 Found 202 hits with Last Name = 'kish' and Initial = 'kf'
Found 202 hits with Last Name = 'kish' and Initial = 'kf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM29321
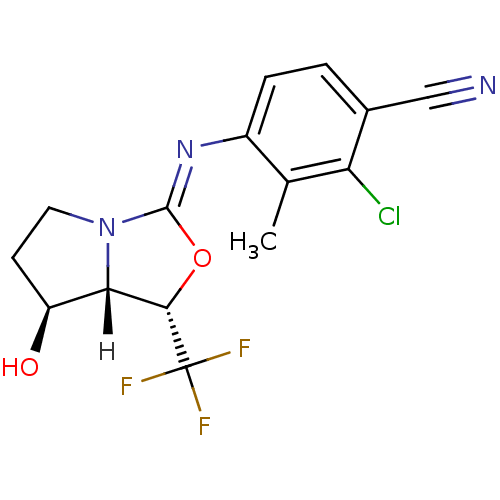
(oxazolidin-2-imine, 6d)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29323
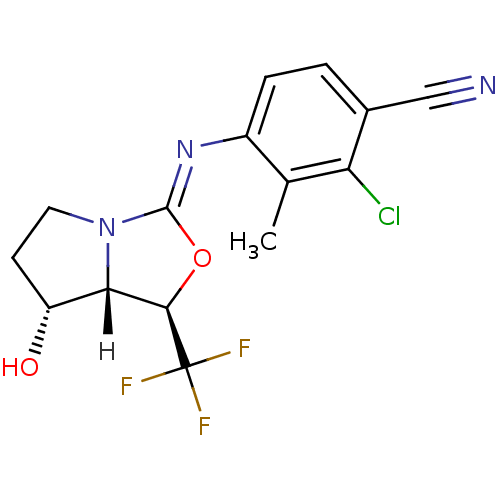
(oxazolidin-2-imine, 6f)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12+,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29319
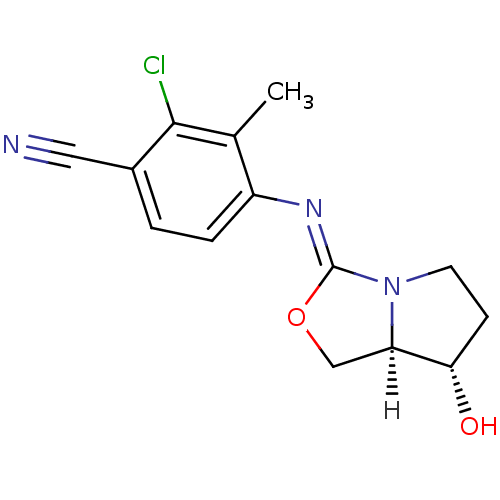
(oxazolidin-2-imine, 6b)Show SMILES [H][C@]12CO\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@@H]2O |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)17-14-18-5-4-12(19)11(18)7-20-14/h2-3,11-12,19H,4-5,7H2,1H3/b17-14-/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29320

(BMS-665139 | oxazolidin-2-imine, 6c)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376456
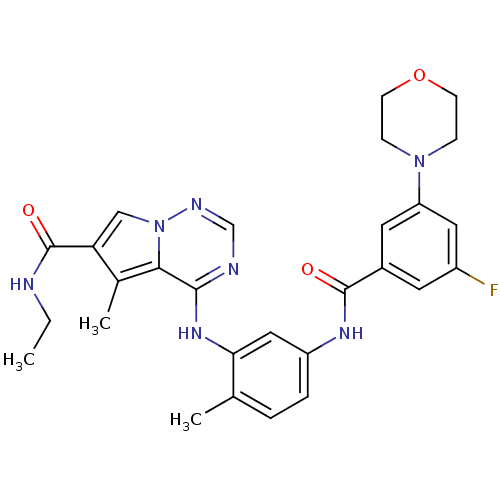
(CHEMBL262592)Show SMILES CCNC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C28H30FN7O3/c1-4-30-28(38)23-15-36-25(18(23)3)26(31-16-32-36)34-24-14-21(6-5-17(24)2)33-27(37)19-11-20(29)13-22(12-19)35-7-9-39-10-8-35/h5-6,11-16H,4,7-10H2,1-3H3,(H,30,38)(H,33,37)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376433

(CHEMBL258895)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4ccnc(c4)N4CCOCC4)ccc3C)c2c1C)c1ccccc1 Show InChI InChI=1S/C33H34N8O3/c1-21-9-10-26(38-32(42)25-11-12-34-29(17-25)40-13-15-44-16-14-40)18-28(21)39-31-30-22(2)27(19-41(30)36-20-35-31)33(43)37-23(3)24-7-5-4-6-8-24/h4-12,17-20,23H,13-16H2,1-3H3,(H,37,43)(H,38,42)(H,35,36,39)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376434

(CHEMBL408150)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cccc(c4)N4CCOCC4)ccc3C)c2c1C)c1ccccc1 |r| Show InChI InChI=1S/C34H35N7O3/c1-22-12-13-27(38-33(42)26-10-7-11-28(18-26)40-14-16-44-17-15-40)19-30(22)39-32-31-23(2)29(20-41(31)36-21-35-32)34(43)37-24(3)25-8-5-4-6-9-25/h4-13,18-21,24H,14-17H2,1-3H3,(H,37,43)(H,38,42)(H,35,36,39)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376435
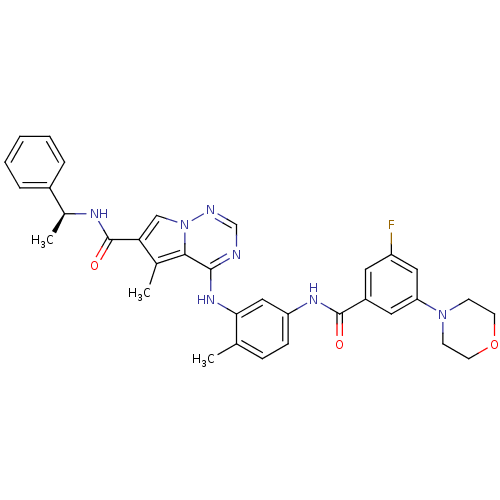
(CHEMBL261845)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C)c1ccccc1 Show InChI InChI=1S/C34H34FN7O3/c1-21-9-10-27(39-33(43)25-15-26(35)17-28(16-25)41-11-13-45-14-12-41)18-30(21)40-32-31-22(2)29(19-42(31)37-20-36-32)34(44)38-23(3)24-7-5-4-6-8-24/h4-10,15-20,23H,11-14H2,1-3H3,(H,38,44)(H,39,43)(H,36,37,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29324
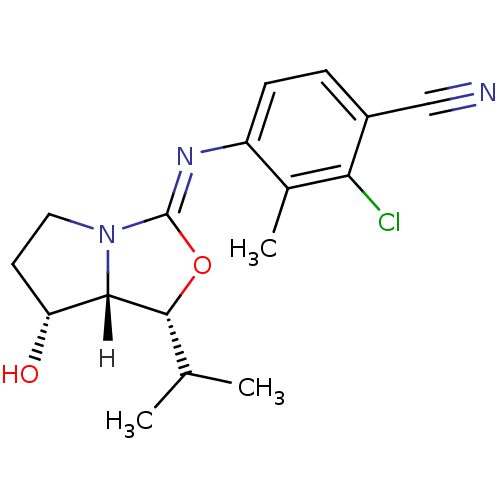
(oxazolidin-2-imine, 6g)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@@H]2C(C)C)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C17H20ClN3O2/c1-9(2)16-15-13(22)6-7-21(15)17(23-16)20-12-5-4-11(8-19)14(18)10(12)3/h4-5,9,13,15-16,22H,6-7H2,1-3H3/b20-17-/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 3.70 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29318

(oxazolidin-2-imine, 6a)Show SMILES [H][C@]12CO\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@H]2O |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)17-14-18-5-4-12(19)11(18)7-20-14/h2-3,11-12,19H,4-5,7H2,1H3/b17-14-/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | -51.4 | n/a | n/a | 4.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376432
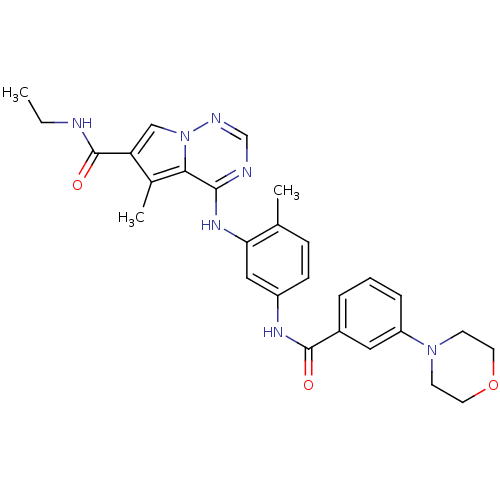
(CHEMBL258748)Show SMILES CCNC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cccc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C28H31N7O3/c1-4-29-28(37)23-16-35-25(19(23)3)26(30-17-31-35)33-24-15-21(9-8-18(24)2)32-27(36)20-6-5-7-22(14-20)34-10-12-38-13-11-34/h5-9,14-17H,4,10-13H2,1-3H3,(H,29,37)(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18176

(4-[(1S,7S,7aR)-7-hydroxy-1-methyl-3-oxo-hexahydro-...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2C)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H16ClN3O2/c1-8-11(4-3-10(7-17)13(8)16)19-9(2)14-12(20)5-6-18(14)15(19)21/h3-4,9,12,14,20H,5-6H2,1-2H3/t9-,12-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | -49.7 | n/a | n/a | 5.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29326

