

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236280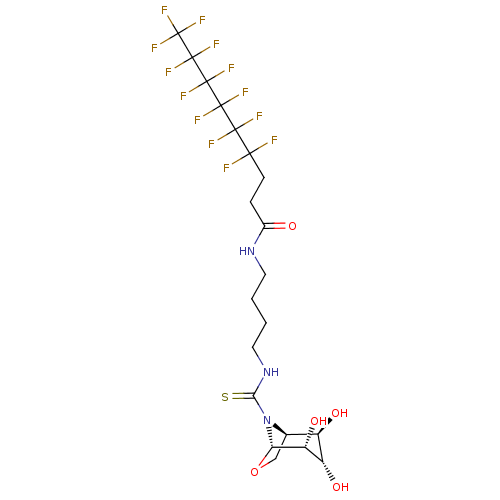 (CHEMBL4100971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236278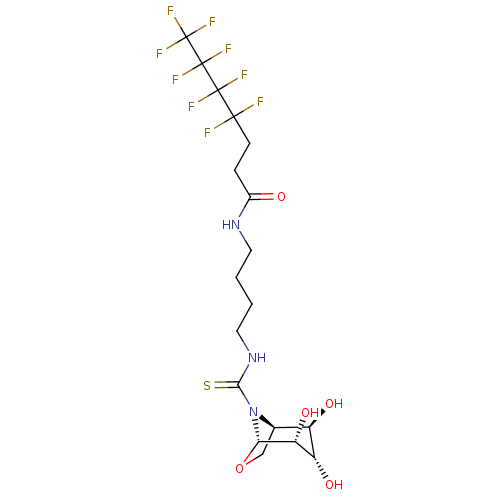 (CHEMBL4077472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525017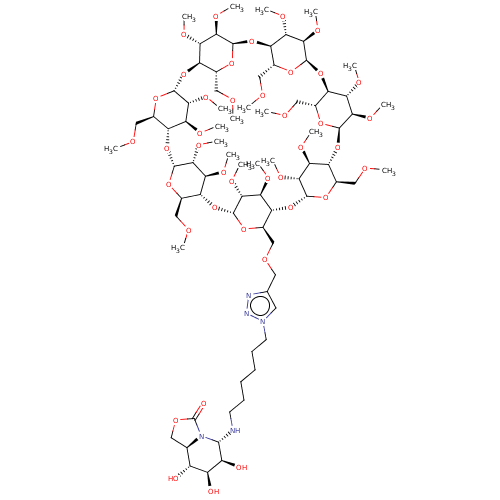 (CHEMBL4462117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525014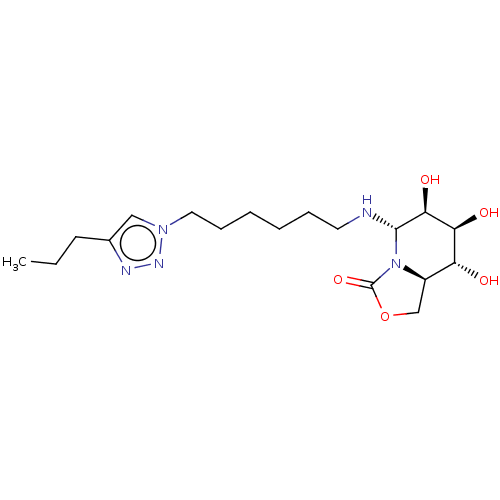 (CHEMBL4475419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525016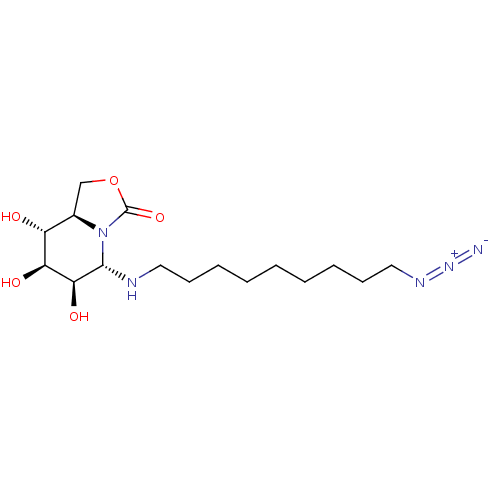 (CHEMBL4457217) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525018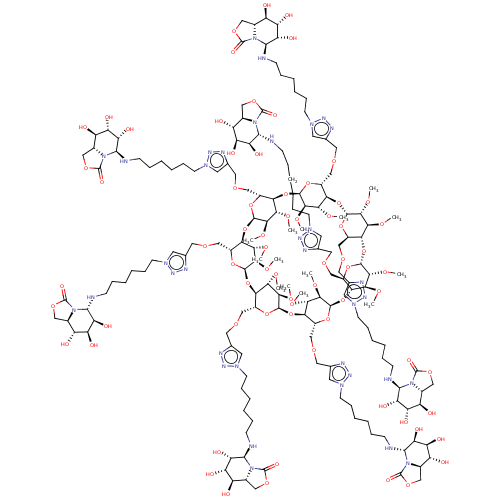 (CHEMBL4465842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525020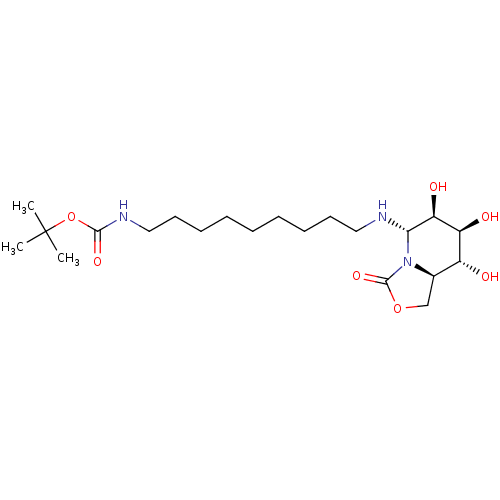 (CHEMBL4518572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525015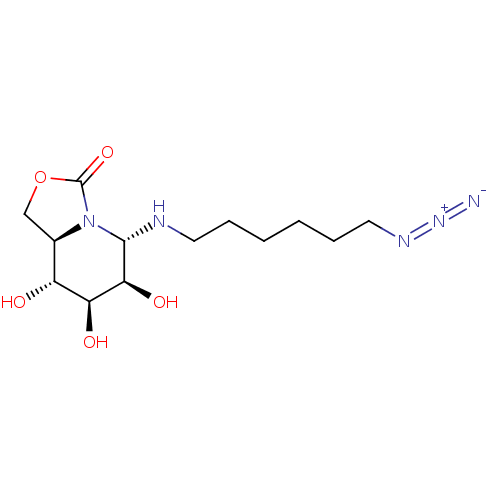 (CHEMBL4435118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236279 (CHEMBL4093241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236281 (CHEMBL4076141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 in presence of bet... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677,R201C] (Homo sapiens (Human)) | BDBM228800 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 940 | -35.8 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677] (Homo sapiens (Human)) | BDBM228800 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | -35.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677,I51T] (Homo sapiens (Human)) | BDBM228800 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | -35.2 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236280 (CHEMBL4100971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50375511 (CHEMBL406973) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Seville Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase using o-nitrophenyl alpha-D-galactopyranoside after 10 to 30 mins by spectrophotometric method | Eur J Med Chem 121: 880-891 (2016) Article DOI: 10.1016/j.ejmech.2015.08.038 BindingDB Entry DOI: 10.7270/Q2FT8P07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525019 (CHEMBL4471561) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236279 (CHEMBL4093241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli (Enterobacteria)) | BDBM50375511 (CHEMBL406973) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Seville Curated by ChEMBL | Assay Description Inhibition of Escherichia coli beta-galactosidase using o-nitrophenyl beta-D-galactopyranoside after 10 to 30 mins by spectrophotometric method | Eur J Med Chem 121: 880-891 (2016) Article DOI: 10.1016/j.ejmech.2015.08.038 BindingDB Entry DOI: 