

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323215 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclohexylethyl)-1,2,4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323216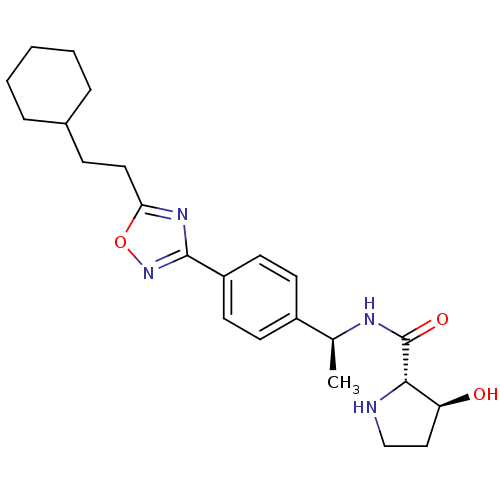 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclohexylethyl)-1,2,4-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323223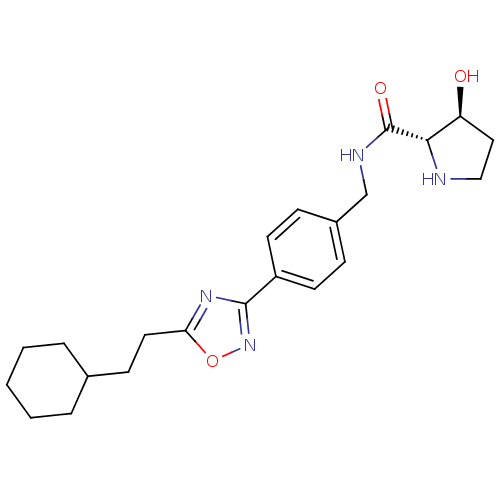 ((2S,3S)-N-(4-(5-(2-cyclohexylethyl)-1,2,4-oxadiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323219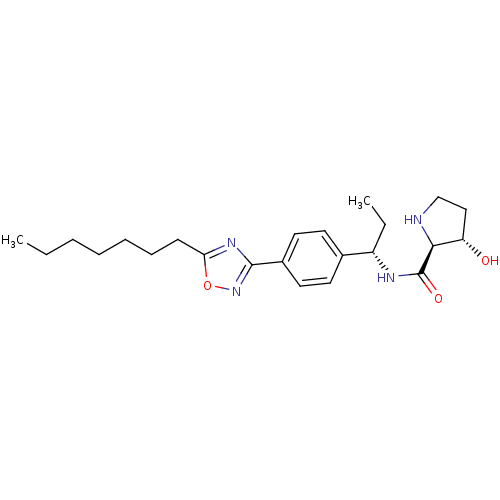 ((2S,3S)-N-((S)-1-(4-(5-heptyl-1,2,4-oxadiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120351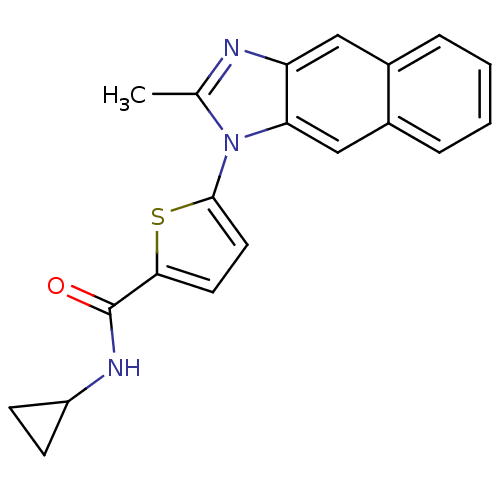 (US8703811, 93) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120317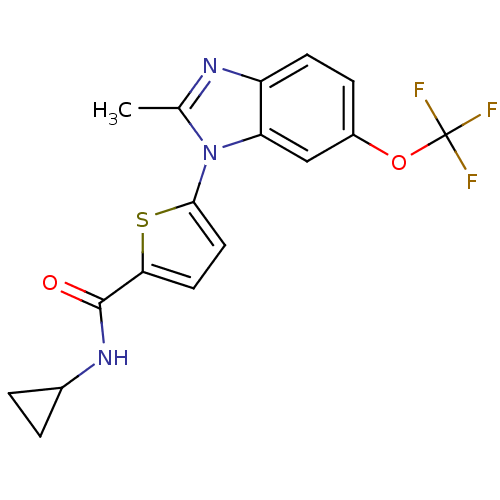 (US8703811, 58) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120319 (US8703811, 60) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120301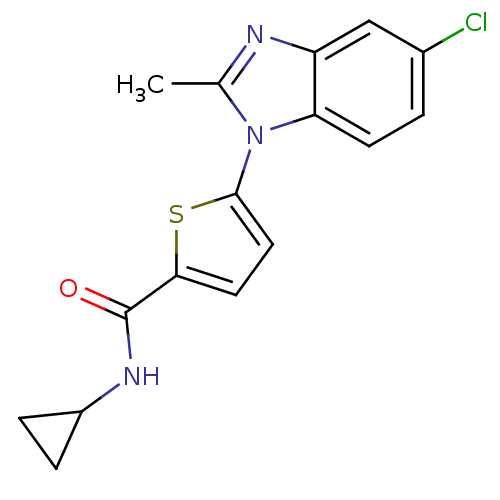 (US8703811, 40 | US8703811, 41) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium vivax) | BDBM92606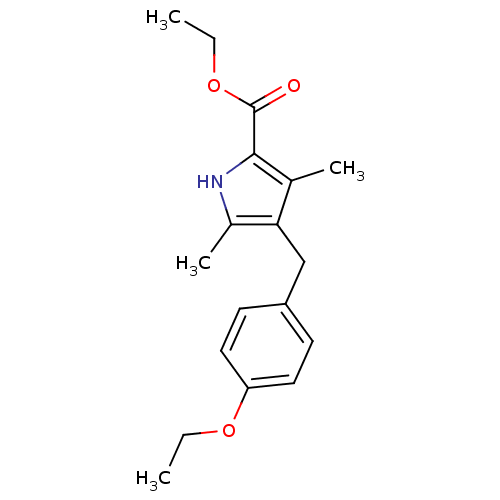 (DHOD Inhibitor, 5 | US8703811, 6) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium vivax) | BDBM92606 (DHOD Inhibitor, 5 | US8703811, 6) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Assay was determined using a continuous assay. | J Biol Chem 283: 35078-85 (2008) Article DOI: 10.1074/jbc.M804990200 BindingDB Entry DOI: 10.7270/Q2VH5MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120323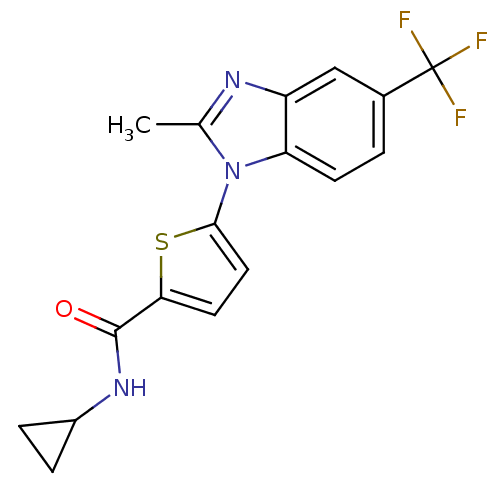 (US8703811, 64) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120324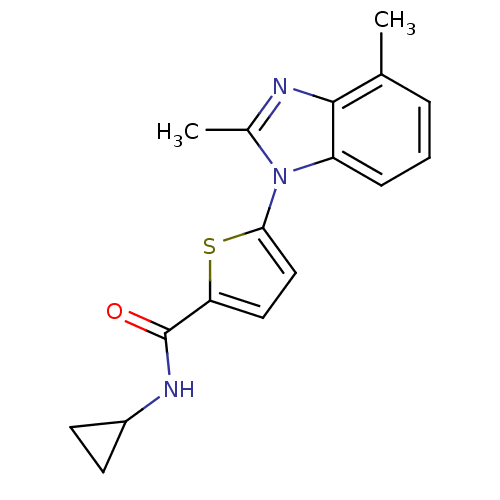 (US8703811, 65) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323220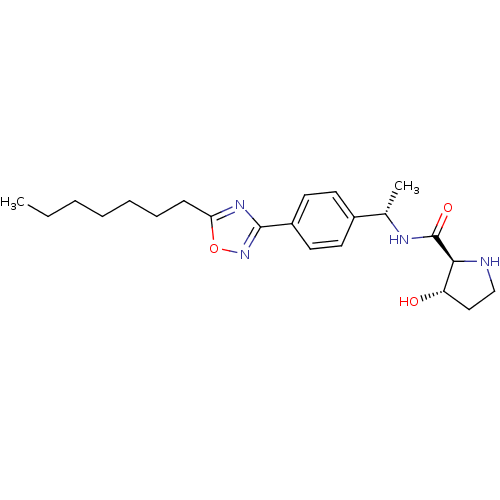 ((2S,3S)-N-((S)-1-(4-(5-heptyl-1,2,4-oxadiazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323231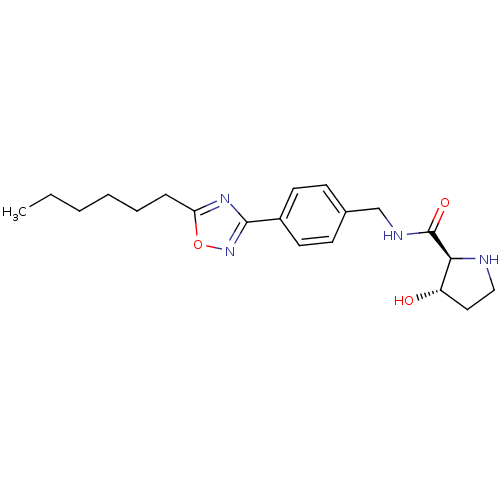 ((2S,3S)-N-(4-(5-hexyl-1,2,4-oxadiazol-3-yl)benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299323 ((S)-2-amino-4-hydroxy-N-(4-octylphenyl)butanamide ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of human sphingosine kinase 1 by off chip mobility shift assay | Bioorg Med Chem Lett 19: 6119-21 (2009) Article DOI: 10.1016/j.bmcl.2009.09.022 BindingDB Entry DOI: 10.7270/Q29Z94ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120315 (US8703811, 55) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323242 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclopentylethyl)-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50379142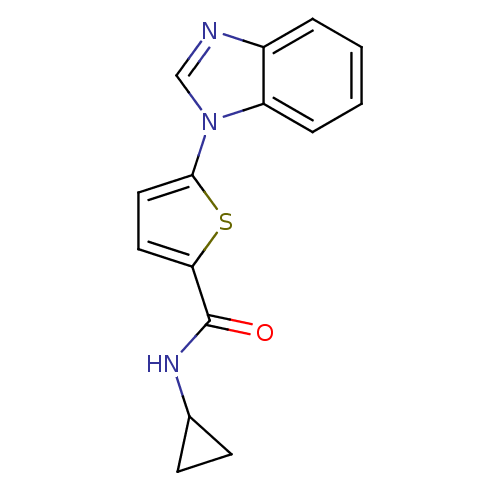 (CHEMBL2012824 | US8703811, 23) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120305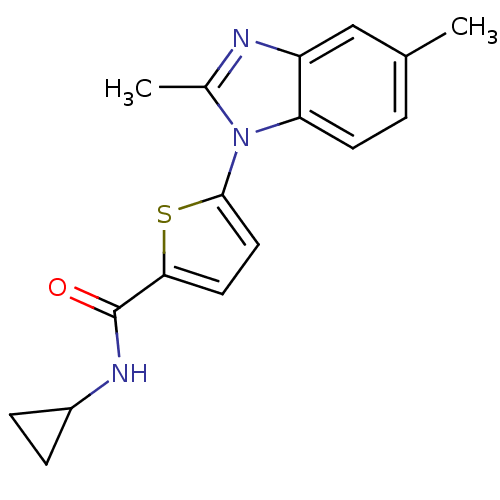 (US8703811, 44) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120328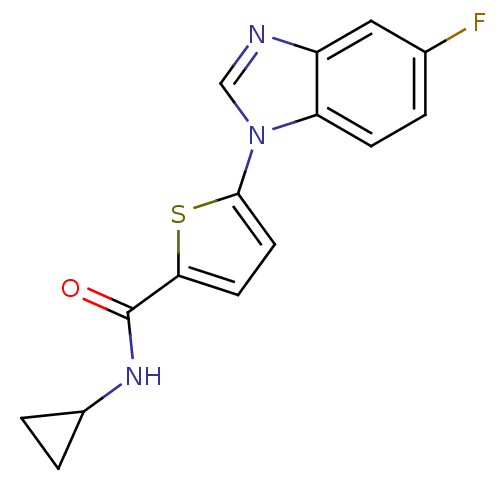 (US8703811, 70) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120301 (US8703811, 40 | US8703811, 41) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323217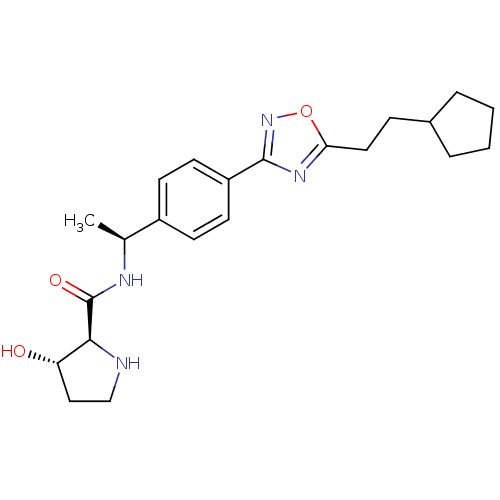 ((2S,3S)-N-((S)-1-(4-(5-(2-cyclopentylethyl)-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [100-517] (Plasmodium berghei) | BDBM92606 (DHOD Inhibitor, 5 | US8703811, 6) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Assay was determined using a continuous assay. | J Biol Chem 283: 35078-85 (2008) Article DOI: 10.1074/jbc.M804990200 BindingDB Entry DOI: 10.7270/Q2VH5MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [100-517] (Plasmodium berghei) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Assay was determined using a continuous assay. | J Biol Chem 283: 35078-85 (2008) Article DOI: 10.1074/jbc.M804990200 BindingDB Entry DOI: 10.7270/Q2VH5MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [100-517] (Plasmodium berghei) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial [100-517] (Plasmodium berghei) | BDBM92606 (DHOD Inhibitor, 5 | US8703811, 6) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323234 ((2S,3S)-N-(4-(3-heptyl-1,2,4-oxadiazol-5-yl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299328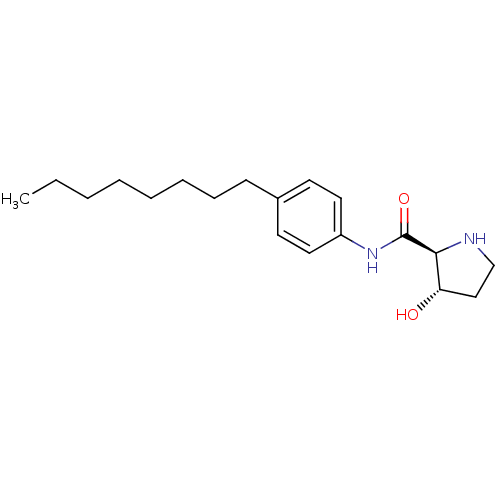 ((2S,3S)-3-hydroxy-N-(4-octylphenyl)pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50299328 ((2S,3S)-3-hydroxy-N-(4-octylphenyl)pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of human sphingosine kinase 1 by off chip mobility shift assay | Bioorg Med Chem Lett 19: 6119-21 (2009) Article DOI: 10.1016/j.bmcl.2009.09.022 BindingDB Entry DOI: 10.7270/Q29Z94ZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50379157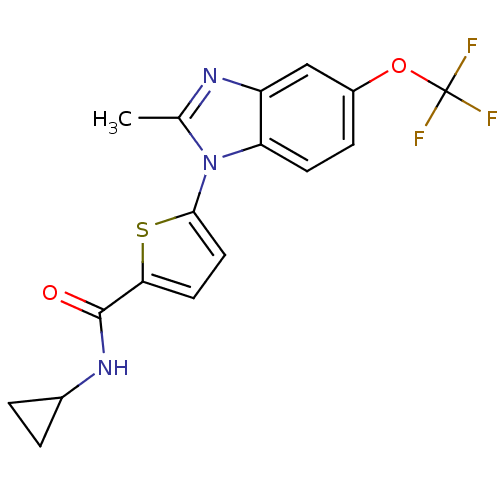 (CHEMBL1234899 | US8703811, 57) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120325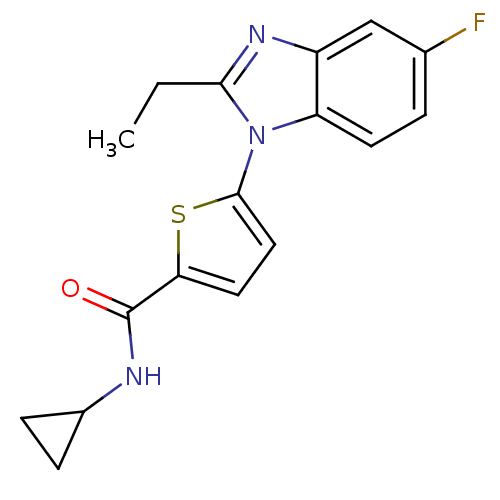 (US8703811, 66) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323225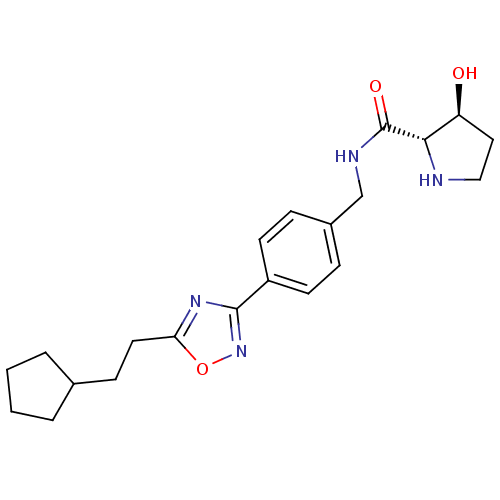 ((2S,3S)-N-(4-(5-(2-cyclopentylethyl)-1,2,4-oxadiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323240 ((2S,3S)-N-(4-(5-heptyl-1,2,4-oxadiazol-3-yl)benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM92604 (DHOD Inhibitor, 3 | US8703811, 1) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323233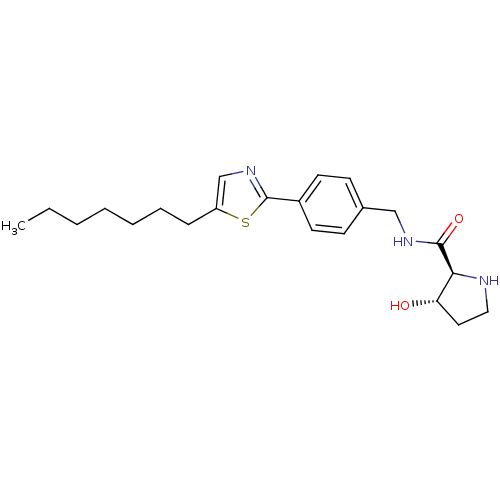 ((2S,3S)-N-(4-(5-heptylthiazol-2-yl)benzyl)-3-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50379146 (CHEMBL2012829 | US8703811, 31 | US8703811, 32) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120320 (US8703811, 61) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM92606 (DHOD Inhibitor, 5 | US8703811, 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM92606 (DHOD Inhibitor, 5 | US8703811, 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School | Assay Description Assay was determined using a continuous assay. | J Biol Chem 283: 35078-85 (2008) Article DOI: 10.1074/jbc.M804990200 BindingDB Entry DOI: 10.7270/Q2VH5MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50323238 ((2S,3S)-3-hydroxy-N-(4-(5-octyl-1,2,4-oxadiazol-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation Curated by ChEMBL | Assay Description Inhibition of sphingosine kinase 1 | Bioorg Med Chem Lett 20: 4550-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.019 BindingDB Entry DOI: 10.7270/Q23T9HDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120303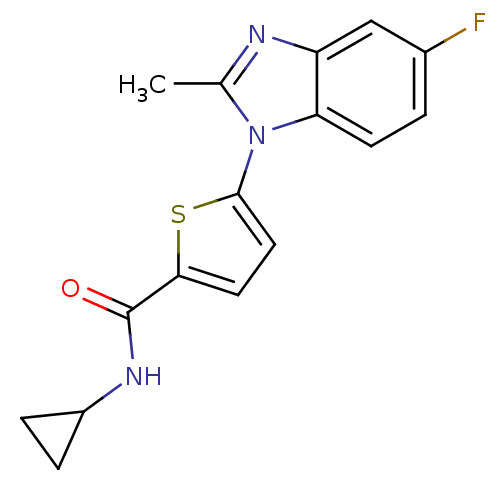 (US8703811, 42 | US8703811, 43) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120356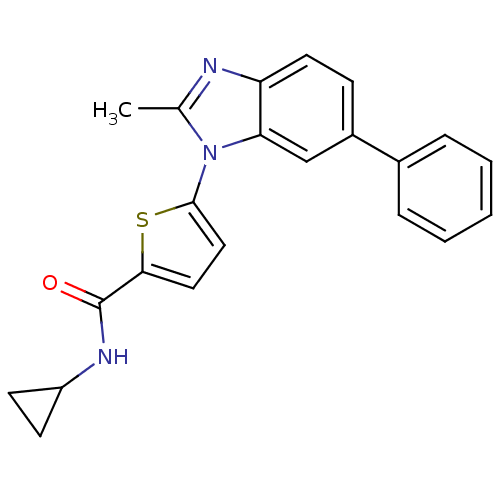 (US8703811, 98) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120312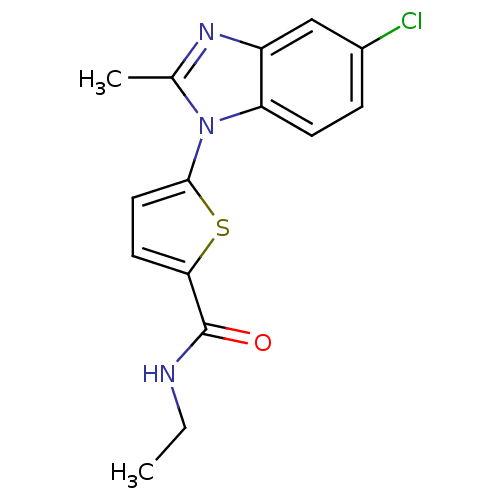 (US8703811, 52) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM50379146 (CHEMBL2012829 | US8703811, 31 | US8703811, 32) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50379157 (CHEMBL1234899 | US8703811, 57) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 using bufuralol as substrate after 10 mins by LC-MS/MS analysis | ACS Med Chem Lett 2: 708-713 (2011) Article DOI: 10.1021/ml200143c BindingDB Entry DOI: 10.7270/Q2C24XDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120303 (US8703811, 42 | US8703811, 43) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120326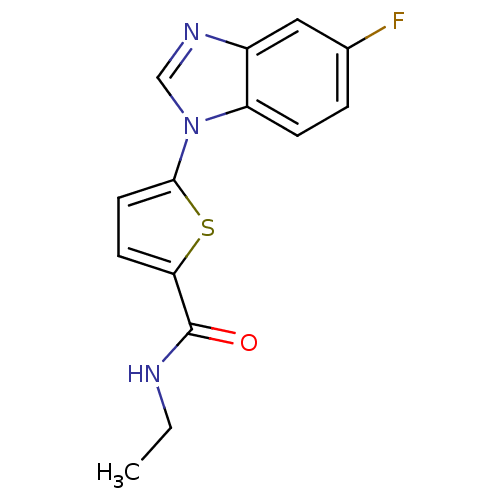 (US8703811, 68) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (quinone), mitochondrial (Plasmodium falciparum) | BDBM120353 (US8703811, 95) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corporation; Massachusetts Institute of Technology; President and Fellows of Harvard College US Patent | Assay Description Type 2 DHODH activity was monitored with either the direct assay measuring the formation of orotate or via a chromogen reduction assay using DCIP. Al... | US Patent US8703811 (2014) BindingDB Entry DOI: 10.7270/Q2WD3Z7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 265 total ) | Next | Last >> |