(guanidine derivative, 12)Show SMILES [H][C@]12CN(C)\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@H]2O |r| Show InChI InChI=1S/C15H17ClN4O/c1-9-11(4-3-10(7-17)14(9)16)18-15-19(2)8-12-13(21)5-6-20(12)15/h3-4,12-13,21H,5-6,8H2,1-2H3/b18-15+/t12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | -49.3 | n/a | n/a | 44 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29322
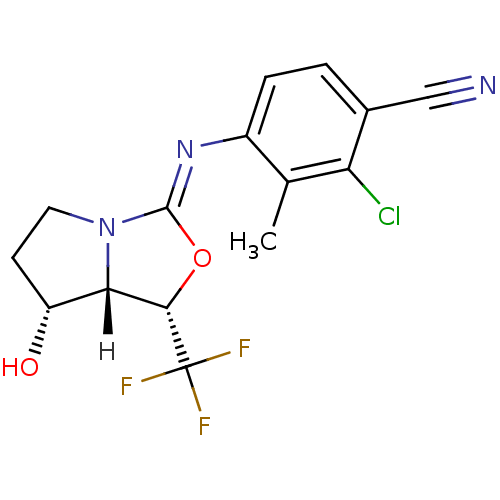
(oxazolidin-2-imine, 6e)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | -48.8 | n/a | n/a | 1.10 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376457
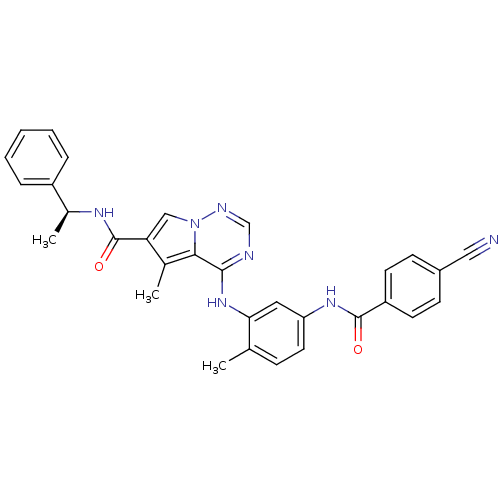
(CHEMBL259596)Show SMILES C[C@H](NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4ccc(cc4)C#N)ccc3C)c2c1C)c1ccccc1 Show InChI InChI=1S/C31H27N7O2/c1-19-9-14-25(36-30(39)24-12-10-22(16-32)11-13-24)15-27(19)37-29-28-20(2)26(17-38(28)34-18-33-29)31(40)35-21(3)23-7-5-4-6-8-23/h4-15,17-18,21H,1-3H3,(H,35,40)(H,36,39)(H,33,34,37)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18174
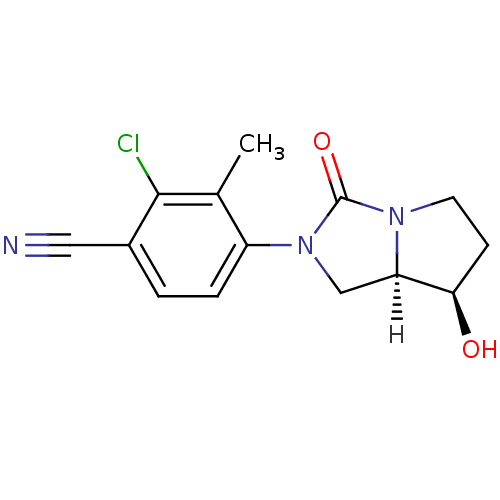
(4-[(7R,7aR)-7-hydroxy-3-oxo-hexahydro-1H-pyrrolo[1...)Show SMILES [H][C@]12CN(C(=O)N1CC[C@H]2O)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)18-7-11-12(19)4-5-17(11)14(18)20/h2-3,11-12,19H,4-5,7H2,1H3/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | 6.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29325
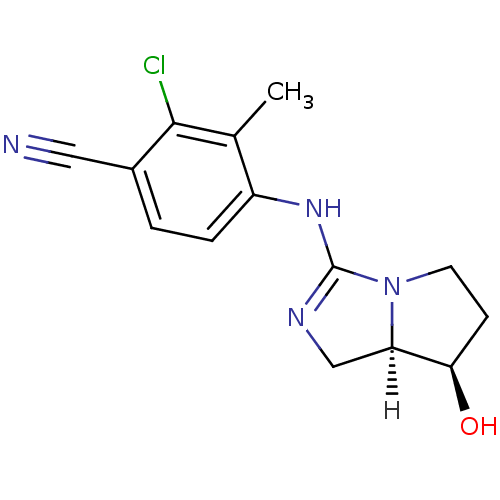
(guanidine derivative, 11)Show SMILES [H][C@]12CN=C(Nc3ccc(C#N)c(Cl)c3C)N1CC[C@H]2O |r,t:3| Show InChI InChI=1S/C14H15ClN4O/c1-8-10(3-2-9(6-16)13(8)15)18-14-17-7-11-12(20)4-5-19(11)14/h2-3,11-12,20H,4-5,7H2,1H3,(H,17,18)/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 1.80E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18173
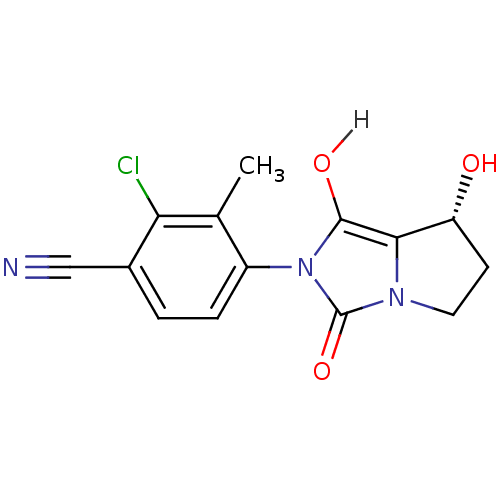
(4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...)Show SMILES Cc1c(Cl)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |r,wU:12.13,(.01,.36,;.81,1.67,;2.35,1.63,;3.09,.28,;3.16,2.94,;2.42,4.3,;.88,4.34,;.08,3.02,;-1.46,3.02,;-1.94,4.49,;-1.03,5.73,;-3.48,4.49,;-4.72,5.39,;-4.72,6.93,;-5.97,4.49,;-5.5,3.02,;-3.95,3.02,;-2.71,2.12,;-2.71,.58,;4.7,2.9,;6.24,2.9,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 14 | -44.4 | n/a | n/a | 7.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM29328
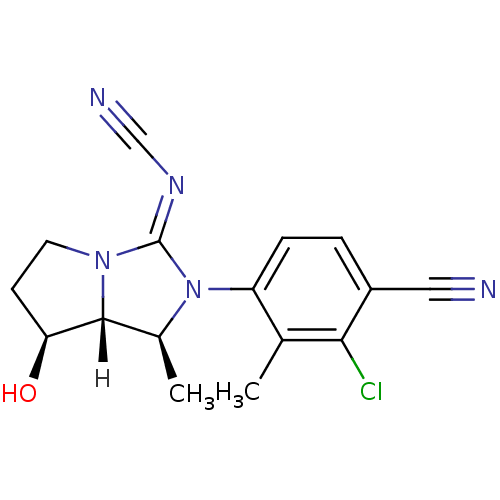
(cyanoguanidine, 15)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(=N/C#N)N([C@H]2C)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H16ClN5O/c1-9-12(4-3-11(7-18)14(9)17)22-10(2)15-13(23)5-6-21(15)16(22)20-8-19/h3-4,10,13,15,23H,5-6H2,1-2H3/b20-16+/t10-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 17 | -43.9 | n/a | n/a | 1.11E+3 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29327
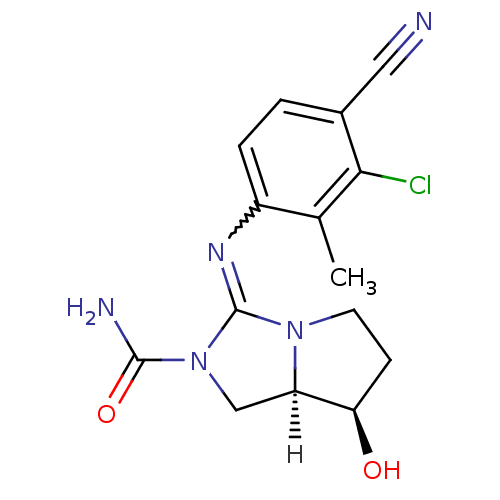
(guanidine derivative, 13)Show SMILES [H][C@]12CN(C(N)=O)C(=Nc3ccc(C#N)c(Cl)c3C)N1CC[C@H]2O |r,w:8.8| Show InChI InChI=1S/C15H16ClN5O2/c1-8-10(3-2-9(6-17)13(8)16)19-15-20-5-4-12(22)11(20)7-21(15)14(18)23/h2-3,11-12,22H,4-5,7H2,1H3,(H2,18,23)/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492385
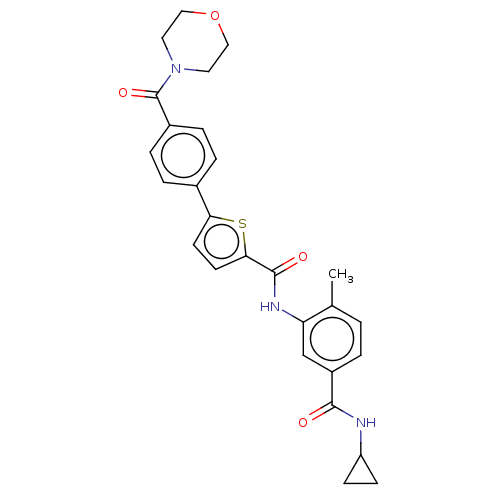
(CHEMBL2401994)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1ccc(cc1)C(=O)N1CCOCC1)C(=O)NC1CC1 Show InChI InChI=1S/C27H27N3O4S/c1-17-2-3-20(25(31)28-21-8-9-21)16-22(17)29-26(32)24-11-10-23(35-24)18-4-6-19(7-5-18)27(33)30-12-14-34-15-13-30/h2-7,10-11,16,21H,8-9,12-15H2,1H3,(H,28,31)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50492380

(CHEMBL2402001)Show SMILES Cc1ccc(cn1)-c1ccc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H21N3O2S/c1-13-3-5-15(21(26)24-17-7-8-17)11-18(13)25-22(27)20-10-9-19(28-20)16-6-4-14(2)23-12-16/h3-6,9-12,17H,7-8H2,1-2H3,(H,24,26)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAPK-mediated TNFalpha production in LPS-induced human whole blood preincubated for 5 mins prior to LPS-treatment measured after 6 ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492384
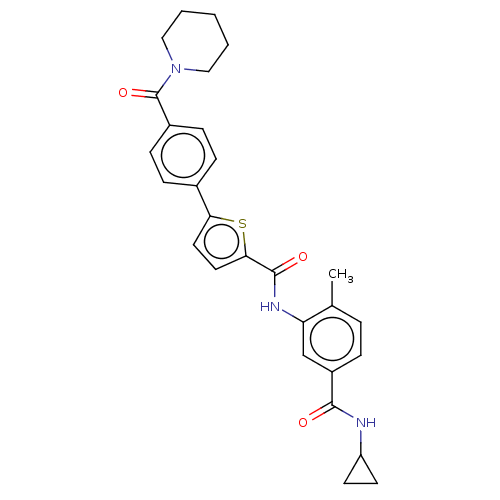
(CHEMBL2401995)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1ccc(cc1)C(=O)N1CCCCC1)C(=O)NC1CC1 Show InChI InChI=1S/C28H29N3O3S/c1-18-5-6-21(26(32)29-22-11-12-22)17-23(18)30-27(33)25-14-13-24(35-25)19-7-9-20(10-8-19)28(34)31-15-3-2-4-16-31/h5-10,13-14,17,22H,2-4,11-12,15-16H2,1H3,(H,29,32)(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376455
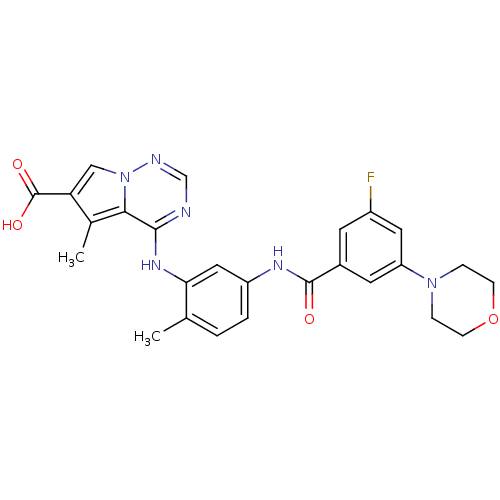
(CHEMBL261007)Show SMILES Cc1c(cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c12)C(O)=O Show InChI InChI=1S/C26H25FN6O4/c1-15-3-4-19(30-25(34)17-9-18(27)11-20(10-17)32-5-7-37-8-6-32)12-22(15)31-24-23-16(2)21(26(35)36)13-33(23)29-14-28-24/h3-4,9-14H,5-8H2,1-2H3,(H,30,34)(H,35,36)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50492375
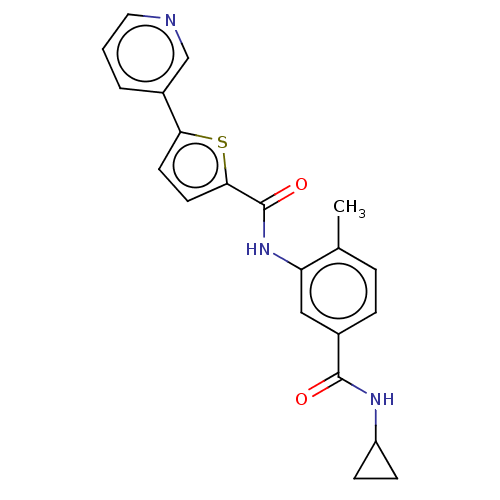
(CHEMBL2401997)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1cccnc1)C(=O)NC1CC1 Show InChI InChI=1S/C21H19N3O2S/c1-13-4-5-14(20(25)23-16-6-7-16)11-17(13)24-21(26)19-9-8-18(27-19)15-3-2-10-22-12-15/h2-5,8-12,16H,6-7H2,1H3,(H,23,25)(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38 MAPK-mediated TNFalpha production in LPS-induced human whole blood preincubated for 5 mins prior to LPS-treatment measured after 6 ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50327416
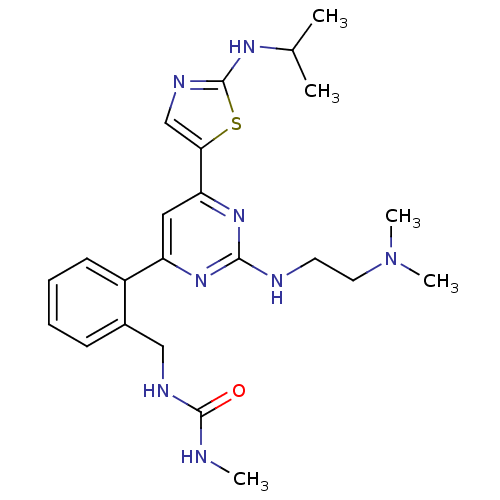
(1-(2-(2-(2-(dimethylamino)ethylamino)-6-(2-(isopro...)Show SMILES CNC(=O)NCc1ccccc1-c1cc(nc(NCCN(C)C)n1)-c1cnc(NC(C)C)s1 Show InChI InChI=1S/C23H32N8OS/c1-15(2)28-23-27-14-20(33-23)19-12-18(29-21(30-19)25-10-11-31(4)5)17-9-7-6-8-16(17)13-26-22(32)24-3/h6-9,12,14-15H,10-11,13H2,1-5H3,(H,27,28)(H2,24,26,32)(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bacterially expressed activated p38alpha pre-incubated 10 mins measured after 45 mins by scintillation counting |
Bioorg Med Chem Lett 20: 5864-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.102
BindingDB Entry DOI: 10.7270/Q22F7NP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50236473
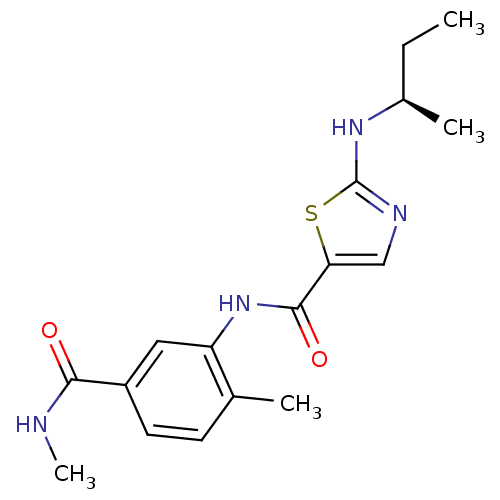
((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492382
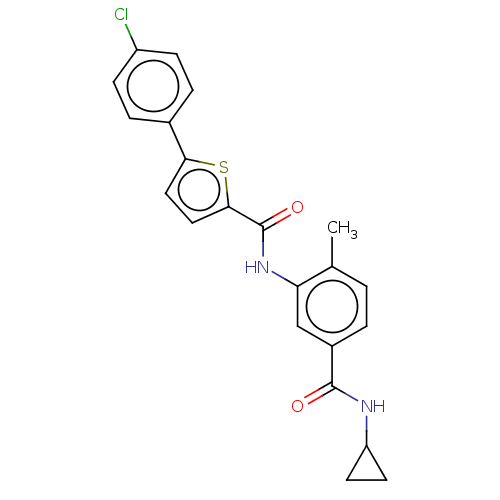
(CHEMBL2401981)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1ccc(Cl)cc1)C(=O)NC1CC1 Show InChI InChI=1S/C22H19ClN2O2S/c1-13-2-3-15(21(26)24-17-8-9-17)12-18(13)25-22(27)20-11-10-19(28-20)14-4-6-16(23)7-5-14/h2-7,10-12,17H,8-9H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376431

(CHEMBL260447)Show SMILES CCOC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C28H29FN6O4/c1-4-39-28(37)23-15-35-25(18(23)3)26(30-16-31-35)33-24-14-21(6-5-17(24)2)32-27(36)19-11-20(29)13-22(12-19)34-7-9-38-10-8-34/h5-6,11-16H,4,7-10H2,1-3H3,(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50236473

((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376438

(CHEMBL260411)Show SMILES COC[C@H](C)NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C30H34FN7O4/c1-18-5-6-23(35-29(39)21-11-22(31)13-24(12-21)37-7-9-42-10-8-37)14-26(18)36-28-27-20(3)25(15-38(27)33-17-32-28)30(40)34-19(2)16-41-4/h5-6,11-15,17,19H,7-10,16H2,1-4H3,(H,34,40)(H,35,39)(H,32,33,36)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376441

(CHEMBL262608)Show SMILES CCNC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4ccnc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C27H30N8O3/c1-4-28-27(37)21-15-35-24(18(21)3)25(30-16-31-35)33-22-14-20(6-5-17(22)2)32-26(36)19-7-8-29-23(13-19)34-9-11-38-12-10-34/h5-8,13-16H,4,9-12H2,1-3H3,(H,28,37)(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376438

(CHEMBL260411)Show SMILES COC[C@H](C)NC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C30H34FN7O4/c1-18-5-6-23(35-29(39)21-11-22(31)13-24(12-21)37-7-9-42-10-8-37)14-26(18)36-28-27-20(3)25(15-38(27)33-17-32-28)30(40)34-19(2)16-41-4/h5-6,11-15,17,19H,7-10,16H2,1-4H3,(H,34,40)(H,35,39)(H,32,33,36)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM20657
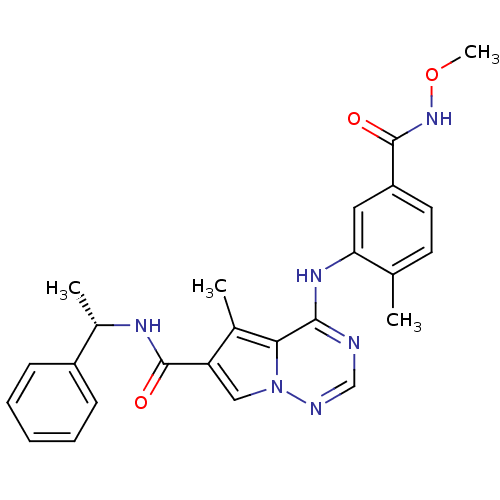
(4-{[5-(methoxycarbamoyl)-2-methylphenyl]amino}-5-m...)Show SMILES CONC(=O)c1ccc(C)c(Nc2ncnn3cc(C(=O)N[C@@H](C)c4ccccc4)c(C)c23)c1 |r| Show InChI InChI=1S/C25H26N6O3/c1-15-10-11-19(24(32)30-34-4)12-21(15)29-23-22-16(2)20(13-31(22)27-14-26-23)25(33)28-17(3)18-8-6-5-7-9-18/h5-14,17H,1-4H3,(H,28,33)(H,30,32)(H,26,27,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of bacterially expressed p38alpha pretreated for 10 mins measured after 45 mins |
J Med Chem 53: 6629-39 (2010)
Article DOI: 10.1021/jm100540x
BindingDB Entry DOI: 10.7270/Q2JM29T1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492376

(CHEMBL2402000)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1cnccc1N1CCOCC1)C(=O)NC1CC1 Show InChI InChI=1S/C25H26N4O3S/c1-16-2-3-17(24(30)27-18-4-5-18)14-20(16)28-25(31)23-7-6-22(33-23)19-15-26-9-8-21(19)29-10-12-32-13-11-29/h2-3,6-9,14-15,18H,4-5,10-13H2,1H3,(H,27,30)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376454
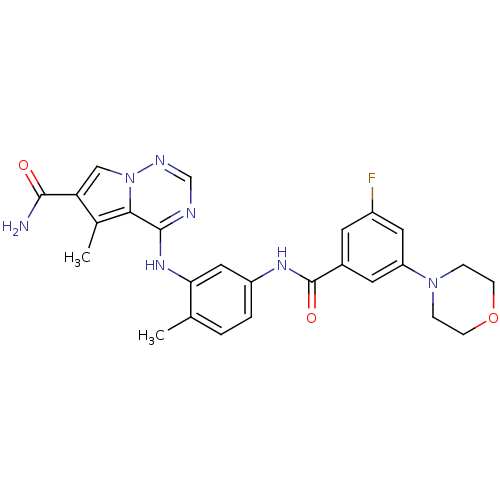
(CHEMBL261006)Show SMILES Cc1c(cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c12)C(N)=O Show InChI InChI=1S/C26H26FN7O3/c1-15-3-4-19(31-26(36)17-9-18(27)11-20(10-17)33-5-7-37-8-6-33)12-22(15)32-25-23-16(2)21(24(28)35)13-34(23)30-14-29-25/h3-4,9-14H,5-8H2,1-2H3,(H2,28,35)(H,31,36)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50327391
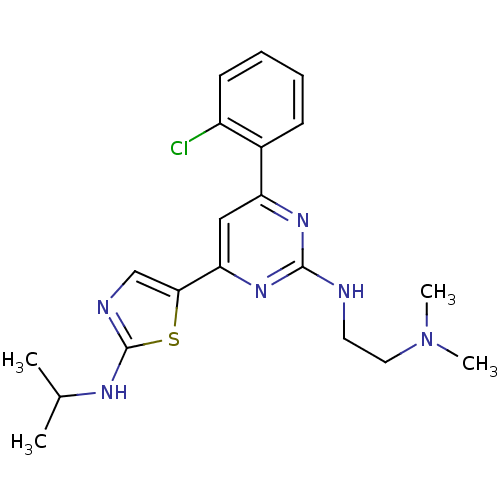
(CHEMBL1258976 | N1-(4-(2-chlorophenyl)-6-(2-(isopr...)Show SMILES CC(C)Nc1ncc(s1)-c1cc(nc(NCCN(C)C)n1)-c1ccccc1Cl Show InChI InChI=1S/C20H25ClN6S/c1-13(2)24-20-23-12-18(28-20)17-11-16(14-7-5-6-8-15(14)21)25-19(26-17)22-9-10-27(3)4/h5-8,11-13H,9-10H2,1-4H3,(H,23,24)(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LIMK2 |
Bioorg Med Chem Lett 20: 5864-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.102
BindingDB Entry DOI: 10.7270/Q22F7NP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM20649
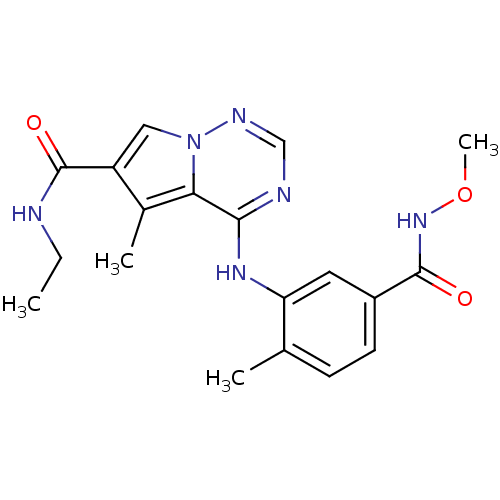
(N-ethyl-4-{[5-(methoxycarbamoyl)-2-methylphenyl]am...)Show SMILES CCNC(=O)c1cn2ncnc(Nc3cc(ccc3C)C(=O)NOC)c2c1C Show InChI InChI=1S/C19H22N6O3/c1-5-20-19(27)14-9-25-16(12(14)3)17(21-10-22-25)23-15-8-13(7-6-11(15)2)18(26)24-28-4/h6-10H,5H2,1-4H3,(H,20,27)(H,24,26)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of bacterially expressed p38alpha pretreated for 10 mins measured after 45 mins |
J Med Chem 53: 6629-39 (2010)
Article DOI: 10.1021/jm100540x
BindingDB Entry DOI: 10.7270/Q2JM29T1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492379
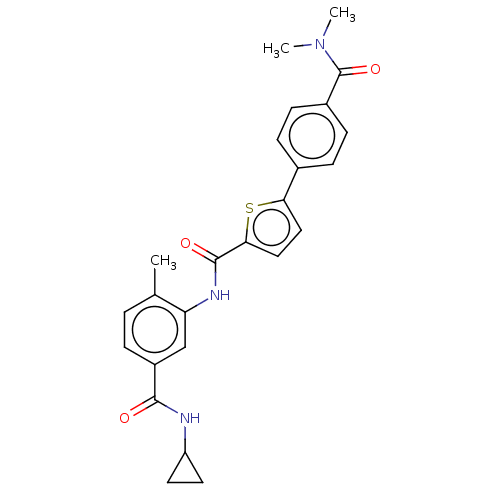
(CHEMBL2401993)Show SMILES CN(C)C(=O)c1ccc(cc1)-c1ccc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C25H25N3O3S/c1-15-4-5-18(23(29)26-19-10-11-19)14-20(15)27-24(30)22-13-12-21(32-22)16-6-8-17(9-7-16)25(31)28(2)3/h4-9,12-14,19H,10-11H2,1-3H3,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492392
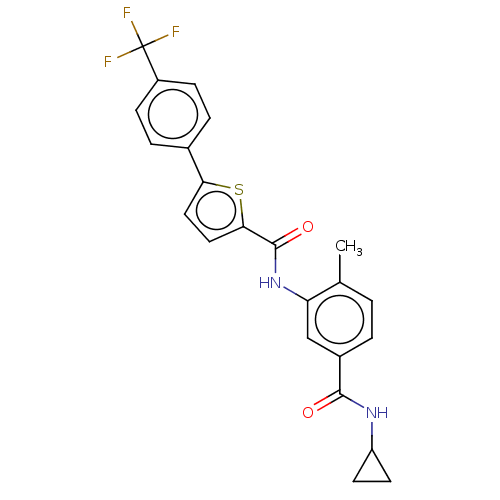
(CHEMBL2401985)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1ccc(cc1)C(F)(F)F)C(=O)NC1CC1 Show InChI InChI=1S/C23H19F3N2O2S/c1-13-2-3-15(21(29)27-17-8-9-17)12-18(13)28-22(30)20-11-10-19(31-20)14-4-6-16(7-5-14)23(24,25)26/h2-7,10-12,17H,8-9H2,1H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50327020
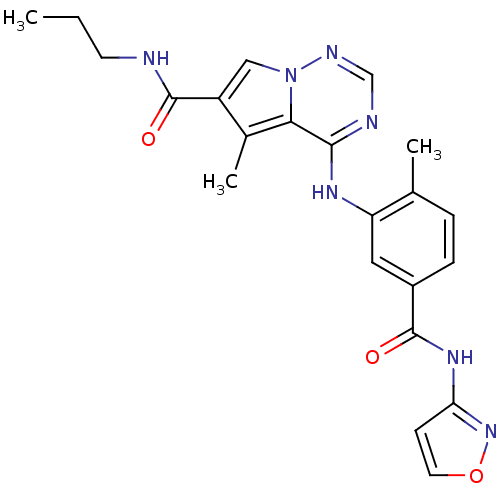
(4-(5-(Isoxazol-3-ylcarbamoyl)-2-methylphenylamino)...)Show SMILES CCCNC(=O)c1cn2ncnc(Nc3cc(ccc3C)C(=O)Nc3ccon3)c2c1C Show InChI InChI=1S/C22H23N7O3/c1-4-8-23-22(31)16-11-29-19(14(16)3)20(24-12-25-29)26-17-10-15(6-5-13(17)2)21(30)27-18-7-9-32-28-18/h5-7,9-12H,4,8H2,1-3H3,(H,23,31)(H,24,25,26)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of bacterially expressed p38alpha pretreated for 10 mins measured after 45 mins |
J Med Chem 53: 6629-39 (2010)
Article DOI: 10.1021/jm100540x
BindingDB Entry DOI: 10.7270/Q2JM29T1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50492386
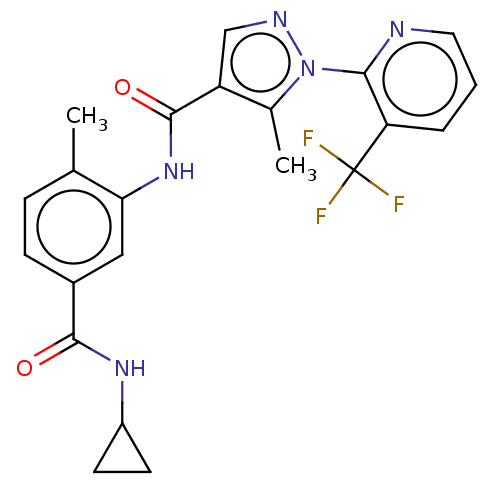
(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492378
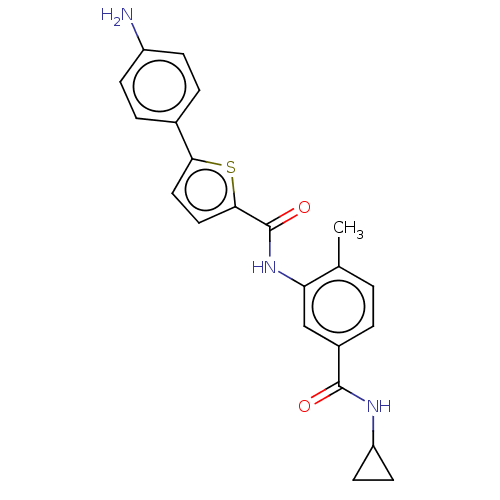
(CHEMBL2401988)Show SMILES Cc1ccc(cc1NC(=O)c1ccc(s1)-c1ccc(N)cc1)C(=O)NC1CC1 Show InChI InChI=1S/C22H21N3O2S/c1-13-2-3-15(21(26)24-17-8-9-17)12-18(13)25-22(27)20-11-10-19(28-20)14-4-6-16(23)7-5-14/h2-7,10-12,17H,8-9,23H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50236473

((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492386

(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50327390
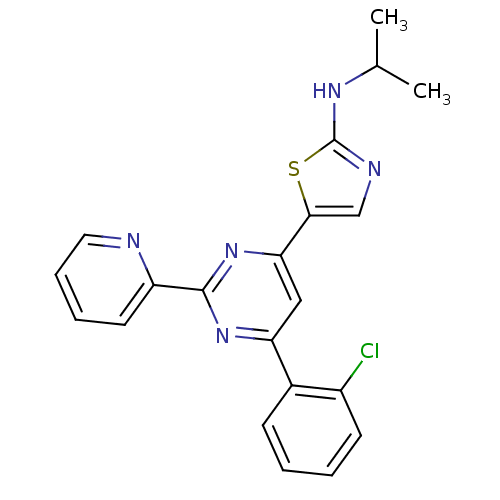
(5-(6-(2-chlorophenyl)-2-(pyridin-2-yl)pyrimidin-4-...)Show SMILES CC(C)Nc1ncc(s1)-c1cc(nc(n1)-c1ccccn1)-c1ccccc1Cl Show InChI InChI=1S/C21H18ClN5S/c1-13(2)25-21-24-12-19(28-21)18-11-17(14-7-3-4-8-15(14)22)26-20(27-18)16-9-5-6-10-23-16/h3-13H,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of bacterially expressed activated p38alpha pre-incubated 10 mins measured after 45 mins by scintillation counting |
Bioorg Med Chem Lett 20: 5864-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.102
BindingDB Entry DOI: 10.7270/Q22F7NP6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50492386

(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50236473

((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50376430

(CHEMBL261005)Show SMILES CNC(=O)c1cn2ncnc(Nc3cc(NC(=O)c4cc(F)cc(c4)N4CCOCC4)ccc3C)c2c1C Show InChI InChI=1S/C27H28FN7O3/c1-16-4-5-20(32-26(36)18-10-19(28)12-21(11-18)34-6-8-38-9-7-34)13-23(16)33-25-24-17(2)22(27(37)29-3)14-35(24)31-15-30-25/h4-5,10-15H,6-9H2,1-3H3,(H,29,37)(H,32,36)(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 18: 2739-44 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.067
BindingDB Entry DOI: 10.7270/Q29887X6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50492386

(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data