

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333148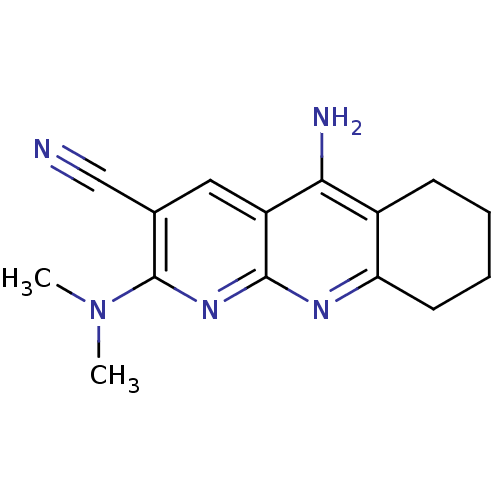 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333150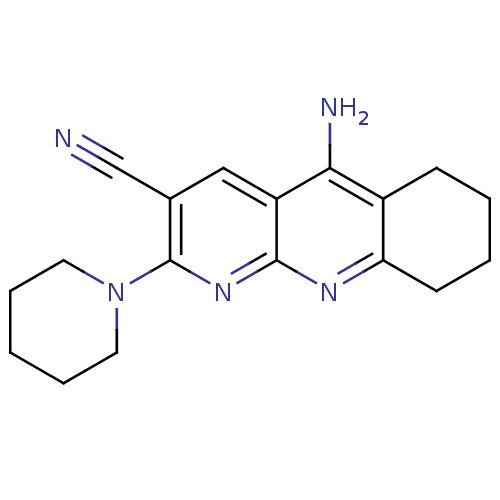 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333151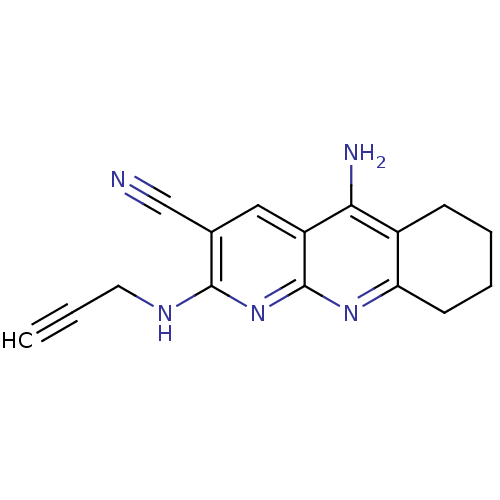 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of AChE in bovine erythrocytes using acetylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333149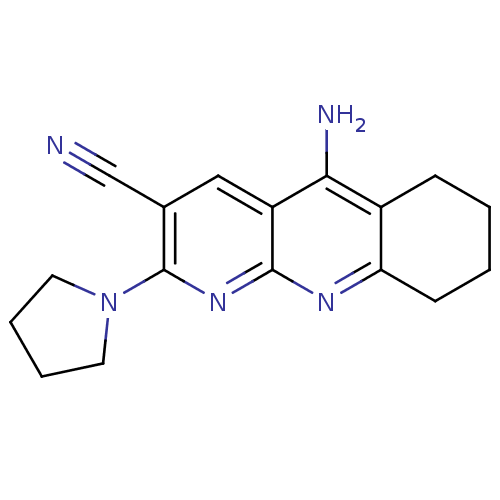 (5-Amino-2-pyrrolidin-1-yl-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333154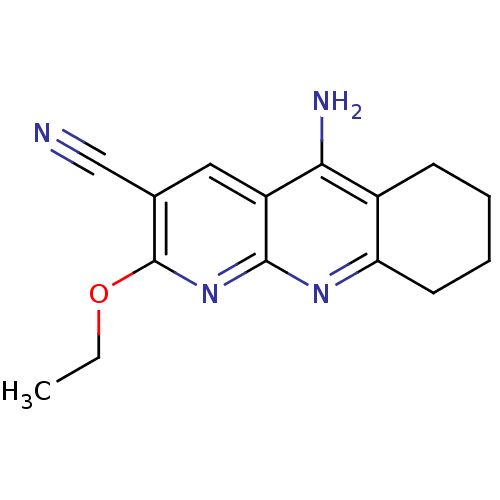 (5-Amino-2-(ethyloxy)-6,7,8,9-tetrahydrobenzo[1,8-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333153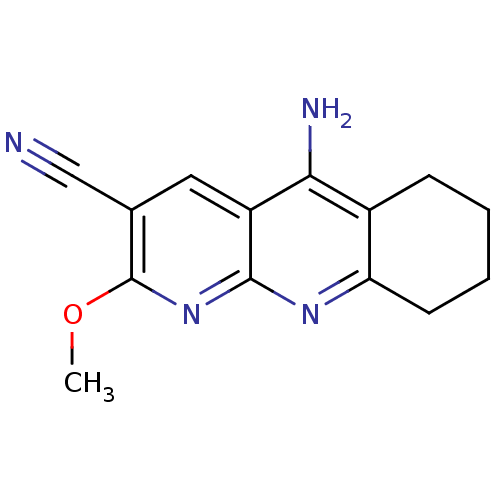 (5-Amino-2-(methyloxy)-6,7,8,9-tetrahydrobenzo[1,8-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333155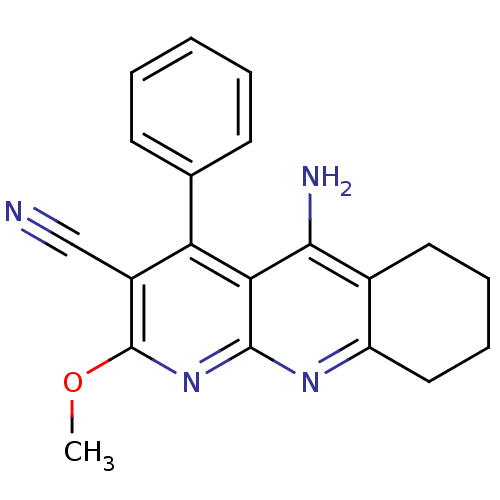 (5-Amino-2-methoxy-4-phenyl-6,7,8,9-tetrahydrobenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333152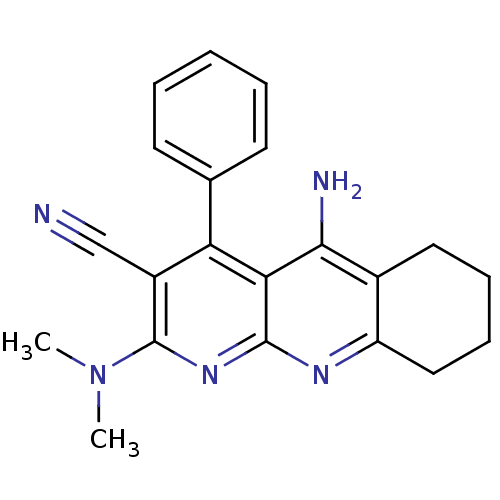 (5-Amino-2-(dimethylamino)-4-phenyl-6,7,8,9-tetrahy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333148 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333149 (5-Amino-2-pyrrolidin-1-yl-6,7,8,9-tetrahydrobenzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333158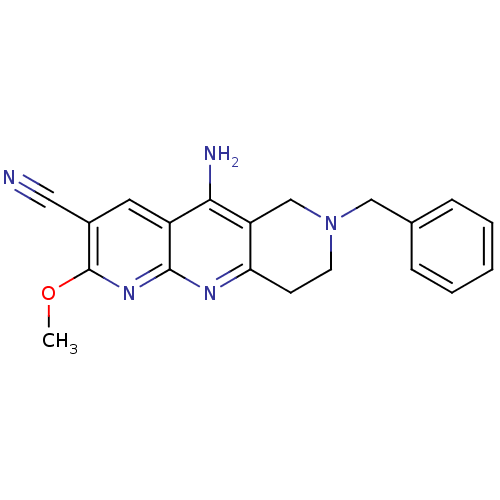 (5-Amino-7-benzyl-2-methoxy-6,7,8,9-tetrahydropyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333159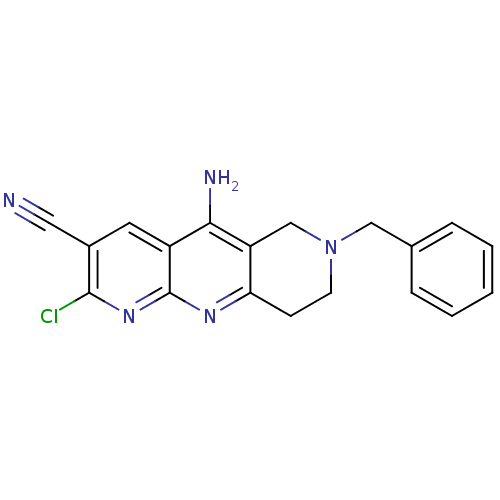 (5-Amino-7-benzyl-2-chloro-6,7,8,9-tetrahydropyrido...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361459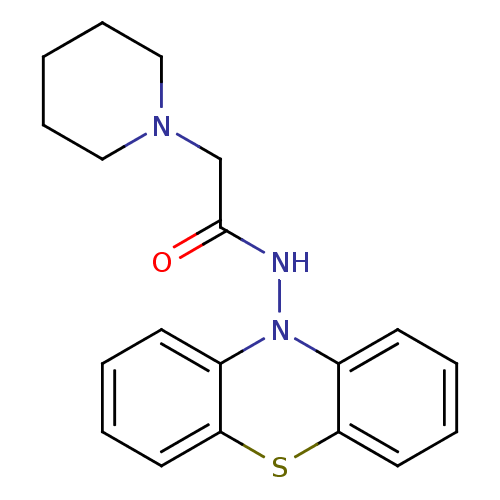 (CHEMBL1938454) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361456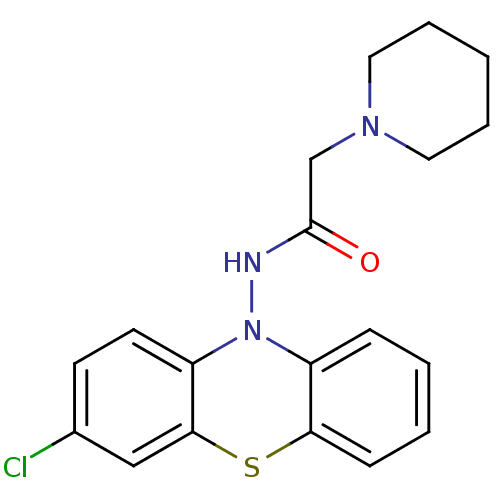 (CHEMBL1938457) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361455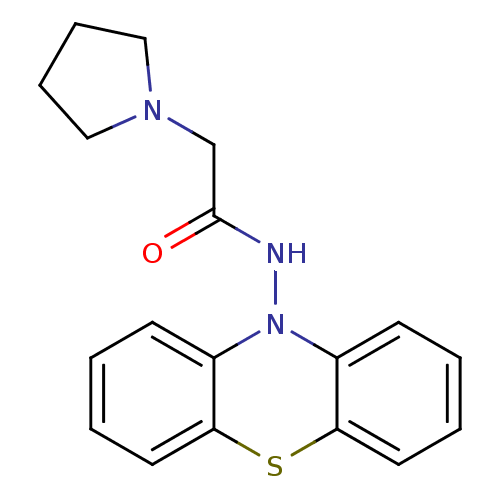 (CHEMBL1938458) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361449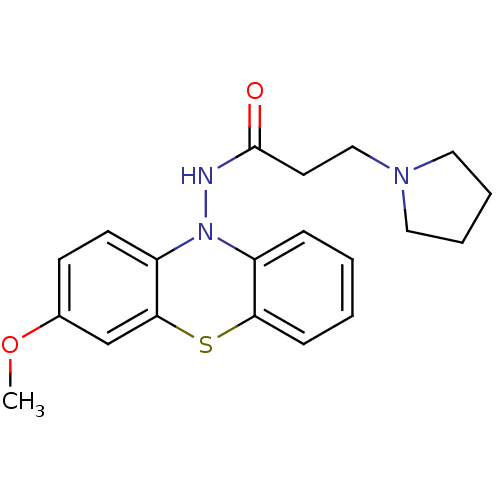 (CHEMBL1938464) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361447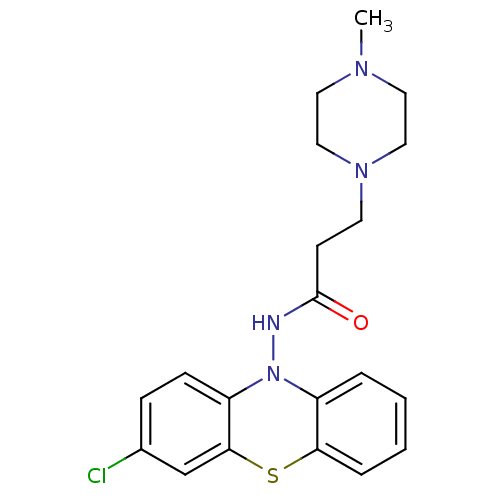 (CHEMBL1938397) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361453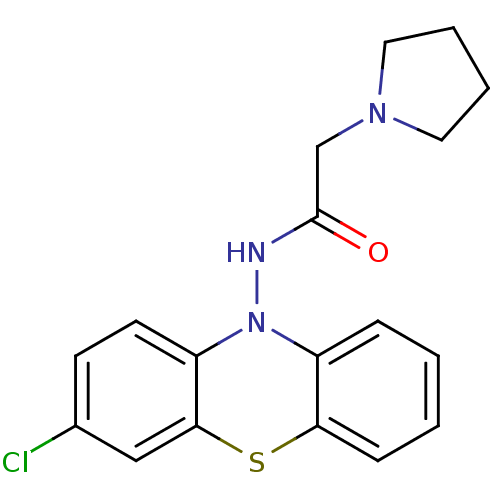 (CHEMBL1938460) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333150 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333154 (5-Amino-2-(ethyloxy)-6,7,8,9-tetrahydrobenzo[1,8-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361448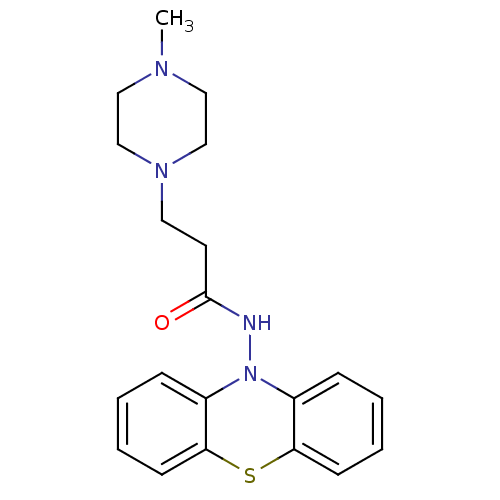 (CHEMBL1938465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361451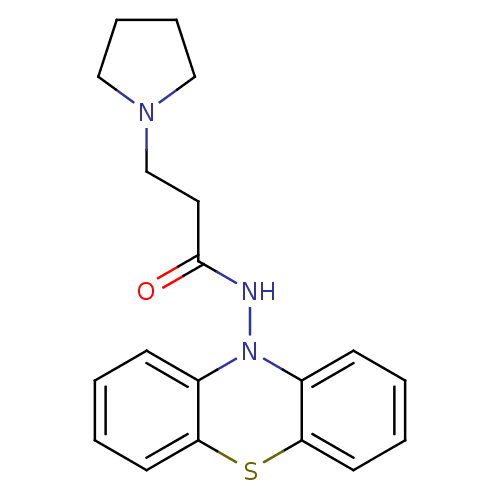 (CHEMBL1938462) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361445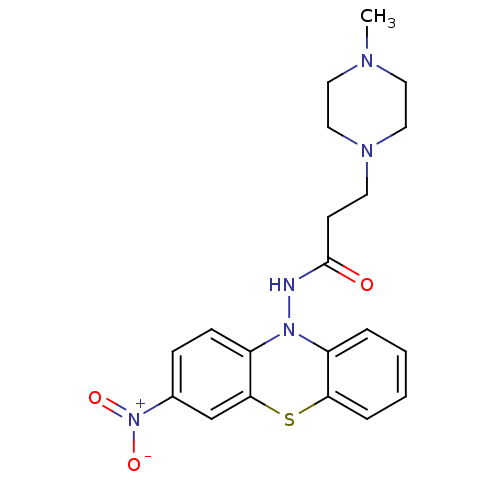 (CHEMBL1938467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333151 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333153 (5-Amino-2-(methyloxy)-6,7,8,9-tetrahydrobenzo[1,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361462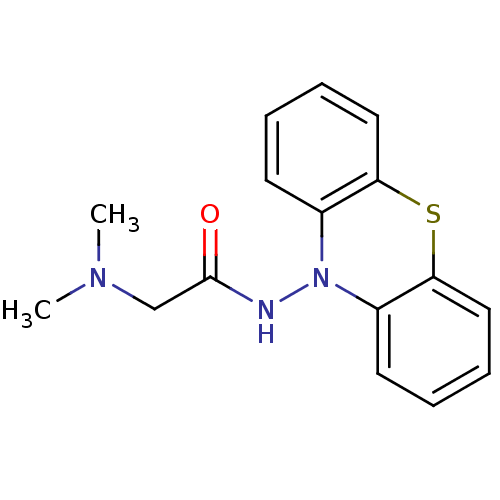 (CHEMBL1938451) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361446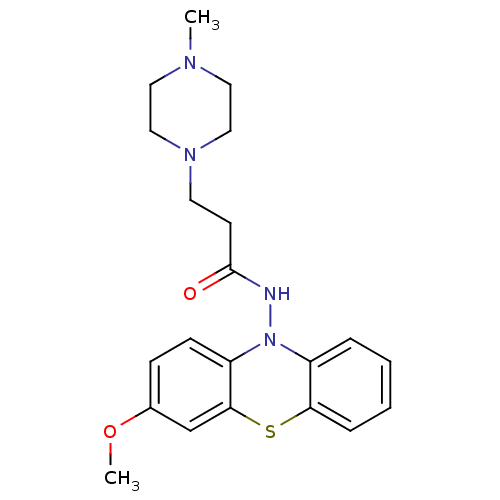 (CHEMBL1938466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361458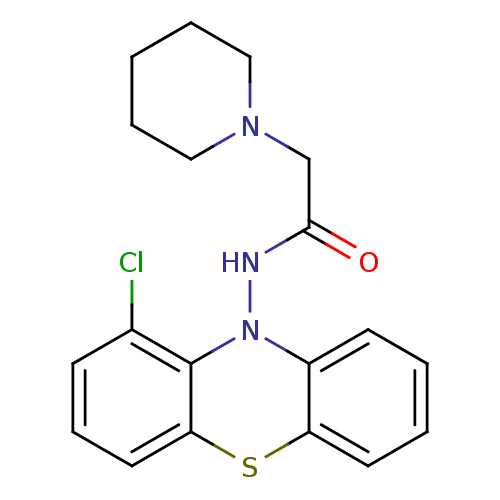 (CHEMBL1938455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333151 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361450 (CHEMBL1938463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333150 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361454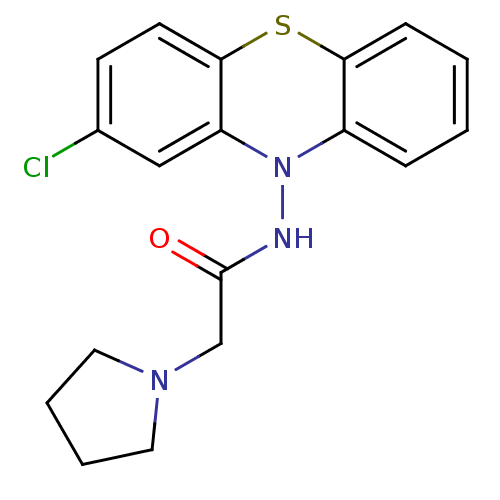 (CHEMBL1938459) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333152 (5-Amino-2-(dimethylamino)-4-phenyl-6,7,8,9-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333156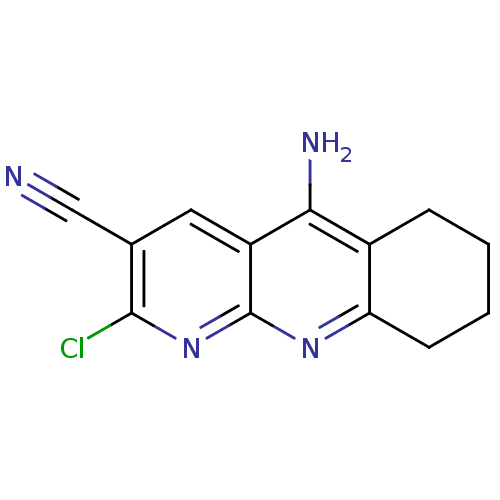 (5-Amino-2-chloro-6,7,8,9-tetrahydrobenzo[1,8-b]-na...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333158 (5-Amino-7-benzyl-2-methoxy-6,7,8,9-tetrahydropyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333155 (5-Amino-2-methoxy-4-phenyl-6,7,8,9-tetrahydrobenzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361460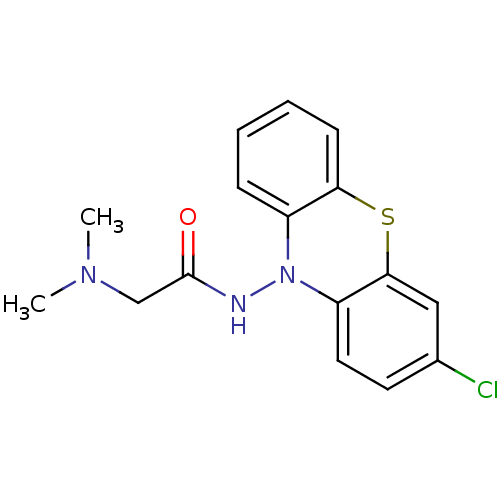 (CHEMBL1938453) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333155 (5-Amino-2-methoxy-4-phenyl-6,7,8,9-tetrahydrobenzo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333148 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333149 (5-Amino-2-pyrrolidin-1-yl-6,7,8,9-tetrahydrobenzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50361461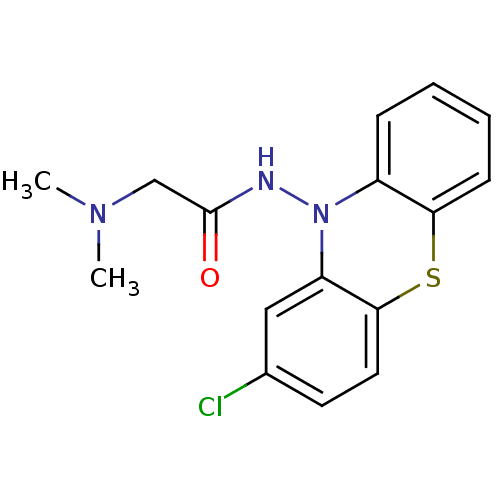 (CHEMBL1938452) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of BuChE in horse serum using butyrylthiocholine as substrate by Ellman method | Eur J Med Chem 46: 2224-35 (2011) Article DOI: 10.1016/j.ejmech.2011.03.003 BindingDB Entry DOI: 10.7270/Q2X34XWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333152 (5-Amino-2-(dimethylamino)-4-phenyl-6,7,8,9-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333153 (5-Amino-2-(methyloxy)-6,7,8,9-tetrahydrobenzo[1,8-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333157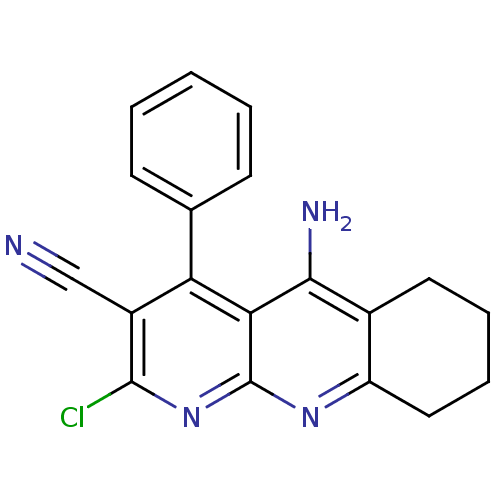 (5-Amino-2-chloro-4-phenyl-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333157 (5-Amino-2-chloro-4-phenyl-6,7,8,9-tetrahydrobenzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |