 Found 136 hits with Last Name = 'sakaguchi' and Initial = 'k'
Found 136 hits with Last Name = 'sakaguchi' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118477
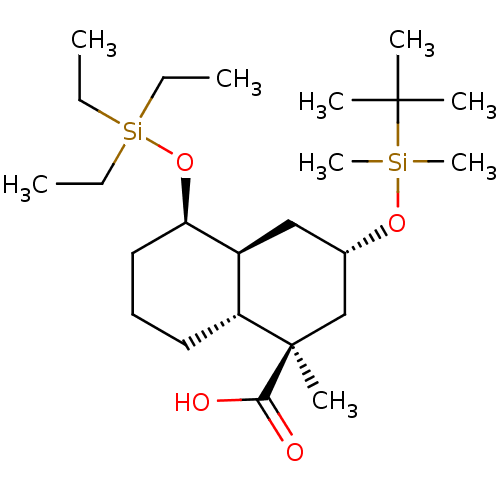
(CHEMBL3613748)Show SMILES [H][C@]12C[C@@H](C[C@@](C)(C(O)=O)[C@]1([H])CCC[C@H]2O[Si](CC)(CC)CC)O[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C24H48O4Si2/c1-10-30(11-2,12-3)28-21-15-13-14-20-19(21)16-18(17-24(20,7)22(25)26)27-29(8,9)23(4,5)6/h18-21H,10-17H2,1-9H3,(H,25,26)/t18-,19-,20+,21+,24+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 228 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEP... |
Bioorg Med Chem 23: 6246-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.042
BindingDB Entry DOI: 10.7270/Q26975C7 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416570
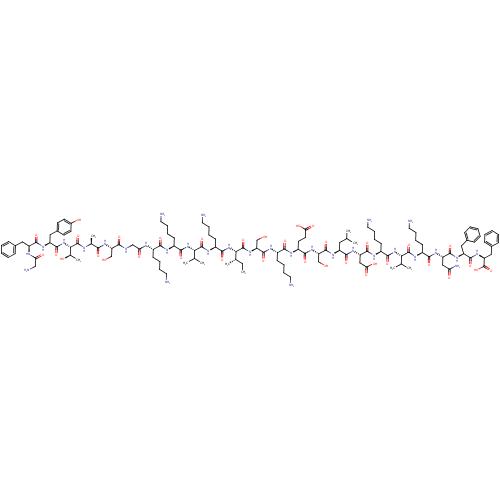
(CHEMBL1215819)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C127H201N31O35/c1-11-72(8)104(157-114(179)85(44-26-32-56-133)145-124(189)103(71(6)7)155-112(177)83(42-24-30-54-131)140-108(173)80(39-21-27-51-128)138-99(166)65-136-107(172)94(66-159)152-106(171)73(9)137-126(191)105(74(10)162)158-120(185)90(60-78-45-47-79(163)48-46-78)148-116(181)88(139-98(165)64-134)58-75-33-15-12-16-34-75)125(190)154-96(68-161)121(186)142-81(40-22-28-52-129)109(174)143-86(49-50-100(167)168)111(176)153-95(67-160)122(187)146-87(57-69(2)3)115(180)150-92(63-101(169)170)119(184)141-84(43-25-31-55-132)113(178)156-102(70(4)5)123(188)144-82(41-23-29-53-130)110(175)149-91(62-97(135)164)118(183)147-89(59-76-35-17-13-18-36-76)117(182)151-93(127(192)193)61-77-37-19-14-20-38-77/h12-20,33-38,45-48,69-74,80-96,102-105,159-163H,11,21-32,39-44,49-68,128-134H2,1-10H3,(H2,135,164)(H,136,172)(H,137,191)(H,138,166)(H,139,165)(H,140,173)(H,141,184)(H,142,186)(H,143,174)(H,144,188)(H,145,189)(H,146,187)(H,147,183)(H,148,181)(H,149,175)(H,150,180)(H,151,182)(H,152,171)(H,153,176)(H,154,190)(H,155,177)(H,156,178)(H,157,179)(H,158,185)(H,167,168)(H,169,170)(H,192,193)/t72-,73-,74+,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,102-,103-,104-,105-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416568
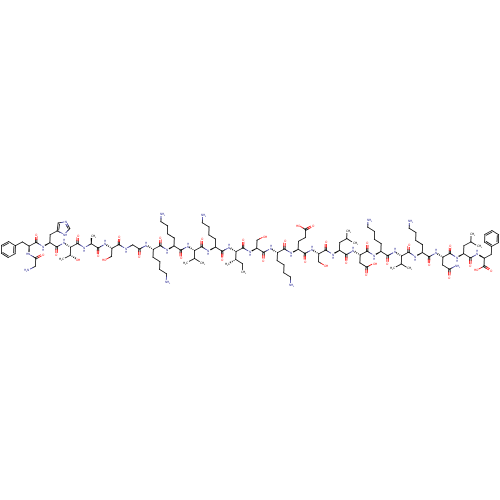
(CHEMBL1215817)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C121H201N33O34/c1-13-68(10)98(153-108(174)79(41-25-31-49-127)141-118(184)97(67(8)9)151-106(172)77(39-23-29-47-125)136-102(168)74(36-20-26-44-122)134-93(161)59-131-101(167)88(60-155)148-100(166)69(11)133-120(186)99(70(12)158)154-114(180)84(54-73-58-130-63-132-73)144-111(177)83(135-92(160)57-128)52-71-32-16-14-17-33-71)119(185)150-90(62-157)115(181)138-75(37-21-27-45-123)103(169)139-80(42-43-94(162)163)105(171)149-89(61-156)116(182)143-81(50-64(2)3)109(175)146-86(56-95(164)165)113(179)137-78(40-24-30-48-126)107(173)152-96(66(6)7)117(183)140-76(38-22-28-46-124)104(170)145-85(55-91(129)159)112(178)142-82(51-65(4)5)110(176)147-87(121(187)188)53-72-34-18-15-19-35-72/h14-19,32-35,58,63-70,74-90,96-99,155-158H,13,20-31,36-57,59-62,122-128H2,1-12H3,(H2,129,159)(H,130,132)(H,131,167)(H,133,186)(H,134,161)(H,135,160)(H,136,168)(H,137,179)(H,138,181)(H,139,169)(H,140,183)(H,141,184)(H,142,178)(H,143,182)(H,144,177)(H,145,170)(H,146,175)(H,147,176)(H,148,166)(H,149,171)(H,150,185)(H,151,172)(H,152,173)(H,153,174)(H,154,180)(H,162,163)(H,164,165)(H,187,188)/t68-,69-,70+,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153106
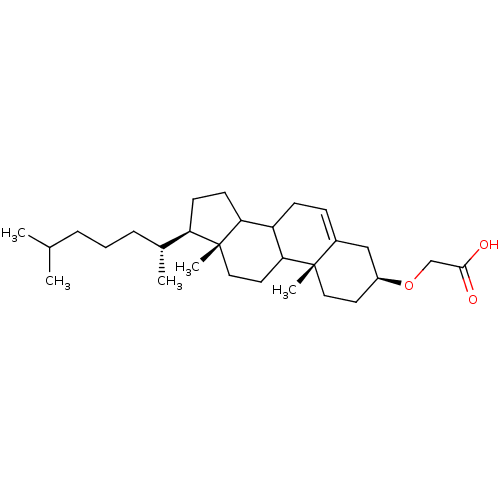
(CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O |t:14| Show InChI InChI=1S/C29H48O3/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(32-18-27(30)31)13-15-28(21,4)26(23)14-16-29(24,25)5/h9,19-20,22-26H,6-8,10-18H2,1-5H3,(H,30,31)/t20-,22+,23?,24-,25?,26?,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416569
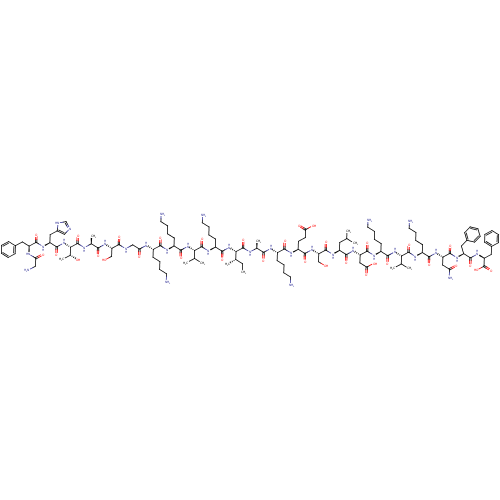
(CHEMBL1215818)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C124H199N33O33/c1-12-70(8)101(156-112(177)83(45-27-33-53-130)145-121(186)100(69(6)7)154-110(175)81(43-25-31-51-128)141-106(171)78(40-22-28-48-125)138-96(163)63-134-105(170)92(64-158)152-104(169)72(10)137-123(188)102(73(11)160)157-118(183)88(58-77-62-133-66-135-77)148-114(179)86(139-95(162)61-131)55-74-34-16-13-17-35-74)122(187)136-71(9)103(168)140-79(41-23-29-49-126)107(172)143-84(46-47-97(164)165)109(174)153-93(65-159)119(184)146-85(54-67(2)3)113(178)150-90(60-98(166)167)117(182)142-82(44-26-32-52-129)111(176)155-99(68(4)5)120(185)144-80(42-24-30-50-127)108(173)149-89(59-94(132)161)116(181)147-87(56-75-36-18-14-19-37-75)115(180)151-91(124(189)190)57-76-38-20-15-21-39-76/h13-21,34-39,62,66-73,78-93,99-102,158-160H,12,22-33,40-61,63-65,125-131H2,1-11H3,(H2,132,161)(H,133,135)(H,134,170)(H,136,187)(H,137,188)(H,138,163)(H,139,162)(H,140,168)(H,141,171)(H,142,182)(H,143,172)(H,144,185)(H,145,186)(H,146,184)(H,147,181)(H,148,179)(H,149,173)(H,150,178)(H,151,180)(H,152,169)(H,153,174)(H,154,175)(H,155,176)(H,156,177)(H,157,183)(H,164,165)(H,166,167)(H,189,190)/t70-,71-,72-,73+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-,102-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50109030

(4-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES [H][C@@](C)(CCC(O)=O)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CCC4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16?,17+,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer dose |
Bioorg Med Chem Lett 12: 287-90 (2002)
BindingDB Entry DOI: 10.7270/Q2K936TC |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416566
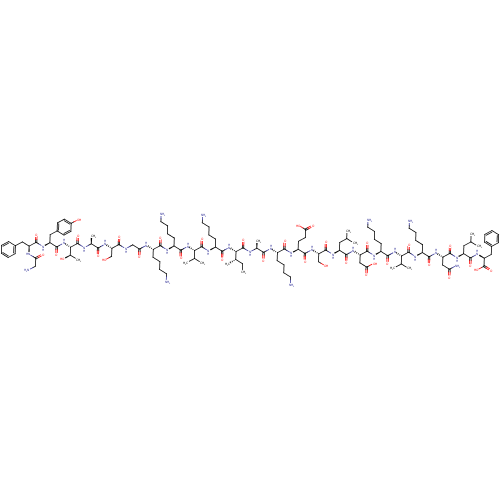
(CHEMBL1215815)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C124H203N31O34/c1-14-70(10)101(154-112(176)83(42-26-32-54-130)143-121(185)100(69(8)9)152-110(174)81(40-24-30-52-128)139-106(170)78(37-21-27-49-125)136-96(162)63-133-105(169)92(64-156)150-104(168)72(12)135-123(187)102(73(13)158)155-118(182)88(58-76-43-45-77(159)46-44-76)146-115(179)87(137-95(161)62-131)57-74-33-17-15-18-34-74)122(186)134-71(11)103(167)138-79(38-22-28-50-126)107(171)141-84(47-48-97(163)164)109(173)151-93(65-157)119(183)145-85(55-66(2)3)113(177)148-90(61-98(165)166)117(181)140-82(41-25-31-53-129)111(175)153-99(68(6)7)120(184)142-80(39-23-29-51-127)108(172)147-89(60-94(132)160)116(180)144-86(56-67(4)5)114(178)149-91(124(188)189)59-75-35-19-16-20-36-75/h15-20,33-36,43-46,66-73,78-93,99-102,156-159H,14,21-32,37-42,47-65,125-131H2,1-13H3,(H2,132,160)(H,133,169)(H,134,186)(H,135,187)(H,136,162)(H,137,161)(H,138,167)(H,139,170)(H,140,181)(H,141,171)(H,142,184)(H,143,185)(H,144,180)(H,145,183)(H,146,179)(H,147,172)(H,148,177)(H,149,178)(H,150,168)(H,151,173)(H,152,174)(H,153,175)(H,154,176)(H,155,182)(H,163,164)(H,165,166)(H,188,189)/t70-,71-,72-,73+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-,102-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50109030

(4-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES [H][C@@](C)(CCC(O)=O)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CCC4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C Show InChI InChI=1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16?,17+,18+,19-,20+,21+,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of nucleotide substrate c... |
Bioorg Med Chem Lett 12: 287-90 (2002)
BindingDB Entry DOI: 10.7270/Q2K936TC |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153111
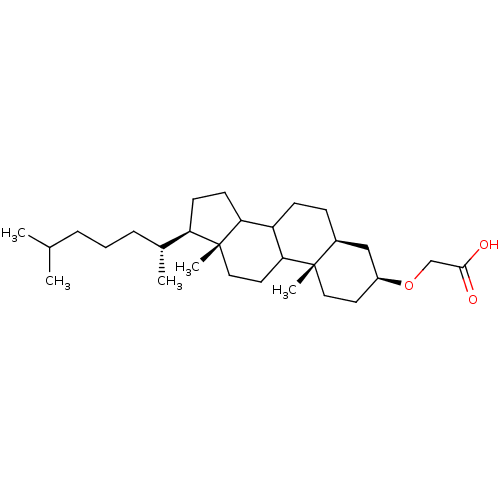
(CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2C3CC[C@H]4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O Show InChI InChI=1S/C29H50O3/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(32-18-27(30)31)13-15-28(21,4)26(23)14-16-29(24,25)5/h19-26H,6-18H2,1-5H3,(H,30,31)/t20-,21+,22+,23?,24-,25?,26?,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416564
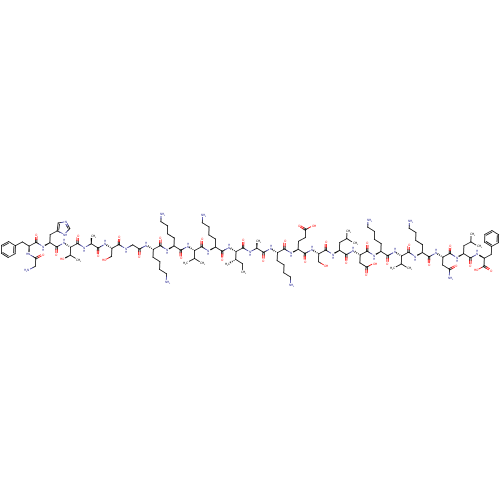
(CHEMBL1215813)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C121H201N33O33/c1-14-68(10)98(153-109(174)80(42-26-32-50-127)142-118(183)97(67(8)9)151-107(172)78(40-24-30-48-125)138-103(168)75(37-21-27-45-122)135-93(160)60-131-102(167)89(61-155)149-101(166)70(12)134-120(185)99(71(13)157)154-115(180)85(55-74-59-130-63-132-74)145-112(177)84(136-92(159)58-128)53-72-33-17-15-18-34-72)119(184)133-69(11)100(165)137-76(38-22-28-46-123)104(169)140-81(43-44-94(161)162)106(171)150-90(62-156)116(181)144-82(51-64(2)3)110(175)147-87(57-95(163)164)114(179)139-79(41-25-31-49-126)108(173)152-96(66(6)7)117(182)141-77(39-23-29-47-124)105(170)146-86(56-91(129)158)113(178)143-83(52-65(4)5)111(176)148-88(121(186)187)54-73-35-19-16-20-36-73/h15-20,33-36,59,63-71,75-90,96-99,155-157H,14,21-32,37-58,60-62,122-128H2,1-13H3,(H2,129,158)(H,130,132)(H,131,167)(H,133,184)(H,134,185)(H,135,160)(H,136,159)(H,137,165)(H,138,168)(H,139,179)(H,140,169)(H,141,182)(H,142,183)(H,143,178)(H,144,181)(H,145,177)(H,146,170)(H,147,175)(H,148,176)(H,149,166)(H,150,171)(H,151,172)(H,152,173)(H,153,174)(H,154,180)(H,161,162)(H,163,164)(H,186,187)/t68-,69-,70-,71+,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153113
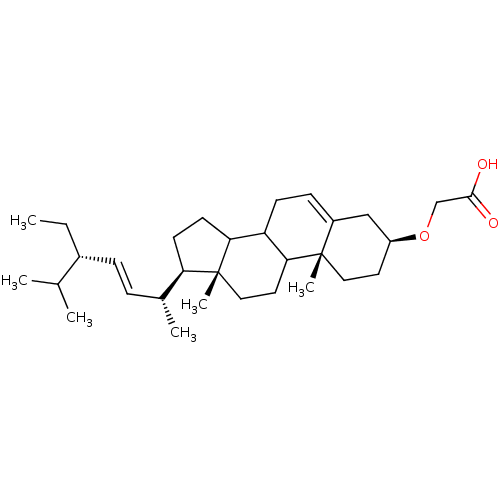
(CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O)C(C)C |t:13| Show InChI InChI=1S/C31H50O3/c1-7-22(20(2)3)9-8-21(4)26-12-13-27-25-11-10-23-18-24(34-19-29(32)33)14-16-30(23,5)28(25)15-17-31(26,27)6/h8-10,20-22,24-28H,7,11-19H2,1-6H3,(H,32,33)/b9-8+/t21-,22-,24+,25?,26-,27?,28?,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha non competitively on dNTP substrate |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50109029
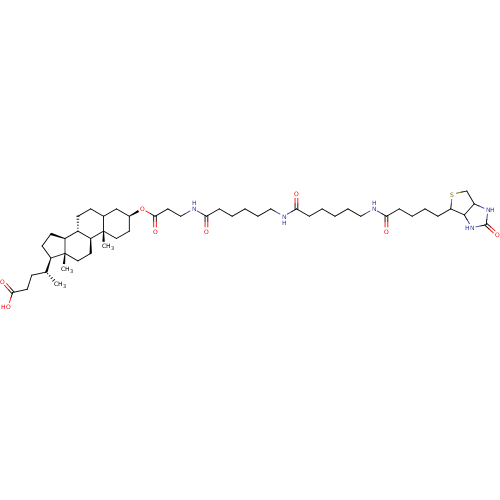
(4-{10,13-Dimethyl-3-[3-(6-{6-[5-(2-oxo-hexahydro-t...)Show SMILES [H][C@@](C)(CCC(O)=O)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CCC4C[C@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)CCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC1SCC2NC(=O)NC12 Show InChI InChI=1S/C49H81N5O8S/c1-32(16-21-44(58)59)36-19-20-37-35-18-17-33-30-34(22-25-48(33,2)38(35)23-26-49(36,37)3)62-45(60)24-29-52-43(57)14-7-5-11-27-50-41(55)13-6-4-10-28-51-42(56)15-9-8-12-40-46-39(31-63-40)53-47(61)54-46/h32-40,46H,4-31H2,1-3H3,(H,50,55)(H,51,56)(H,52,57)(H,58,59)(H2,53,54,61)/t32-,33?,34+,35+,36-,37+,38+,39?,40?,46?,48+,49-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer dose |
Bioorg Med Chem Lett 12: 287-90 (2002)
BindingDB Entry DOI: 10.7270/Q2K936TC |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153111

(CHEMBL189667 | [(3S,5S,10S,13R,17R)-17-((R)-1,5-Di...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2C3CC[C@H]4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O Show InChI InChI=1S/C29H50O3/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(32-18-27(30)31)13-15-28(21,4)26(23)14-16-29(24,25)5/h19-26H,6-18H2,1-5H3,(H,30,31)/t20-,21+,22+,23?,24-,25?,26?,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha competitively on DNA template |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153106

(CHEMBL189703 | [(3S,10R,13R,17R)-17-((R)-1,5-Dimet...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O |t:14| Show InChI InChI=1S/C29H48O3/c1-19(2)7-6-8-20(3)24-11-12-25-23-10-9-21-17-22(32-18-27(30)31)13-15-28(21,4)26(23)14-16-29(24,25)5/h9,19-20,22-26H,6-8,10-18H2,1-5H3,(H,30,31)/t20-,22+,23?,24-,25?,26?,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha competitively on DNA template |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50109029

(4-{10,13-Dimethyl-3-[3-(6-{6-[5-(2-oxo-hexahydro-t...)Show SMILES [H][C@@](C)(CCC(O)=O)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CCC4C[C@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)CCNC(=O)CCCCCNC(=O)CCCCCNC(=O)CCCCC1SCC2NC(=O)NC12 Show InChI InChI=1S/C49H81N5O8S/c1-32(16-21-44(58)59)36-19-20-37-35-18-17-33-30-34(22-25-48(33,2)38(35)23-26-49(36,37)3)62-45(60)24-29-52-43(57)14-7-5-11-27-50-41(55)13-6-4-10-28-51-42(56)15-9-8-12-40-46-39(31-63-40)53-47(61)54-46/h32-40,46H,4-31H2,1-3H3,(H,50,55)(H,51,56)(H,52,57)(H,58,59)(H2,53,54,61)/t32-,33?,34+,35+,36-,37+,38+,39?,40?,46?,48+,49-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science
Curated by ChEMBL
| Assay Description
Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of template primer dose |
Bioorg Med Chem Lett 12: 287-90 (2002)
BindingDB Entry DOI: 10.7270/Q2K936TC |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50153113

(CHEMBL364611 | [(3S,10R,13R,17R)-17-((E)-(1R,4S)-4...)Show SMILES CC[C@H](\C=C\[C@@H](C)[C@H]1CCC2C3CC=C4C[C@H](CC[C@]4(C)C3CC[C@]12C)OCC(O)=O)C(C)C |t:13| Show InChI InChI=1S/C31H50O3/c1-7-22(20(2)3)9-8-21(4)26-12-13-27-25-11-10-23-18-24(34-19-29(32)33)14-16-30(23,5)28(25)15-17-31(26,27)6/h8-10,20-22,24-28H,7,11-19H2,1-6H3,(H,32,33)/b9-8+/t21-,22-,24+,25?,26-,27?,28?,30+,31-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
| Assay Description
Inhibition constant against DNA polymerase alpha competitively on DNA template |
J Med Chem 47: 4971-4 (2004)
Article DOI: 10.1021/jm030553v
BindingDB Entry DOI: 10.7270/Q2RN37BH |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416567
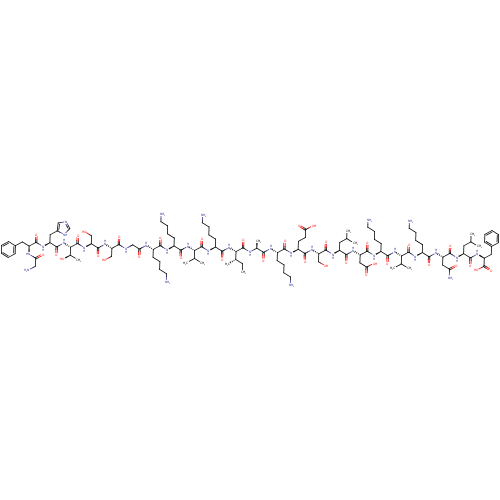
(CHEMBL1215816)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C121H201N33O34/c1-13-68(10)98(153-108(174)79(41-25-31-49-127)141-118(184)97(67(8)9)151-106(172)77(39-23-29-47-125)137-102(168)74(36-20-26-44-122)134-93(161)59-131-101(167)88(60-155)148-116(182)90(62-157)150-120(186)99(70(12)158)154-114(180)84(54-73-58-130-63-132-73)144-111(177)83(135-92(160)57-128)52-71-32-16-14-17-33-71)119(185)133-69(11)100(166)136-75(37-21-27-45-123)103(169)139-80(42-43-94(162)163)105(171)149-89(61-156)115(181)143-81(50-64(2)3)109(175)146-86(56-95(164)165)113(179)138-78(40-24-30-48-126)107(173)152-96(66(6)7)117(183)140-76(38-22-28-46-124)104(170)145-85(55-91(129)159)112(178)142-82(51-65(4)5)110(176)147-87(121(187)188)53-72-34-18-15-19-35-72/h14-19,32-35,58,63-70,74-90,96-99,155-158H,13,20-31,36-57,59-62,122-128H2,1-12H3,(H2,129,159)(H,130,132)(H,131,167)(H,133,185)(H,134,161)(H,135,160)(H,136,166)(H,137,168)(H,138,179)(H,139,169)(H,140,183)(H,141,184)(H,142,178)(H,143,181)(H,144,177)(H,145,170)(H,146,175)(H,147,176)(H,148,182)(H,149,171)(H,150,186)(H,151,172)(H,152,173)(H,153,174)(H,154,180)(H,162,163)(H,164,165)(H,187,188)/t68-,69-,70+,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p... |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118478
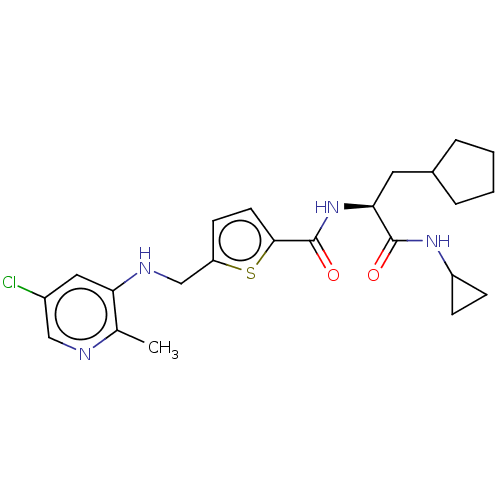
(CHEMBL3613749)Show SMILES Cc1ncc(Cl)cc1NCc1ccc(s1)C(=O)N[C@@H](CC1CCCC1)C(=O)NC1CC1 |r| Show InChI InChI=1S/C23H29ClN4O2S/c1-14-19(11-16(24)12-25-14)26-13-18-8-9-21(31-18)23(30)28-20(10-15-4-2-3-5-15)22(29)27-17-6-7-17/h8-9,11-12,15,17,20,26H,2-7,10,13H2,1H3,(H,27,29)(H,28,30)/t20-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N... |
Bioorg Med Chem 23: 6246-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.042
BindingDB Entry DOI: 10.7270/Q26975C7 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50361941
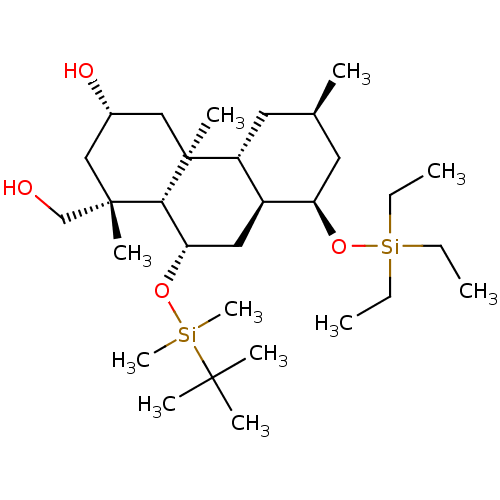
(CHEMBL1939361)Show SMILES CC[Si](CC)(CC)O[C@@H]1C[C@H](C)C[C@@H]2[C@@H]1C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1[C@](C)(CO)C[C@H](O)C[C@@]21C |r| Show InChI InChI=1S/C30H60O4Si2/c1-12-36(13-2,14-3)34-25-16-21(4)15-24-23(25)17-26(33-35(10,11)28(5,6)7)27-29(8,20-31)18-22(32)19-30(24,27)9/h21-27,31-32H,12-20H2,1-11H3/t21-,22+,23+,24-,25-,26+,27-,29+,30+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N... |
Bioorg Med Chem 23: 6246-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.042
BindingDB Entry DOI: 10.7270/Q26975C7 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118477

(CHEMBL3613748)Show SMILES [H][C@]12C[C@@H](C[C@@](C)(C(O)=O)[C@]1([H])CCC[C@H]2O[Si](CC)(CC)CC)O[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C24H48O4Si2/c1-10-30(11-2,12-3)28-21-15-13-14-20-19(21)16-18(17-24(20,7)22(25)26)27-29(8,9)23(4,5)6/h18-21H,10-17H2,1-9H3,(H,25,26)/t18-,19-,20+,21+,24+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N... |
Bioorg Med Chem 23: 6246-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.042
BindingDB Entry DOI: 10.7270/Q26975C7 |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50118476
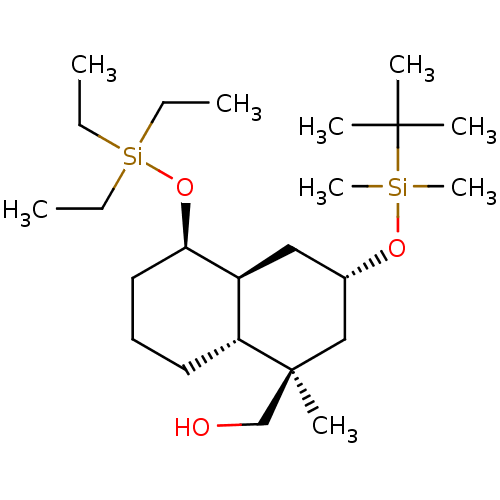
(CHEMBL3613747)Show SMILES [H][C@]12C[C@@H](C[C@@](C)(CO)[C@]1([H])CCC[C@H]2O[Si](CC)(CC)CC)O[Si](C)(C)C(C)(C)C |r| Show InChI InChI=1S/C24H50O3Si2/c1-10-29(11-2,12-3)27-22-15-13-14-21-20(22)16-19(17-24(21,7)18-25)26-28(8,9)23(4,5)6/h19-22,25H,10-18H2,1-9H3/t19-,20-,21+,22+,24-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N... |
Bioorg Med Chem 23: 6246-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.042
BindingDB Entry DOI: 10.7270/Q26975C7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481610
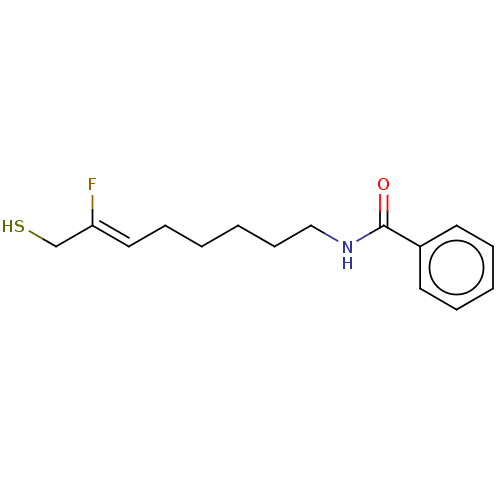
(CHEMBL609609)Show InChI InChI=1S/C15H20FNOS/c16-14(12-19)10-6-1-2-7-11-17-15(18)13-8-4-3-5-9-13/h3-5,8-10,19H,1-2,6-7,11-12H2,(H,17,18)/b14-10- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assay |
Bioorg Med Chem 18: 605-11 (2010)
Article DOI: 10.1016/j.bmc.2009.12.005
BindingDB Entry DOI: 10.7270/Q20C4ZMK |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481612
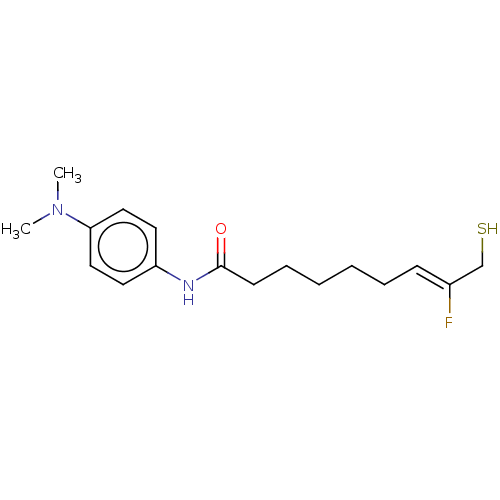
(CHEMBL596660)Show InChI InChI=1S/C17H25FN2OS/c1-20(2)16-11-9-15(10-12-16)19-17(21)8-6-4-3-5-7-14(18)13-22/h7,9-12,22H,3-6,8,13H2,1-2H3,(H,19,21)/b14-7- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assay |
Bioorg Med Chem 18: 605-11 (2010)
Article DOI: 10.1016/j.bmc.2009.12.005
BindingDB Entry DOI: 10.7270/Q20C4ZMK |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481613
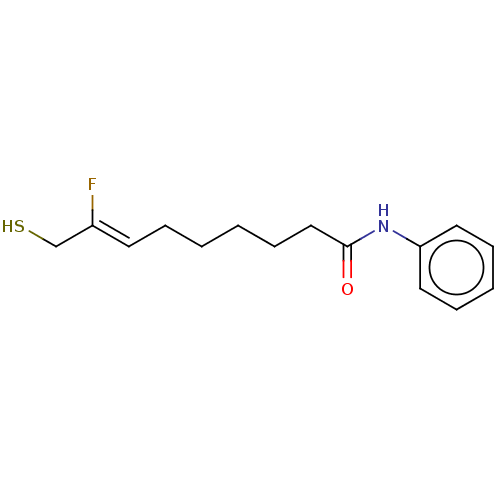
(CHEMBL597443)Show InChI InChI=1S/C15H20FNOS/c16-13(12-19)8-4-1-2-7-11-15(18)17-14-9-5-3-6-10-14/h3,5-6,8-10,19H,1-2,4,7,11-12H2,(H,17,18)/b13-8- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assay |
Bioorg Med Chem 18: 605-11 (2010)
Article DOI: 10.1016/j.bmc.2009.12.005
BindingDB Entry DOI: 10.7270/Q20C4ZMK |
More data for this
Ligand-Target Pair | |
Protein phosphatase 1D
(Homo sapiens (Human)) | BDBM50361941

(CHEMBL1939361)Show SMILES CC[Si](CC)(CC)O[C@@H]1C[C@H](C)C[C@@H]2[C@@H]1C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1[C@](C)(CO)C[C@H](O)C[C@@]21C |r| Show InChI InChI=1S/C30H60O4Si2/c1-12-36(13-2,14-3)34-25-16-21(4)15-24-23(25)17-26(33-35(10,11)28(5,6)7)27-29(8,20-31)18-22(32)19-30(24,27)9/h21-27,31-32H,12-20H2,1-11H3/t21-,22+,23+,24-,25-,26+,27-,29+,30+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal histidine-tagged PPM1Dc after 10 mins using BIOMOL GREEN assay |
Bioorg Med Chem Lett 22: 729-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.084
BindingDB Entry DOI: 10.7270/Q21N81KJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50481611
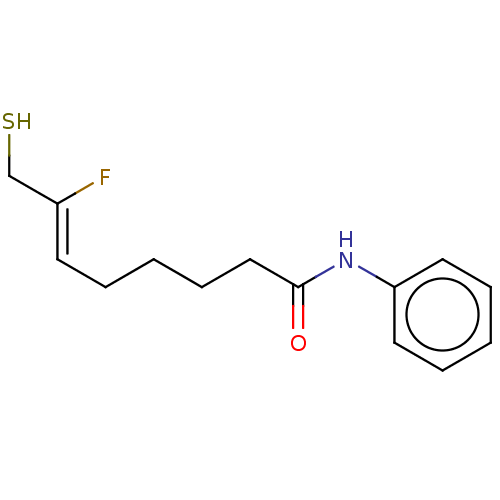
(CHEMBL599968)Show InChI InChI=1S/C14H18FNOS/c15-12(11-18)7-3-1-6-10-14(17)16-13-8-4-2-5-9-13/h2,4-5,7-9,18H,1,3,6,10-11H2,(H,16,17)/b12-7- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assay |
Bioorg Med Chem 18: 605-11 (2010)
Article DOI: 10.1016/j.bmc.2009.12.005
BindingDB Entry DOI: 10.7270/Q20C4ZMK |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416570

(CHEMBL1215819)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C127H201N31O35/c1-11-72(8)104(157-114(179)85(44-26-32-56-133)145-124(189)103(71(6)7)155-112(177)83(42-24-30-54-131)140-108(173)80(39-21-27-51-128)138-99(166)65-136-107(172)94(66-159)152-106(171)73(9)137-126(191)105(74(10)162)158-120(185)90(60-78-45-47-79(163)48-46-78)148-116(181)88(139-98(165)64-134)58-75-33-15-12-16-34-75)125(190)154-96(68-161)121(186)142-81(40-22-28-52-129)109(174)143-86(49-50-100(167)168)111(176)153-95(67-160)122(187)146-87(57-69(2)3)115(180)150-92(63-101(169)170)119(184)141-84(43-25-31-55-132)113(178)156-102(70(4)5)123(188)144-82(41-23-29-53-130)110(175)149-91(62-97(135)164)118(183)147-89(59-76-35-17-13-18-36-76)117(182)151-93(127(192)193)61-77-37-19-14-20-38-77/h12-20,33-38,45-48,69-74,80-96,102-105,159-163H,11,21-32,39-44,49-68,128-134H2,1-10H3,(H2,135,164)(H,136,172)(H,137,191)(H,138,166)(H,139,165)(H,140,173)(H,141,184)(H,142,186)(H,143,174)(H,144,188)(H,145,189)(H,146,187)(H,147,183)(H,148,181)(H,149,175)(H,150,180)(H,151,182)(H,152,171)(H,153,176)(H,154,190)(H,155,177)(H,156,178)(H,157,179)(H,158,185)(H,167,168)(H,169,170)(H,192,193)/t72-,73-,74+,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,102-,103-,104-,105-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saga University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assay |
Bioorg Med Chem 18: 605-11 (2010)
Article DOI: 10.1016/j.bmc.2009.12.005
BindingDB Entry DOI: 10.7270/Q20C4ZMK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416570

(CHEMBL1215819)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C127H201N31O35/c1-11-72(8)104(157-114(179)85(44-26-32-56-133)145-124(189)103(71(6)7)155-112(177)83(42-24-30-54-131)140-108(173)80(39-21-27-51-128)138-99(166)65-136-107(172)94(66-159)152-106(171)73(9)137-126(191)105(74(10)162)158-120(185)90(60-78-45-47-79(163)48-46-78)148-116(181)88(139-98(165)64-134)58-75-33-15-12-16-34-75)125(190)154-96(68-161)121(186)142-81(40-22-28-52-129)109(174)143-86(49-50-100(167)168)111(176)153-95(67-160)122(187)146-87(57-69(2)3)115(180)150-92(63-101(169)170)119(184)141-84(43-25-31-55-132)113(178)156-102(70(4)5)123(188)144-82(41-23-29-53-130)110(175)149-91(62-97(135)164)118(183)147-89(59-76-35-17-13-18-36-76)117(182)151-93(127(192)193)61-77-37-19-14-20-38-77/h12-20,33-38,45-48,69-74,80-96,102-105,159-163H,11,21-32,39-44,49-68,128-134H2,1-10H3,(H2,135,164)(H,136,172)(H,137,191)(H,138,166)(H,139,165)(H,140,173)(H,141,184)(H,142,186)(H,143,174)(H,144,188)(H,145,189)(H,146,187)(H,147,183)(H,148,181)(H,149,175)(H,150,180)(H,151,182)(H,152,171)(H,153,176)(H,154,190)(H,155,177)(H,156,178)(H,157,179)(H,158,185)(H,167,168)(H,169,170)(H,192,193)/t72-,73-,74+,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,102-,103-,104-,105-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184758
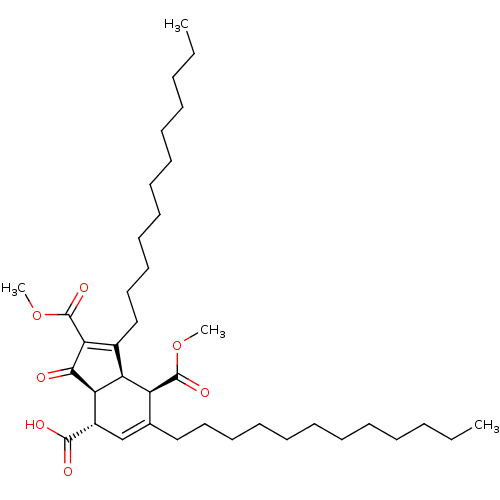
((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184757
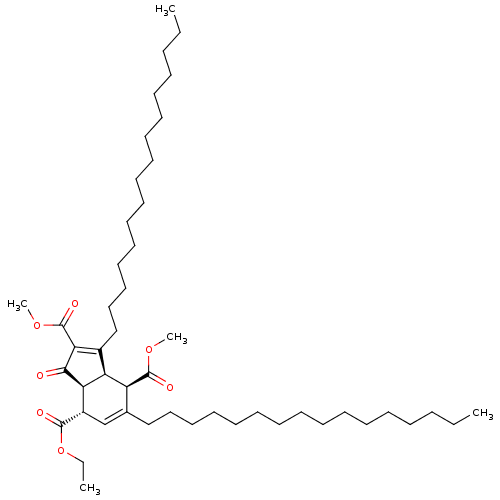
((3aR,4R,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41-,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184755
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416568

(CHEMBL1215817)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C121H201N33O34/c1-13-68(10)98(153-108(174)79(41-25-31-49-127)141-118(184)97(67(8)9)151-106(172)77(39-23-29-47-125)136-102(168)74(36-20-26-44-122)134-93(161)59-131-101(167)88(60-155)148-100(166)69(11)133-120(186)99(70(12)158)154-114(180)84(54-73-58-130-63-132-73)144-111(177)83(135-92(160)57-128)52-71-32-16-14-17-33-71)119(185)150-90(62-157)115(181)138-75(37-21-27-45-123)103(169)139-80(42-43-94(162)163)105(171)149-89(61-156)116(182)143-81(50-64(2)3)109(175)146-86(56-95(164)165)113(179)137-78(40-24-30-48-126)107(173)152-96(66(6)7)117(183)140-76(38-22-28-46-124)104(170)145-85(55-91(129)159)112(178)142-82(51-65(4)5)110(176)147-87(121(187)188)53-72-34-18-15-19-35-72/h14-19,32-35,58,63-70,74-90,96-99,155-158H,13,20-31,36-57,59-62,122-128H2,1-12H3,(H2,129,159)(H,130,132)(H,131,167)(H,133,186)(H,134,161)(H,135,160)(H,136,168)(H,137,179)(H,138,181)(H,139,169)(H,140,183)(H,141,184)(H,142,178)(H,143,182)(H,144,177)(H,145,170)(H,146,175)(H,147,176)(H,148,166)(H,149,171)(H,150,185)(H,151,172)(H,152,173)(H,153,174)(H,154,180)(H,162,163)(H,164,165)(H,187,188)/t68-,69-,70+,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416561
(CHEMBL1215810)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C142H235N37O41/c1-18-79(14)115(178-128(205)92(46-30-36-56-148)164-139(216)114(78(12)13)176-126(203)90(44-28-34-54-146)160-122(199)87(41-25-31-51-143)157-107(184)68-153-121(198)104(70-180)174-118(195)81(16)156-141(218)116(82(17)182)179-136(213)100(63-85-67-151-72-154-85)170-131(208)98(61-83-37-21-19-22-38-83)158-108(185)69-152-120(197)95(58-74(4)5)166-119(196)86(149)57-73(2)3)140(217)155-80(15)117(194)159-88(42-26-32-52-144)123(200)162-93(47-49-109(186)187)125(202)175-105(71-181)137(214)168-97(60-76(8)9)130(207)172-102(65-111(190)191)134(211)161-91(45-29-35-55-147)127(204)177-113(77(10)11)138(215)163-89(43-27-33-53-145)124(201)171-101(64-106(150)183)133(210)167-96(59-75(6)7)129(206)169-99(62-84-39-23-20-24-40-84)132(209)173-103(66-112(192)193)135(212)165-94(142(219)220)48-50-110(188)189/h19-24,37-40,67,72-82,86-105,113-116,180-182H,18,25-36,41-66,68-71,143-149H2,1-17H3,(H2,150,183)(H,151,154)(H,152,197)(H,153,198)(H,155,217)(H,156,218)(H,157,184)(H,158,185)(H,159,194)(H,160,199)(H,161,211)(H,162,200)(H,163,215)(H,164,216)(H,165,212)(H,166,196)(H,167,210)(H,168,214)(H,169,206)(H,170,208)(H,171,201)(H,172,207)(H,173,209)(H,174,195)(H,175,202)(H,176,203)(H,177,204)(H,178,205)(H,179,213)(H,186,187)(H,188,189)(H,190,191)(H,192,193)(H,219,220)/t79-,80-,81-,82+,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,113-,114-,115-,116-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded DNA complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184757

((3aR,4R,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41-,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184758

((3aS,4S,7R,7aR)-1,6-didodecyl-2,7-bis(methoxycarbo...)Show SMILES CCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:12,40| Show InChI InChI=1S/C38H62O7/c1-5-7-9-11-13-15-17-19-21-23-25-28-27-30(36(40)41)33-32(31(28)37(42)44-3)29(34(35(33)39)38(43)45-4)26-24-22-20-18-16-14-12-10-8-6-2/h27,30-33H,5-26H2,1-4H3,(H,40,41)/t30-,31-,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184756
((3aR,4S,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41+,42-,43+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA nucleotidylexotransferase
(Homo sapiens (Human)) | BDBM50184755

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human TdT |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416568

(CHEMBL1215817)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C121H201N33O34/c1-13-68(10)98(153-108(174)79(41-25-31-49-127)141-118(184)97(67(8)9)151-106(172)77(39-23-29-47-125)136-102(168)74(36-20-26-44-122)134-93(161)59-131-101(167)88(60-155)148-100(166)69(11)133-120(186)99(70(12)158)154-114(180)84(54-73-58-130-63-132-73)144-111(177)83(135-92(160)57-128)52-71-32-16-14-17-33-71)119(185)150-90(62-157)115(181)138-75(37-21-27-45-123)103(169)139-80(42-43-94(162)163)105(171)149-89(61-156)116(182)143-81(50-64(2)3)109(175)146-86(56-95(164)165)113(179)137-78(40-24-30-48-126)107(173)152-96(66(6)7)117(183)140-76(38-22-28-46-124)104(170)145-85(55-91(129)159)112(178)142-82(51-65(4)5)110(176)147-87(121(187)188)53-72-34-18-15-19-35-72/h14-19,32-35,58,63-70,74-90,96-99,155-158H,13,20-31,36-57,59-62,122-128H2,1-12H3,(H2,129,159)(H,130,132)(H,131,167)(H,133,186)(H,134,161)(H,135,160)(H,136,168)(H,137,179)(H,138,181)(H,139,169)(H,140,183)(H,141,184)(H,142,178)(H,143,182)(H,144,177)(H,145,170)(H,146,175)(H,147,176)(H,148,166)(H,149,171)(H,150,185)(H,151,172)(H,152,173)(H,153,174)(H,154,180)(H,162,163)(H,164,165)(H,187,188)/t68-,69-,70+,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,96-,97-,98-,99-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA nucleotidylexotransferase
(Homo sapiens (Human)) | BDBM50143524
((3aR,4R,7R,7aS)-3,5-Dihexadecyl-3a,4,7,7a-tetrahyd...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O)C(O)=O |c:43,t:16| Show InChI InChI=1S/C45H78O6/c1-4-6-8-10-12-14-16-18-20-22-24-26-28-30-32-36-34-39(43(46)47)38-35-40(44(48)49)37(42(38)41(36)45(50)51-3)33-31-29-27-25-23-21-19-17-15-13-11-9-7-5-2/h34,38-39,41-42H,4-33,35H2,1-3H3,(H,46,47)(H,48,49)/t38-,39+,41+,42-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Science
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against human terminal deoxynucleotidyltransferase |
Bioorg Med Chem Lett 14: 1975-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.092
BindingDB Entry DOI: 10.7270/Q2W095C3 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416569

(CHEMBL1215818)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C124H199N33O33/c1-12-70(8)101(156-112(177)83(45-27-33-53-130)145-121(186)100(69(6)7)154-110(175)81(43-25-31-51-128)141-106(171)78(40-22-28-48-125)138-96(163)63-134-105(170)92(64-158)152-104(169)72(10)137-123(188)102(73(11)160)157-118(183)88(58-77-62-133-66-135-77)148-114(179)86(139-95(162)61-131)55-74-34-16-13-17-35-74)122(187)136-71(9)103(168)140-79(41-23-29-49-126)107(172)143-84(46-47-97(164)165)109(174)153-93(65-159)119(184)146-85(54-67(2)3)113(178)150-90(60-98(166)167)117(182)142-82(44-26-32-52-129)111(176)155-99(68(4)5)120(185)144-80(42-24-30-50-127)108(173)149-89(59-94(132)161)116(181)147-87(56-75-36-18-14-19-37-75)115(180)151-91(124(189)190)57-76-38-20-15-21-39-76/h13-21,34-39,62,66-73,78-93,99-102,158-160H,12,22-33,40-61,63-65,125-131H2,1-11H3,(H2,132,161)(H,133,135)(H,134,170)(H,136,187)(H,137,188)(H,138,163)(H,139,162)(H,140,168)(H,141,171)(H,142,182)(H,143,172)(H,144,185)(H,145,186)(H,146,184)(H,147,181)(H,148,179)(H,149,173)(H,150,178)(H,151,180)(H,152,169)(H,153,174)(H,154,175)(H,155,176)(H,156,177)(H,157,183)(H,164,165)(H,166,167)(H,189,190)/t70-,71-,72-,73+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-,102-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416566

(CHEMBL1215815)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C124H203N31O34/c1-14-70(10)101(154-112(176)83(42-26-32-54-130)143-121(185)100(69(8)9)152-110(174)81(40-24-30-52-128)139-106(170)78(37-21-27-49-125)136-96(162)63-133-105(169)92(64-156)150-104(168)72(12)135-123(187)102(73(13)158)155-118(182)88(58-76-43-45-77(159)46-44-76)146-115(179)87(137-95(161)62-131)57-74-33-17-15-18-34-74)122(186)134-71(11)103(167)138-79(38-22-28-50-126)107(171)141-84(47-48-97(163)164)109(173)151-93(65-157)119(183)145-85(55-66(2)3)113(177)148-90(61-98(165)166)117(181)140-82(41-25-31-53-129)111(175)153-99(68(6)7)120(184)142-80(39-23-29-51-127)108(172)147-89(60-94(132)160)116(180)144-86(56-67(4)5)114(178)149-91(124(188)189)59-75-35-19-16-20-36-75/h15-20,33-36,43-46,66-73,78-93,99-102,156-159H,14,21-32,37-42,47-65,125-131H2,1-13H3,(H2,132,160)(H,133,169)(H,134,186)(H,135,187)(H,136,162)(H,137,161)(H,138,167)(H,139,170)(H,140,181)(H,141,171)(H,142,184)(H,143,185)(H,144,180)(H,145,183)(H,146,179)(H,147,172)(H,148,177)(H,149,178)(H,150,168)(H,151,173)(H,152,174)(H,153,175)(H,154,176)(H,155,182)(H,163,164)(H,165,166)(H,188,189)/t70-,71-,72-,73+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-,102-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184755

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(methoxycar...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,48| Show InChI InChI=1S/C46H78O7/c1-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-36-35-38(44(48)49)41-40(39(36)45(50)52-3)37(42(43(41)47)46(51)53-4)34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-2/h35,38-41H,5-34H2,1-4H3,(H,48,49)/t38-,39-,40-,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184752
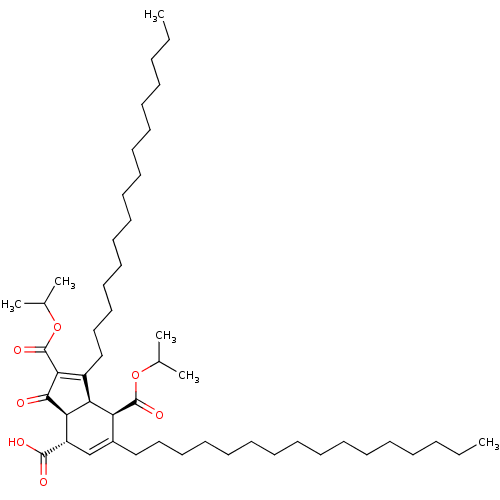
((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(isopropoxy...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC(C)C)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC(C)C)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,52| Show InChI InChI=1S/C50H86O7/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-40-37-42(48(52)53)45-44(43(40)49(54)56-38(3)4)41(46(47(45)51)50(55)57-39(5)6)36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2/h37-39,42-45H,7-36H2,1-6H3,(H,52,53)/t42-,43-,44-,45+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416562
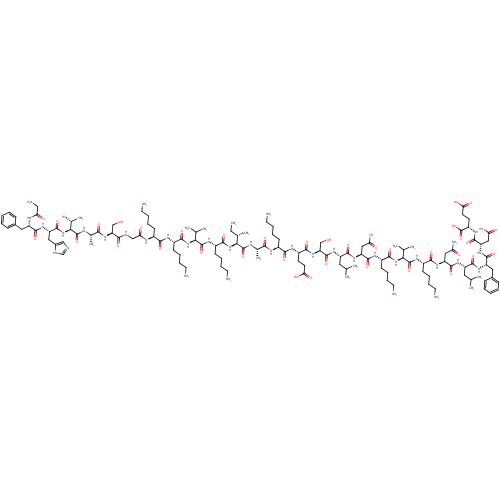
(CHEMBL1215811)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C130H213N35O39/c1-14-71(10)105(164-116(189)83(42-26-32-52-136)151-127(200)104(70(8)9)162-114(187)81(40-24-30-50-134)147-110(183)78(37-21-27-47-131)144-98(171)63-140-109(182)94(64-166)160-108(181)73(12)143-129(202)106(74(13)168)165-124(197)90(57-77-62-139-66-141-77)156-119(192)88(145-97(170)61-137)55-75-33-17-15-18-34-75)128(201)142-72(11)107(180)146-79(38-22-28-48-132)111(184)149-84(43-45-99(172)173)113(186)161-95(65-167)125(198)154-87(54-68(4)5)118(191)158-92(59-101(176)177)122(195)148-82(41-25-31-51-135)115(188)163-103(69(6)7)126(199)150-80(39-23-29-49-133)112(185)157-91(58-96(138)169)121(194)153-86(53-67(2)3)117(190)155-89(56-76-35-19-16-20-36-76)120(193)159-93(60-102(178)179)123(196)152-85(130(203)204)44-46-100(174)175/h15-20,33-36,62,66-74,78-95,103-106,166-168H,14,21-32,37-61,63-65,131-137H2,1-13H3,(H2,138,169)(H,139,141)(H,140,182)(H,142,201)(H,143,202)(H,144,171)(H,145,170)(H,146,180)(H,147,183)(H,148,195)(H,149,184)(H,150,199)(H,151,200)(H,152,196)(H,153,194)(H,154,198)(H,155,190)(H,156,192)(H,157,185)(H,158,191)(H,159,193)(H,160,181)(H,161,186)(H,162,187)(H,163,188)(H,164,189)(H,165,197)(H,172,173)(H,174,175)(H,176,177)(H,178,179)(H,203,204)/t71-,72-,73-,74+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,94-,95-,103-,104-,105-,106-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded DNA complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184756

((3aR,4S,7S,7aS)-7-ethyl 2,4-dimethyl 3,5-dihexadec...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC)C(=O)[C@H]2[C@@H]1[C@H](C(=O)OC)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(=O)OCC |c:16,48| Show InChI InChI=1S/C48H82O7/c1-6-9-11-13-15-17-19-21-23-25-27-29-31-33-35-38-37-40(46(50)55-8-3)43-42(41(38)47(51)53-4)39(44(45(43)49)48(52)54-5)36-34-32-30-28-26-24-22-20-18-16-14-12-10-7-2/h37,40-43H,6-36H2,1-5H3/t40-,41+,42-,43+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50184754
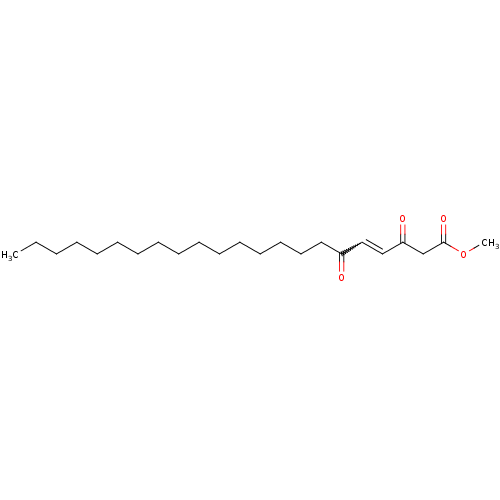
((E)-methyl-3,6-dioxo-4-docosenoate | CHEMBL425779)Show SMILES CCCCCCCCCCCCCCCCC(=O)C=CC(=O)CC(=O)OC |w:18.17| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)18-19-22(25)20-23(26)27-2/h18-19H,3-17,20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50143526

(CHEMBL366792 | Untenone A)Show SMILES CCCCCCCCCCCCCCCC[C@]1(O)C=CC(=O)[C@H]1C(=O)OC |c:18| Show InChI InChI=1S/C23H40O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18-23(26)19-17-20(24)21(23)22(25)27-2/h17,19,21,26H,3-16,18H2,1-2H3/t21-,23-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase alpha |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50184752

((3aS,4S,7R,7aR)-1,6-dihexadecyl-2,7-bis(isopropoxy...)Show SMILES CCCCCCCCCCCCCCCCC1=C(C(=O)OC(C)C)C(=O)[C@H]2[C@@H]1[C@@H](C(=O)OC(C)C)C(CCCCCCCCCCCCCCCC)=C[C@@H]2C(O)=O |c:16,52| Show InChI InChI=1S/C50H86O7/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-40-37-42(48(52)53)45-44(43(40)49(54)56-38(3)4)41(46(47(45)51)50(55)57-39(5)6)36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2/h37-39,42-45H,7-36H2,1-6H3,(H,52,53)/t42-,43-,44-,45+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
Bioorg Med Chem Lett 16: 2877-81 (2006)
Article DOI: 10.1016/j.bmcl.2006.03.005
BindingDB Entry DOI: 10.7270/Q25D8RF8 |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM50416569

(CHEMBL1215818)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CN)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C124H199N33O33/c1-12-70(8)101(156-112(177)83(45-27-33-53-130)145-121(186)100(69(6)7)154-110(175)81(43-25-31-51-128)141-106(171)78(40-22-28-48-125)138-96(163)63-134-105(170)92(64-158)152-104(169)72(10)137-123(188)102(73(11)160)157-118(183)88(58-77-62-133-66-135-77)148-114(179)86(139-95(162)61-131)55-74-34-16-13-17-35-74)122(187)136-71(9)103(168)140-79(41-23-29-49-126)107(172)143-84(46-47-97(164)165)109(174)153-93(65-159)119(184)146-85(54-67(2)3)113(178)150-90(60-98(166)167)117(182)142-82(44-26-32-52-129)111(176)155-99(68(4)5)120(185)144-80(42-24-30-50-127)108(173)149-89(59-94(132)161)116(181)147-87(56-75-36-18-14-19-37-75)115(180)151-91(124(189)190)57-76-38-20-15-21-39-76/h13-21,34-39,62,66-73,78-93,99-102,158-160H,12,22-33,40-61,63-65,125-131H2,1-11H3,(H2,132,161)(H,133,135)(H,134,170)(H,136,187)(H,137,188)(H,138,163)(H,139,162)(H,140,168)(H,141,171)(H,142,182)(H,143,172)(H,144,185)(H,145,186)(H,146,184)(H,147,181)(H,148,179)(H,149,173)(H,150,178)(H,151,180)(H,152,169)(H,153,174)(H,154,175)(H,155,176)(H,156,177)(H,157,183)(H,164,165)(H,166,167)(H,189,190)/t70-,71-,72-,73+,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-,102-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
| Assay Description
Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetry |
J Med Chem 53: 5782-91 (2010)
Article DOI: 10.1021/jm1002974
BindingDB Entry DOI: 10.7270/Q2NG4RV5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data