

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19968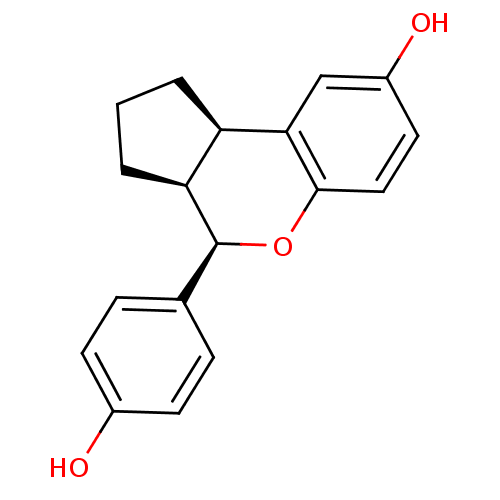 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.170 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19968 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19986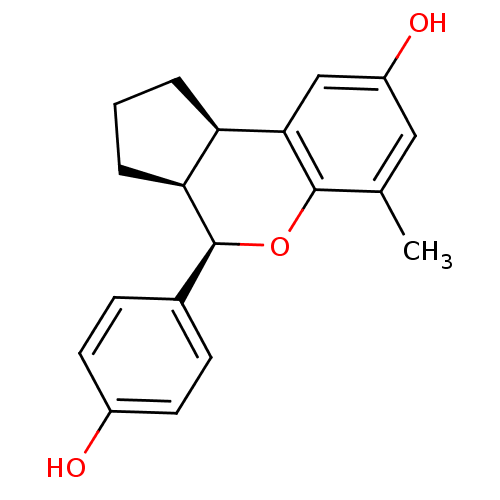 ((2R,6S,7R)-7-(4-hydroxyphenyl)-10-methyl-8-oxatric...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19987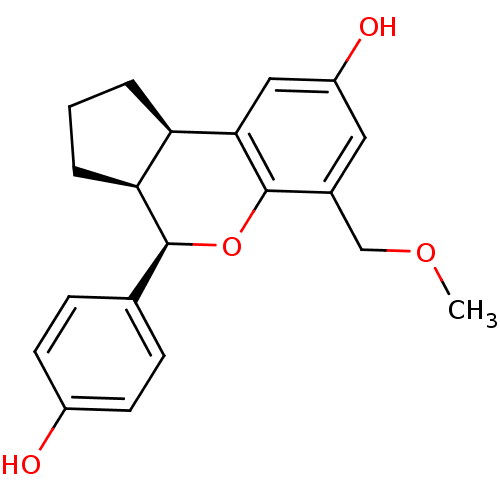 ((2R,6S,7R)-7-(4-hydroxyphenyl)-10-(methoxymethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19985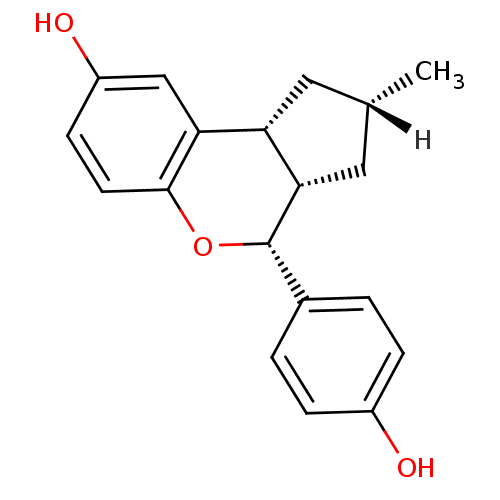 ((2R,4S,6S,7R)-7-(4-hydroxyphenyl)-4-methyl-8-oxatr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19983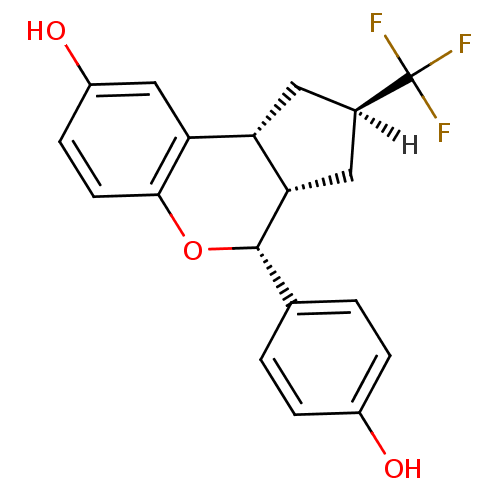 ((2R,4R,6S,7R)-7-(4-hydroxyphenyl)-4-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19982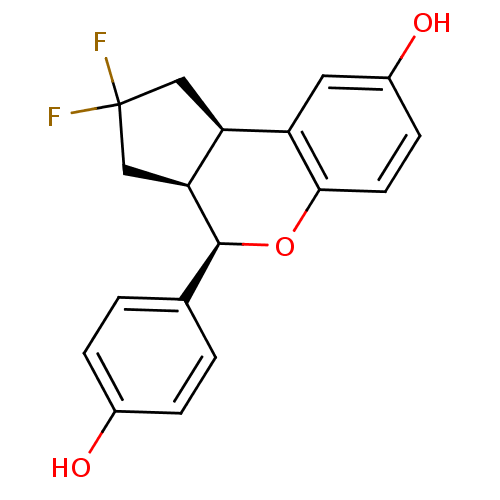 ((2R,6S,7R)-4,4-difluoro-7-(4-hydroxyphenyl)-8-oxat...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.440 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19984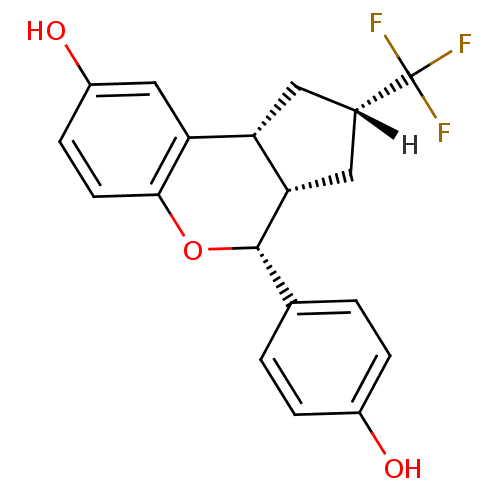 ((2R,4S,6S,7R)-7-(4-hydroxyphenyl)-4-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19988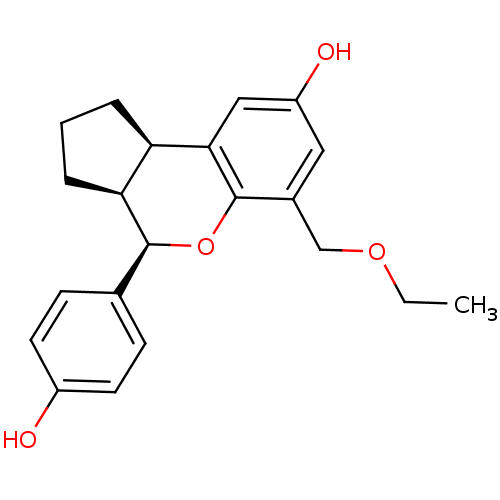 ((2R,6S,7R)-10-(ethoxymethyl)-7-(4-hydroxyphenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.745 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19968 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.29 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19968 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.70 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19986 ((2R,6S,7R)-7-(4-hydroxyphenyl)-10-methyl-8-oxatric...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.82 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19985 ((2R,4S,6S,7R)-7-(4-hydroxyphenyl)-4-methyl-8-oxatr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19989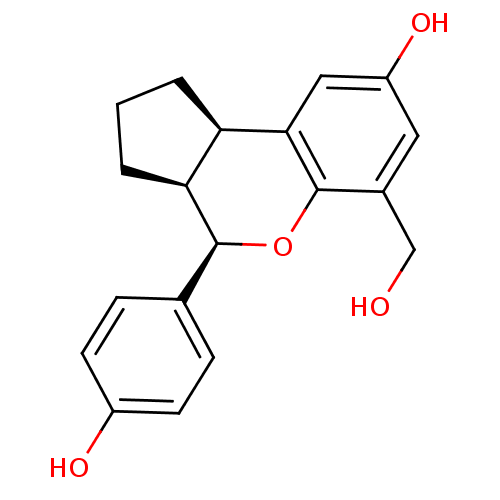 ((2R,6S,7R)-10-(hydroxymethyl)-7-(4-hydroxyphenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.97 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19979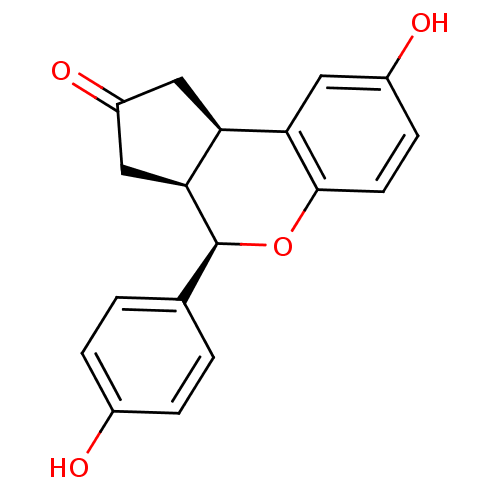 ((2R,6S,7R)-12-hydroxy-7-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.92 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19983 ((2R,4R,6S,7R)-7-(4-hydroxyphenyl)-4-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.20 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19982 ((2R,6S,7R)-4,4-difluoro-7-(4-hydroxyphenyl)-8-oxat...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 8.30 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19981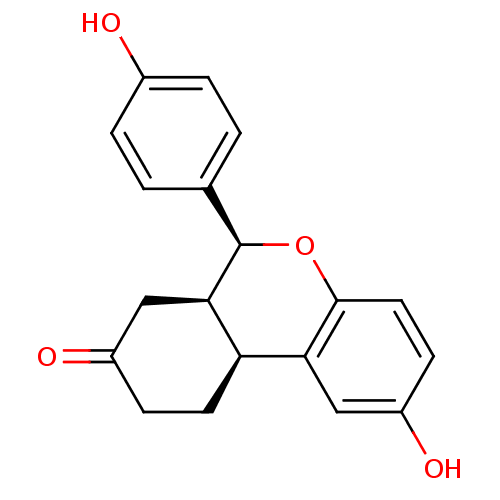 ((1S,9S,10R)-4-hydroxy-9-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10.2 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19987 ((2R,6S,7R)-7-(4-hydroxyphenyl)-10-(methoxymethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19984 ((2R,4S,6S,7R)-7-(4-hydroxyphenyl)-4-(trifluorometh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.4 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19988 ((2R,6S,7R)-10-(ethoxymethyl)-7-(4-hydroxyphenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 27.2 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19989 ((2R,6S,7R)-10-(hydroxymethyl)-7-(4-hydroxyphenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 147 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 5082-5 (2007) Article DOI: 10.1016/j.bmcl.2007.07.009 BindingDB Entry DOI: 10.7270/Q27S7M2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19980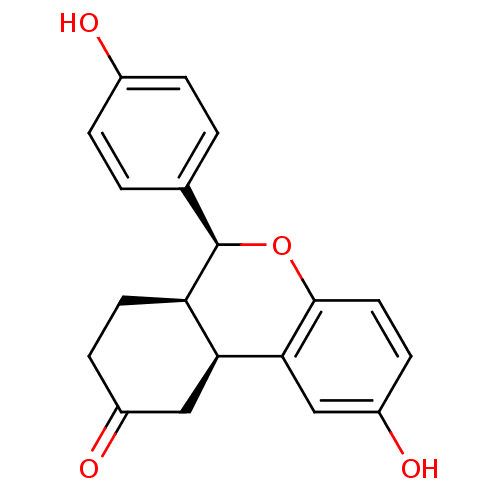 ((1S,9S,10R)-4-hydroxy-9-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 375 | -36.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19981 ((1S,9S,10R)-4-hydroxy-9-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 410 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19979 ((2R,6S,7R)-12-hydroxy-7-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19980 ((1S,9S,10R)-4-hydroxy-9-(4-hydroxyphenyl)-8-oxatri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | Bioorg Med Chem Lett 17: 4824-8 (2007) Article DOI: 10.1016/j.bmcl.2007.06.052 BindingDB Entry DOI: 10.7270/Q2CJ8BRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187689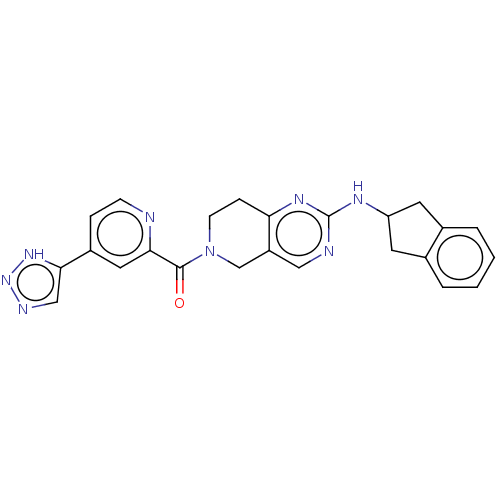 (CHEMBL3827513 | Example 9) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description The purpose of this assay is to detect autotaxin inhibition using a choline release assay.Test compound (10 mM stocks in 100% DMSO) is serially dilut... | US Patent US9550774 (2017) BindingDB Entry DOI: 10.7270/Q23N21KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535210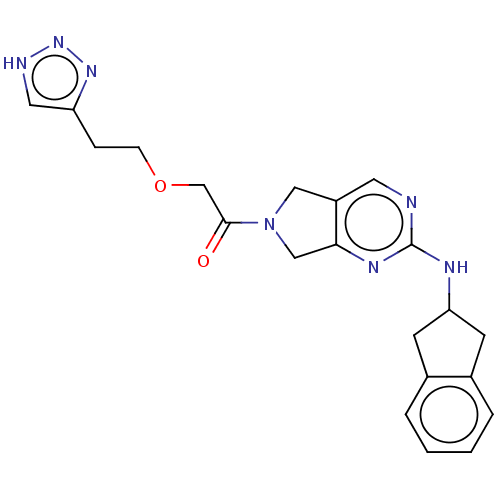 (CHEMBL4448598) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM204931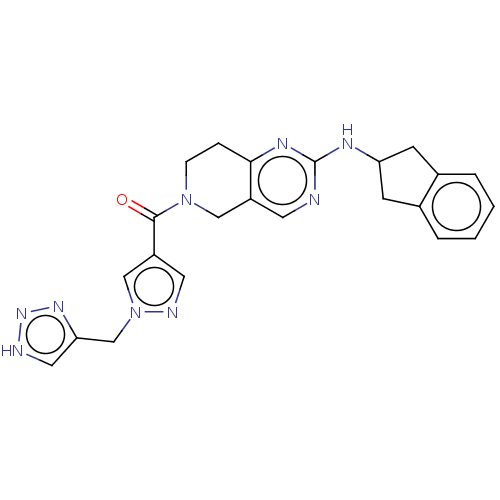 (Example 1 | [2-(indan-2-ylamino)-7,8-dihydro-5H-py...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description The purpose of this assay is to detect autotaxin inhibition using a choline release assay.Test compound (10 mM stocks in 100% DMSO) is serially dilut... | US Patent US9550774 (2017) BindingDB Entry DOI: 10.7270/Q23N21KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535210 (CHEMBL4448598) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535213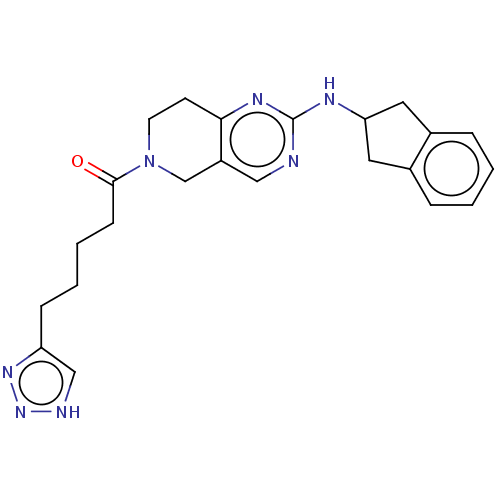 (CHEMBL4453084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535214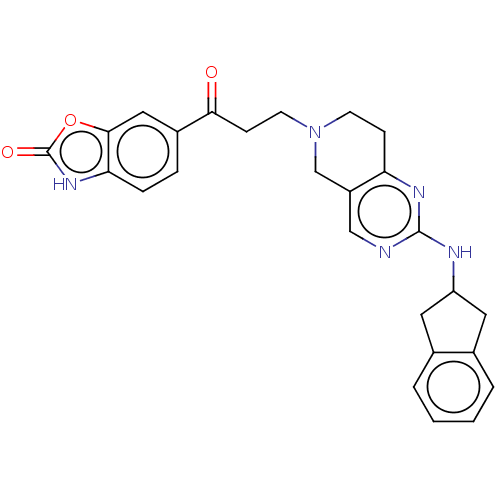 (CHEMBL4549771) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535215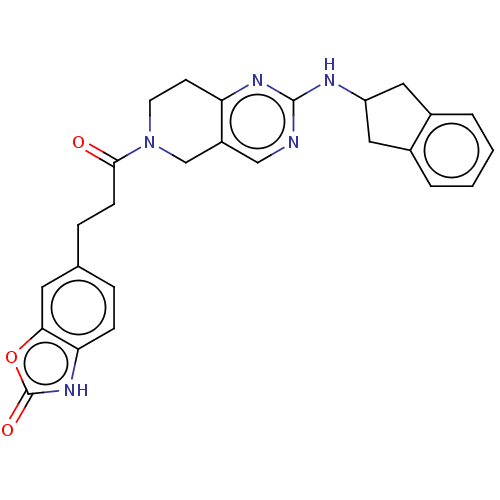 (CHEMBL4476558) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535212 (CHEMBL4569141) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535220 (CHEMBL4454442) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535215 (CHEMBL4476558) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535213 (CHEMBL4453084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535220 (CHEMBL4454442) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187693 (CHEMBL3186509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535221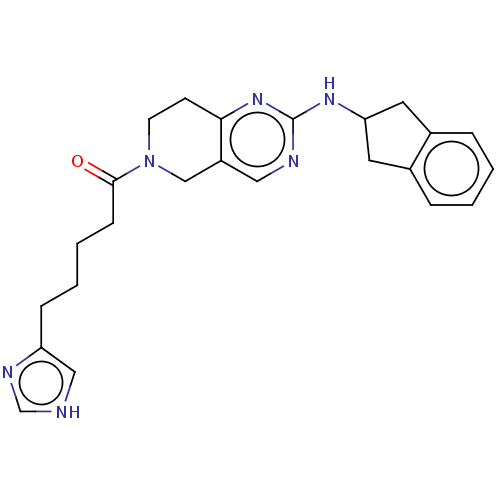 (CHEMBL4462537) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535216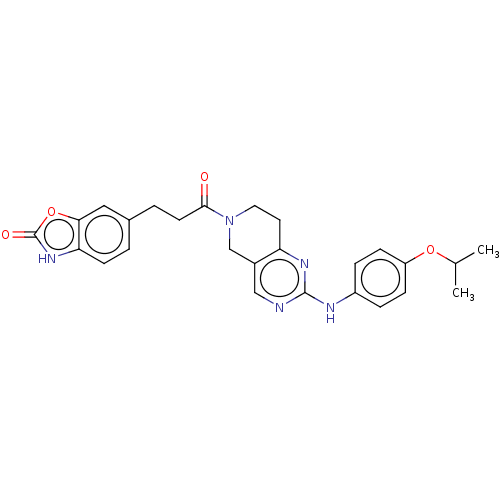 (CHEMBL4472840) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535219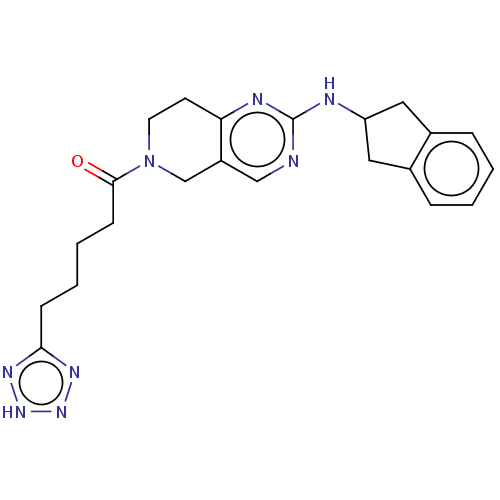 (CHEMBL4446322) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535221 (CHEMBL4462537) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535214 (CHEMBL4549771) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187693 (CHEMBL3186509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535217 (CHEMBL4458386) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535211 (CHEMBL4561467) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of recombinant full length human C-terminal His-tagged autotaxin expressed in human 293E cells assessed as choline release using lysophosp... | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535216 (CHEMBL4472840) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50535211 (CHEMBL4561467) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human plasma assessed as reduction in LPA level after 3 hrs by mass spectrometric analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50187693 (CHEMBL3186509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of autotaxin in healthy human whole blood assessed as reduction in LPA level after 2 hrs by LC-MS/MS analysis | ACS Med Chem Lett 7: 857-61 (2016) Article DOI: 10.1021/acsmedchemlett.6b00207 BindingDB Entry DOI: 10.7270/Q2KD22D0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |