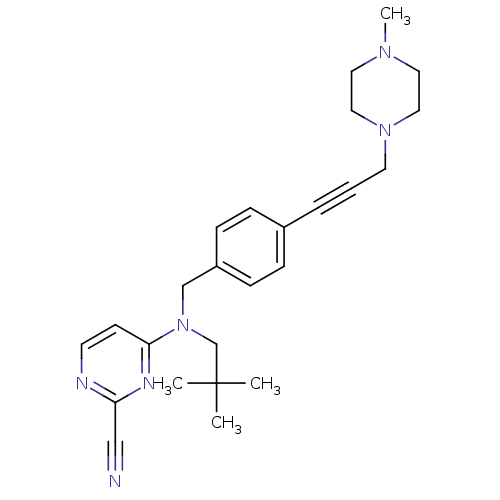
(CHEMBL261516)Show SMILES CN1CCN(CC#Cc2ccc(CN(CC(C)(C)C)c3ccnc(n3)C#N)cc2)CC1 Show InChI InChI=1S/C25H32N6/c1-25(2,3)20-31(24-11-12-27-23(18-26)28-24)19-22-9-7-21(8-10-22)6-5-13-30-16-14-29(4)15-17-30/h7-12H,13-17,19-20H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223936

(6-((5,5-dimethyl-2,4-dioxooxazolidin-3-yl)methyl)-...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)OC(C)(C)C2=O)cc2cnc(nc12)C#N Show InChI InChI=1S/C18H21N5O3/c1-17(2,3)10-23-12(6-11-8-20-13(7-19)21-14(11)23)9-22-15(24)18(4,5)26-16(22)25/h6,8H,9-10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223940
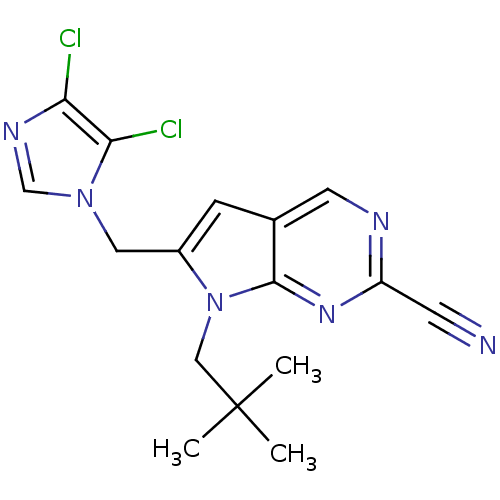
(6-((4,5-dichloro-1H-imidazol-1-yl)methyl)-7-neopen...)Show SMILES CC(C)(C)Cn1c(Cn2cnc(Cl)c2Cl)cc2cnc(nc12)C#N Show InChI InChI=1S/C16H16Cl2N6/c1-16(2,3)8-24-11(7-23-9-21-13(17)14(23)18)4-10-6-20-12(5-19)22-15(10)24/h4,6,9H,7-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223941
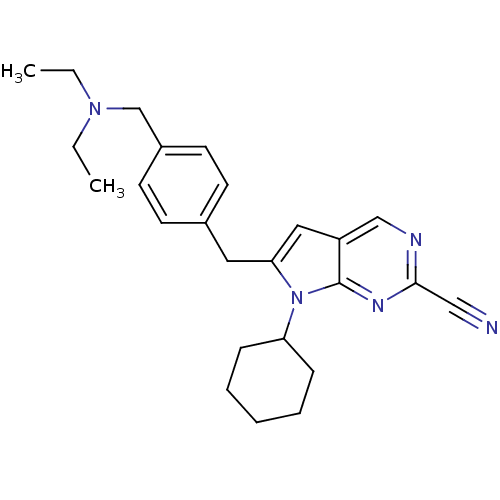
(6-(4-((diethylamino)methyl)benzyl)-7-cyclohexyl-7H...)Show SMILES CCN(CC)Cc1ccc(Cc2cc3cnc(nc3n2C2CCCCC2)C#N)cc1 Show InChI InChI=1S/C25H31N5/c1-3-29(4-2)18-20-12-10-19(11-13-20)14-23-15-21-17-27-24(16-26)28-25(21)30(23)22-8-6-5-7-9-22/h10-13,15,17,22H,3-9,14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
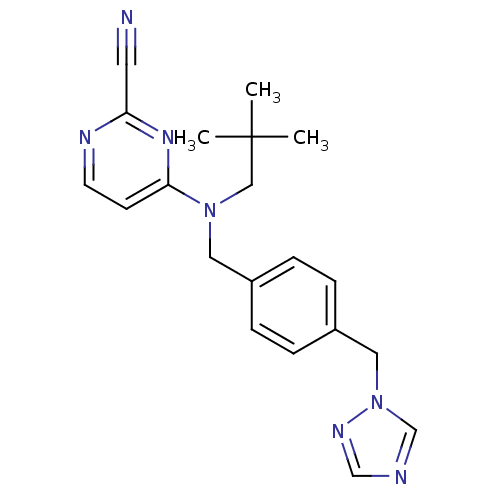
(Homo sapiens (Human)) | BDBM50376504
(CHEMBL258640)Show SMILES CC(C)(C)CN(Cc1ccc(Cn2cncn2)cc1)c1ccnc(n1)C#N Show InChI InChI=1S/C20H23N7/c1-20(2,3)13-26(19-8-9-23-18(10-21)25-19)11-16-4-6-17(7-5-16)12-27-15-22-14-24-27/h4-9,14-15H,11-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223937

(6-((1H-1,2,3-triazol-1-yl)methyl)-7-neopentyl-7H-p...)Show InChI InChI=1S/C15H17N7/c1-15(2,3)10-22-12(9-21-5-4-18-20-21)6-11-8-17-13(7-16)19-14(11)22/h4-6,8H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
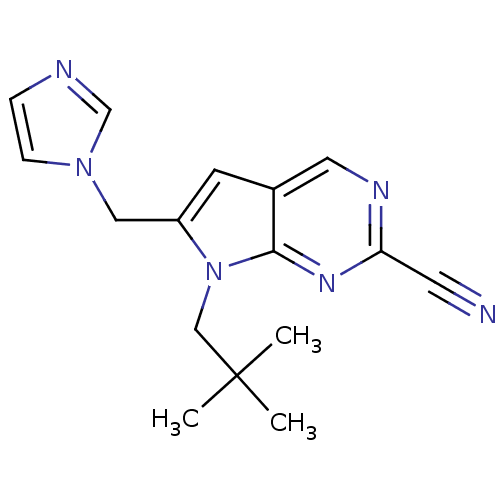
(Homo sapiens (Human)) | BDBM50223918
(6-((1H-imidazol-1-yl)methyl)-7-neopentyl-7H-pyrrol...)Show InChI InChI=1S/C16H18N6/c1-16(2,3)10-22-13(9-21-5-4-18-11-21)6-12-8-19-14(7-17)20-15(12)22/h4-6,8,11H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
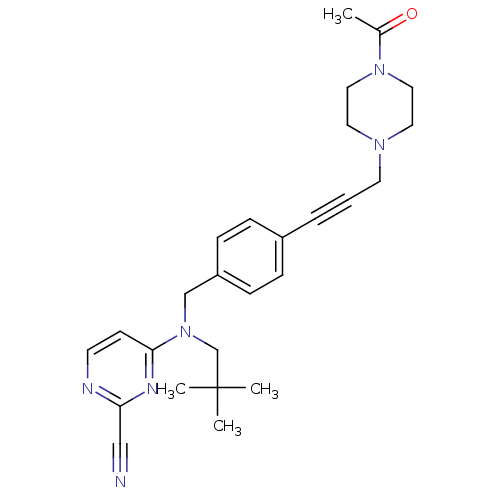
(Homo sapiens (Human)) | BDBM50376502
(CHEMBL410520)Show SMILES CC(=O)N1CCN(CC#Cc2ccc(CN(CC(C)(C)C)c3ccnc(n3)C#N)cc2)CC1 Show InChI InChI=1S/C26H32N6O/c1-21(33)31-16-14-30(15-17-31)13-5-6-22-7-9-23(10-8-22)19-32(20-26(2,3)4)25-11-12-28-24(18-27)29-25/h7-12H,13-17,19-20H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510491

(CHEMBL4552760)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(C)cc4n3C)C2=O)cc1 |r| Show InChI InChI=1S/C36H41N5O5/c1-23(2)35(43)39-18-16-25(17-19-39)21-32(34-37-30-15-6-24(3)20-31(30)38(34)4)41-33(42)22-40(36(41)44)26-7-9-28(10-8-26)46-29-13-11-27(45-5)12-14-29/h6-15,20,23,25,32H,16-19,21-22H2,1-5H3/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced Fluorecein-SRC2-2 coactivator peptide... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00640
BindingDB Entry DOI: 10.7270/Q2QZ2FKJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50510491

(CHEMBL4552760)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N([C@@H](CC3CCN(CC3)C(=O)C(C)C)c3nc4ccc(C)cc4n3C)C2=O)cc1 |r| Show InChI InChI=1S/C36H41N5O5/c1-23(2)35(43)39-18-16-25(17-19-39)21-32(34-37-30-15-6-24(3)20-31(30)38(34)4)41-33(42)22-40(36(41)44)26-7-9-28(10-8-26)46-29-13-11-27(45-5)12-14-29/h6-15,20,23,25,32H,16-19,21-22H2,1-5H3/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... |
Bioorg Med Chem 27: 2220-2227 (2019)
Article DOI: 10.1016/j.bmc.2019.04.029
BindingDB Entry DOI: 10.7270/Q2R49V2G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376498

(CHEMBL259939)Show SMILES CN1CCN(CC1)c1ccc(CC#Cc2cnc(nc2NCC(C)(C)C)C#N)cc1 Show InChI InChI=1S/C24H30N6/c1-24(2,3)18-27-23-20(17-26-22(16-25)28-23)7-5-6-19-8-10-21(11-9-19)30-14-12-29(4)13-15-30/h8-11,17H,6,12-15,18H2,1-4H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376508

(CHEMBL261455)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCN(CC#Cc2cnc(nc2NCC(C)(C)C)C#N)CC1 Show InChI InChI=1S/C24H30N6O3S/c1-24(2,3)18-27-23-19(17-26-22(16-25)28-23)6-5-11-29-12-14-30(15-13-29)34(31,32)21-9-7-20(33-4)8-10-21/h7-10,17H,11-15,18H2,1-4H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376509
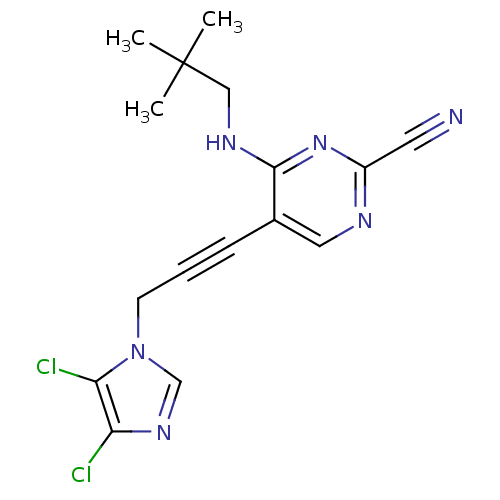
(CHEMBL408562)Show InChI InChI=1S/C16H16Cl2N6/c1-16(2,3)9-21-15-11(8-20-12(7-19)23-15)5-4-6-24-10-22-13(17)14(24)18/h8,10H,6,9H2,1-3H3,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50376501
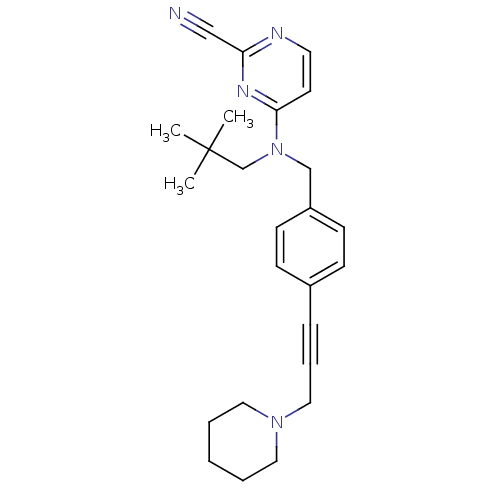
(CHEMBL408974)Show SMILES CC(C)(C)CN(Cc1ccc(cc1)C#CCN1CCCCC1)c1ccnc(n1)C#N Show InChI InChI=1S/C25H31N5/c1-25(2,3)20-30(24-13-14-27-23(18-26)28-24)19-22-11-9-21(10-12-22)8-7-17-29-15-5-4-6-16-29/h9-14H,4-6,15-17,19-20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 18: 2599-603 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.036
BindingDB Entry DOI: 10.7270/Q2HT2Q6P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data