10.7270/Q2FT8P07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236278 (CHEMBL4077472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101405 (CHEMBL3393931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50525021 (CHEMBL4483772) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean Lysosomal alpha-mannosidase assessed as residual hydrolytic activity after 10 to 30 mins by spectrophotometric analysis | J Med Chem 62: 5832-5843 (2019) Article DOI: 10.1021/acs.jmedchem.9b00153 BindingDB Entry DOI: 10.7270/Q22R3W4H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236279 (CHEMBL4093241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 2 | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236281 (CHEMBL4076141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 in presence of bet... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236281 (CHEMBL4076141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101398 (CHEMBL3393930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101399 (CHEMBL3393929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236280 (CHEMBL4100971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101401 (CHEMBL3393928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236278 (CHEMBL4077472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101403 (CHEMBL3393932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101402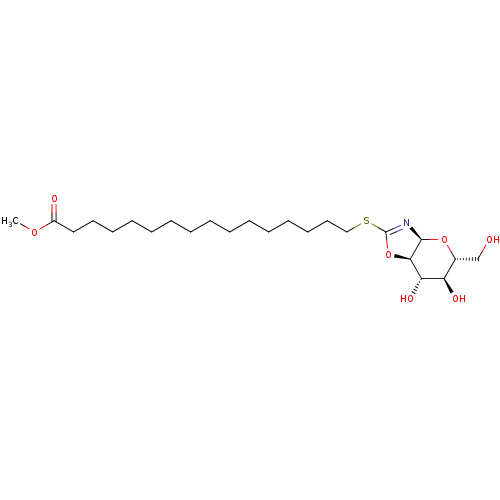 (CHEMBL3393927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 7.3 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236279 (CHEMBL4093241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236278 (CHEMBL4077472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 7 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677,I51T] (Homo sapiens (Human)) | BDBM50163440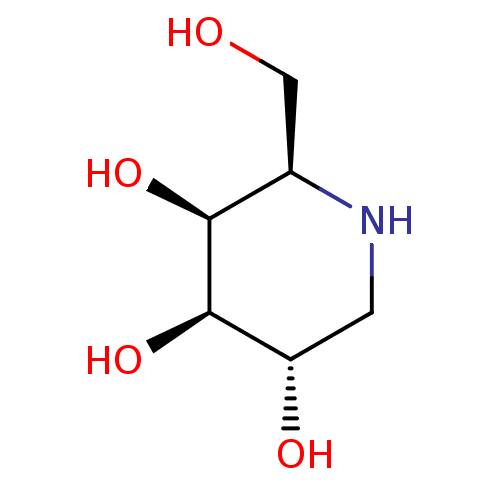 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.47E+4 | -25.8 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101402 (CHEMBL3393927) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677,R201C] (Homo sapiens (Human)) | BDBM50163440 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.90E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase [24-677,R201C] (Homo sapiens (Human)) | BDBM228802 (5N,6S-(N'-butyliminomethylidene)-6-thio-1-deox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 5.33E+4 | -25.4 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101403 (CHEMBL3393932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677] (Homo sapiens (Human)) | BDBM50163440 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.18E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101405 (CHEMBL3393931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101398 (CHEMBL3393930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase [24-677] (Homo sapiens (Human)) | BDBM228802 (5N,6S-(N'-butyliminomethylidene)-6-thio-1-deox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6.83E+4 | -24.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase [24-677,I51T] (Homo sapiens (Human)) | BDBM228802 (5N,6S-(N'-butyliminomethylidene)-6-thio-1-deox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 6.88E+4 | -24.7 | n/a | n/a | n/a | n/a | n/a | 4.5 | 37 |
The University of Tokyo | Assay Description β-Gal activity was measured by using 4-methylumbelliferyll-β-D-galactopyranoside in buffer B (0.15 M sodium citrate, pH 4.5, and 0.2 M NaCl... | J Biol Chem 289: 14560-8 (2014) Article DOI: 10.1074/jbc.M113.529529 BindingDB Entry DOI: 10.7270/Q2WH2NW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236281 (CHEMBL4076141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of human GCase assessed as formation of 4-methylumbelliferone from 4-methylumbelliferyl alpha-D-glucopyranoside at pH 5 by luminescence sp... | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50236280 (CHEMBL4100971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description In vitro inhibitory activity against human whole blood Prostaglandin G/H synthase 1 | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Bos taurus) | BDBM50101401 (CHEMBL3393928) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Universitat Rovira i Virgili Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-glucosidase at pH 5.5 incubated for 10 to 30 mins using beta-D-glycopyranoside substrate by spectrophotometry | Eur J Med Chem 90: 258-66 (2015) Article DOI: 10.1016/j.ejmech.2014.11.002 BindingDB Entry DOI: 10.7270/Q2H996ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50236280 (CHEMBL4100971) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50236278 (CHEMBL4077472) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of green coffee bean alpha-galactosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50236278 (CHEMBL4077472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Binding affinity towards dopamine transporter using [3H]- mazindol as radioligand in rat striatal membranes | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Canavalia ensiformis) | BDBM50236280 (CHEMBL4100971) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sevilla Curated by ChEMBL | Assay Description Inhibition of jack bean alpha-mannosidase using beta-D-glycopyranoside after 10 to 30 mins by spectrophotometry | J Med Chem 60: 1829-1842 (2017) Article DOI: 10.1021/acs.jmedchem.6b01550 BindingDB Entry DOI: 10.7270/Q2K939S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |