

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Homo sapiens (Human)) | BDBM50057514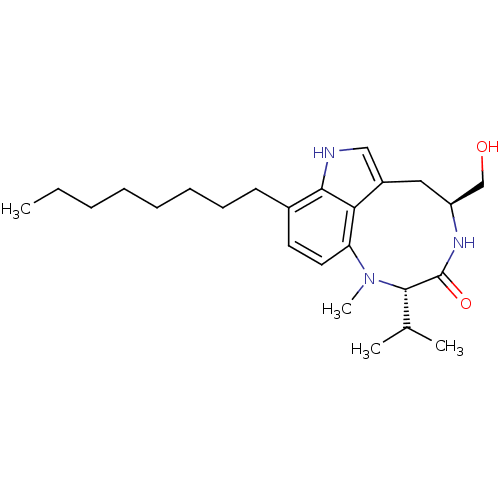 ((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]- PDBu from recombinant PKC beta expressed in baculovirus | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50057514 ((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]- PDBu from recombinant PKC alpha expressed in baculovirus | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135453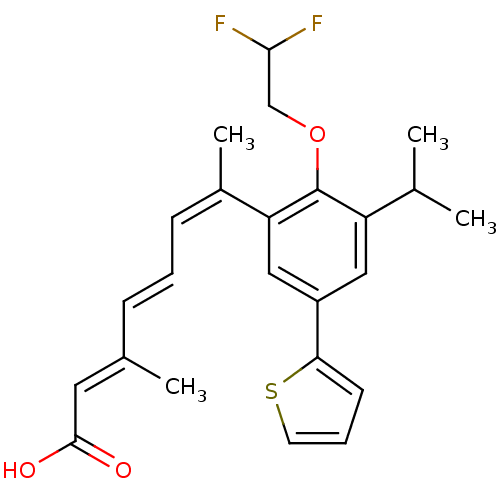 ((2E,4E,6Z)-7-[2-(2,2-Difluoro-ethoxy)-3-isopropyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135460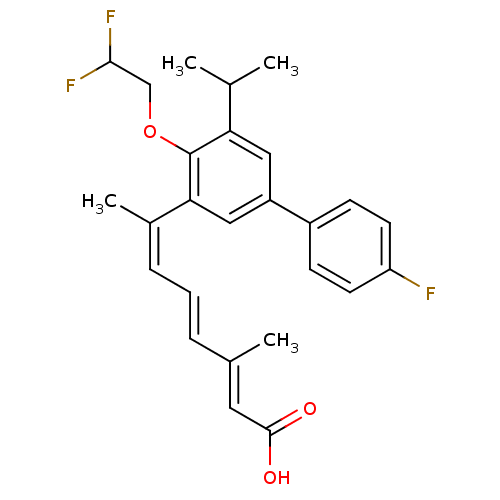 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-4'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50057514 ((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]- PDBu from recombinant PKC delta expressed in baculovirus | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50057512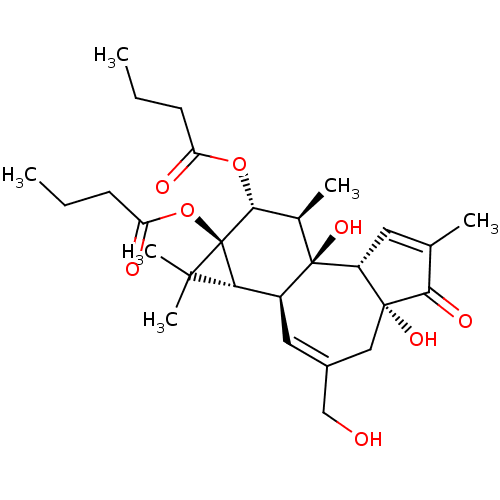 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of [3H]-PDBu binding to PKC delta wild type | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133128 (5-{2-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against Rhizopus chinensis pepsin | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Rattus norvegicus) | BDBM50133119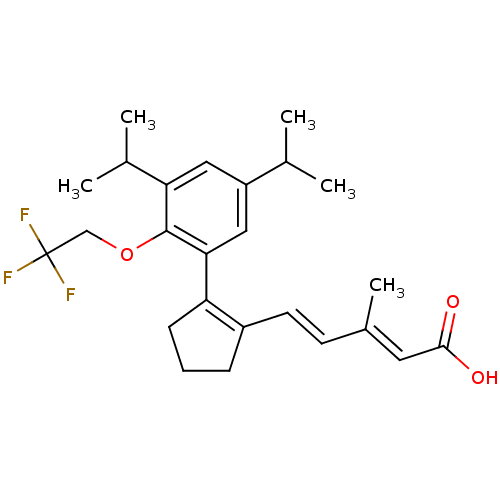 (5-{2-[3,5-Diisopropyl-2-(2,2,2-trifluoro-ethoxy)-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-beta was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135462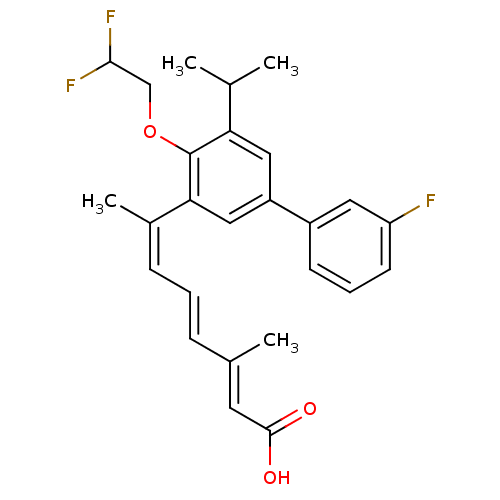 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129719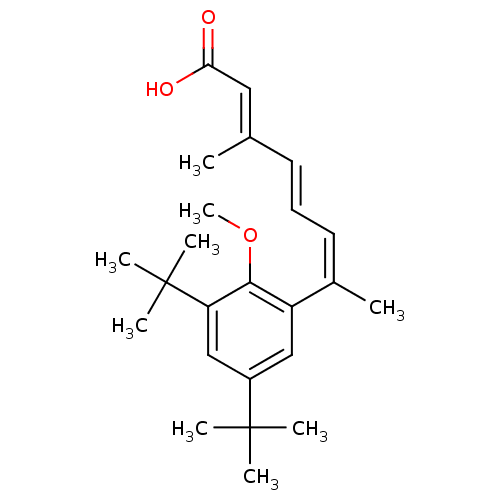 (7-(3,5-Di-tert-butyl-2-methoxy-phenyl)-3-methyl-oc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129723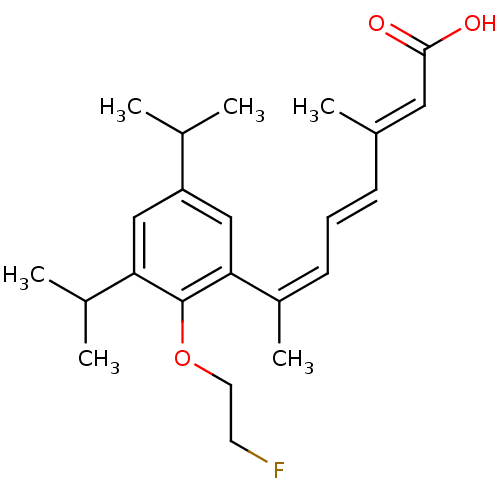 (7-[2-(2-Fluoro-ethoxy)-3,5-diisopropyl-phenyl]-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129717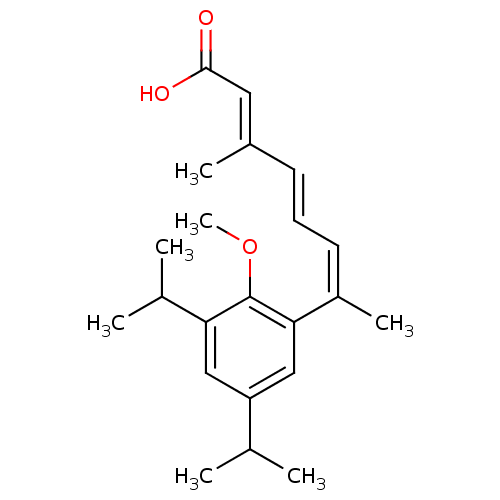 (7-(3,5-Diisopropyl-2-methoxy-phenyl)-3-methyl-octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C epsilon type (Homo sapiens (Human)) | BDBM50057514 ((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]- PDBu from recombinant PKC epsilon expressed in baculovirus | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133119 (5-{2-[3,5-Diisopropyl-2-(2,2,2-trifluoro-ethoxy)-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-alpha was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Homo sapiens (Human)) | BDBM50057514 ((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]- PDBu from recombinant PKC gamma expressed in baculovirus | J Med Chem 40: 1316-26 (1997) Article DOI: 10.1021/jm960875h BindingDB Entry DOI: 10.7270/Q2319TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. | Assay Description PF-00835231 was evaluated against 3CLpro from a variety of other coronaviruses representing alpha, beta and gamma groups of Coronaviridae, using bioc... | bioRxiv (2020) Article DOI: 10.1101/2020.09.12.293498 BindingDB Entry DOI: 10.7270/Q2VQ352Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129720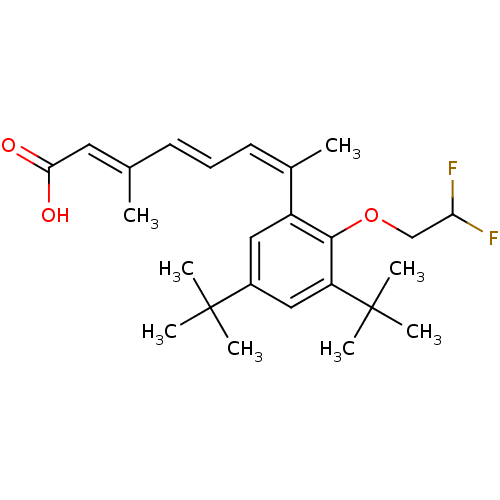 ((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Rattus norvegicus) | BDBM50133118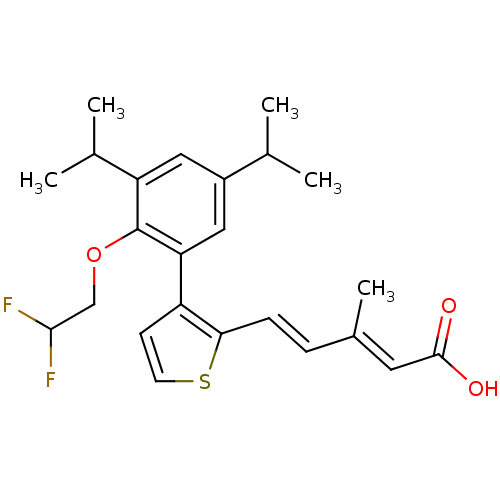 (5-{3-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-beta was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133126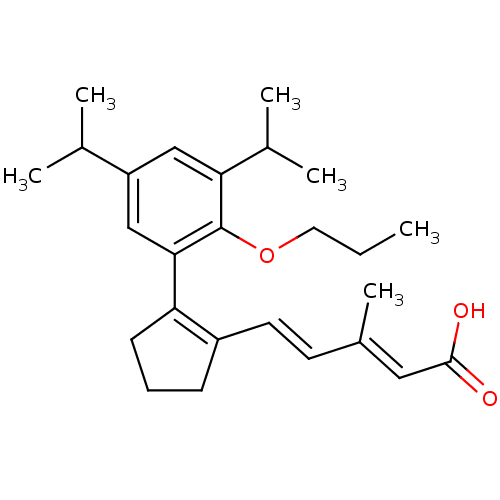 (5-[2-(3,5-Diisopropyl-2-propoxy-phenyl)-cyclopent-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-alpha was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Rattus norvegicus) | BDBM50133128 (5-{2-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-gamma was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133114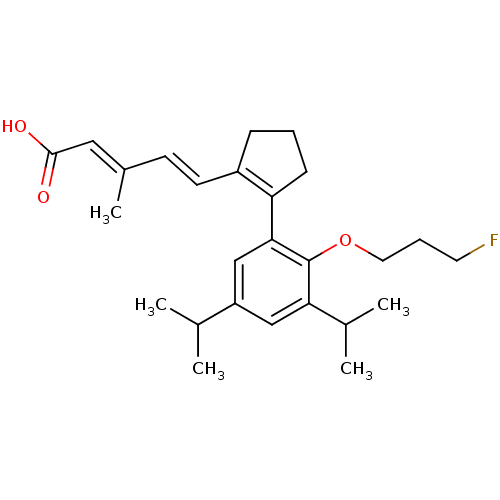 (5-{2-[2-(3-Fluoro-propoxy)-3,5-diisopropyl-phenyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-alpha was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133116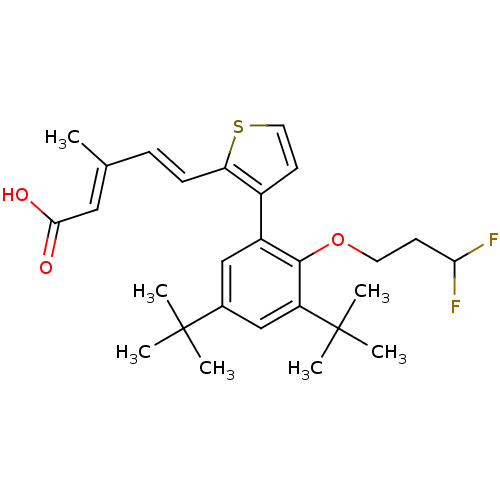 ((2E,4E)-5-{3-[3,5-Di-tert-butyl-2-(3,3-difluoro-pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro transcriptional activation in CV-1 cells expressing RXR-alpha | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129730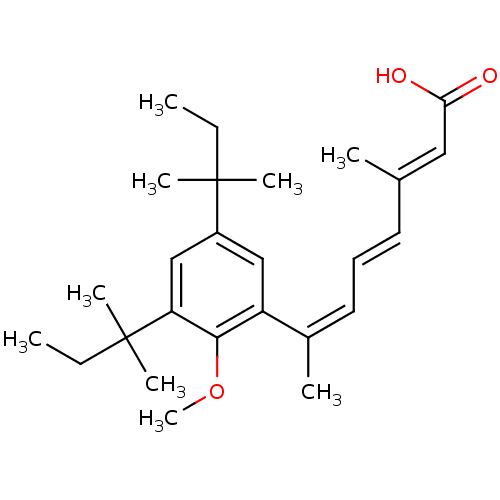 (7-[3,5-Bis-(1,1-dimethyl-propyl)-2-methoxy-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129731 (7-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129726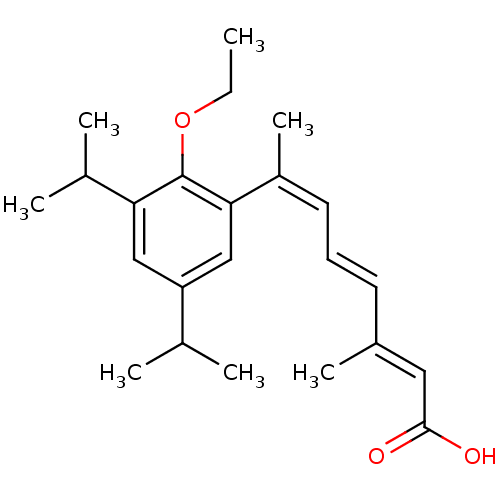 ((2E,4E,6Z)-7-(2-Ethoxy-3,5-diisopropyl-phenyl)-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129722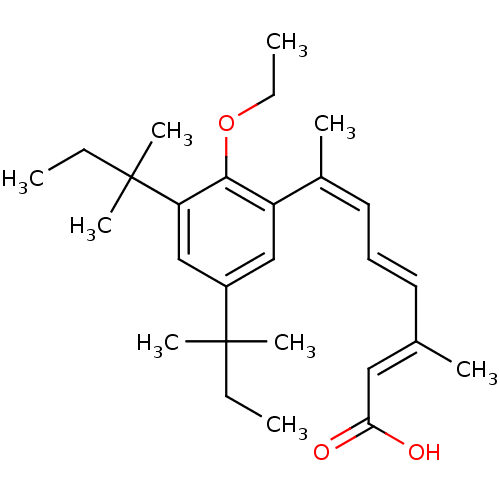 (7-[3,5-Bis-(1,1-dimethyl-propyl)-2-ethoxy-phenyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50133118 (5-{3-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-alpha was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135450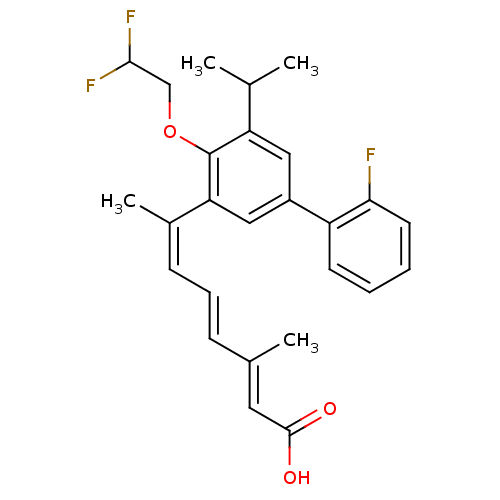 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-2'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50135461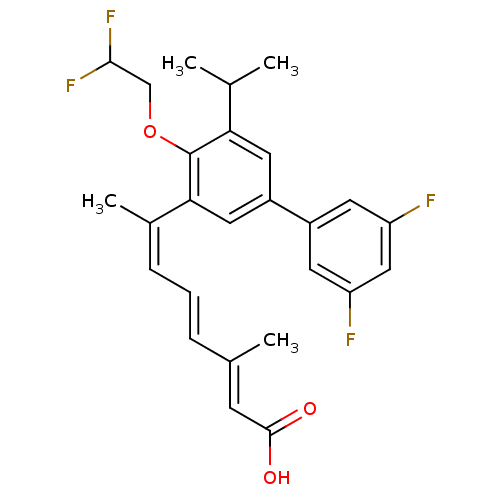 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3',5'-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [3H]-9-cis-RA from RXR beta receptor in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Rattus norvegicus) | BDBM50133128 (5-{2-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-beta was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Rattus norvegicus) | BDBM50133118 (5-{3-[2-(2,2-Difluoro-ethoxy)-3,5-diisopropyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-gamma was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50135461 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3',5'-difluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR alpha receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50135461 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3',5'-difluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR gamma receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Rattus norvegicus) | BDBM50133127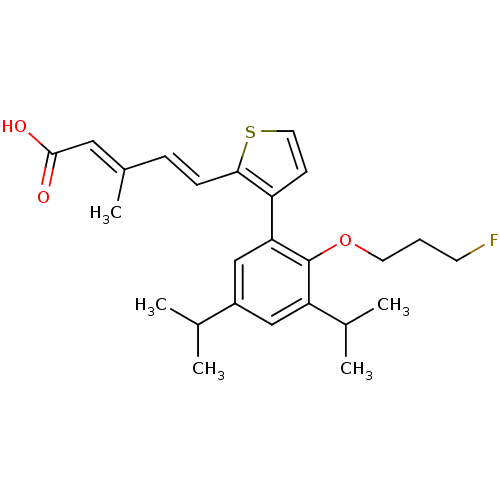 (5-{3-[2-(3-Fluoro-propoxy)-3,5-diisopropyl-phenyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-beta was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50135462 ((2E,4E,6Z)-7-[4-(2,2-Difluoro-ethoxy)-3'-fluoro-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc Curated by ChEMBL | Assay Description Binding affinity against RXR gamma receptor using [3H]-9-cis-RA as radioligand in CV-1 cells | Bioorg Med Chem Lett 13: 4071-5 (2003) BindingDB Entry DOI: 10.7270/Q2V987GP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129733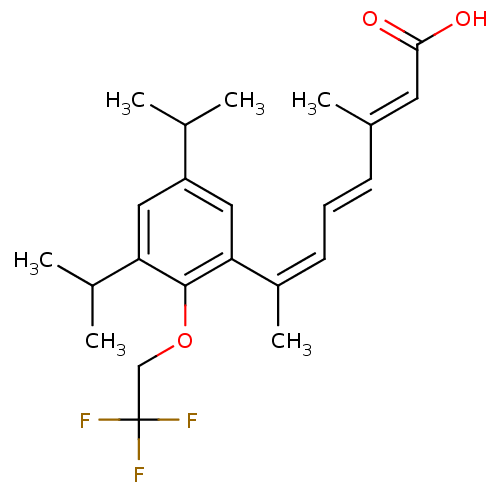 (7-[3,5-Diisopropyl-2-(2,2,2-trifluoro-ethoxy)-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129724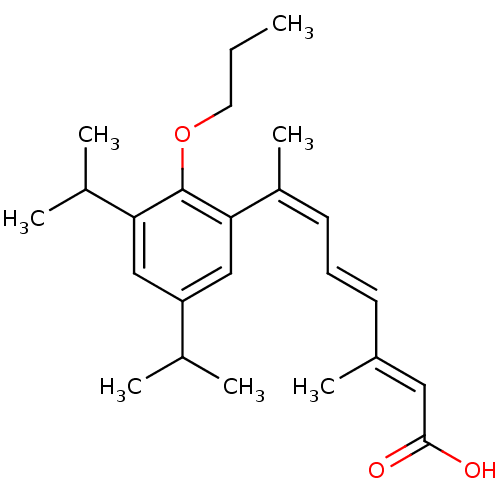 (7-(3,5-Diisopropyl-2-propoxy-phenyl)-3-methyl-octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (RAT) | BDBM50129720 ((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity for Retinoic acid receptor RXR-alpha was determined by competing with 3[H]-9-cis-RA | J Med Chem 46: 4087-103 (2003) Article DOI: 10.1021/jm020401k BindingDB Entry DOI: 10.7270/Q2VM4BNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50129720 ((2E,4E,6Z)-7-[3,5-Di-tert-butyl-2-(2,2-difluoro-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor RXR-alpha was determined in vitro by using [3H]-9-cis-RA as radioligand | J Med Chem 46: 2683-96 (2003) Article DOI: 10.1021/jm020340q BindingDB Entry DOI: 10.7270/Q27H1HZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1662 total ) | Next | Last >> |