

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM182716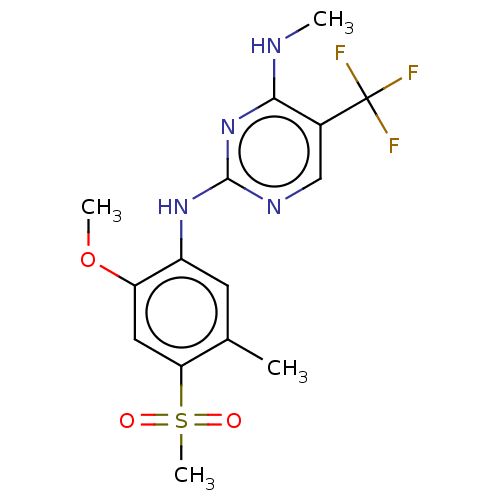 (US9145402, 23 | US9145402, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US9145402 (2015) BindingDB Entry DOI: 10.7270/Q2J67FQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196757 (US9212173, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50110705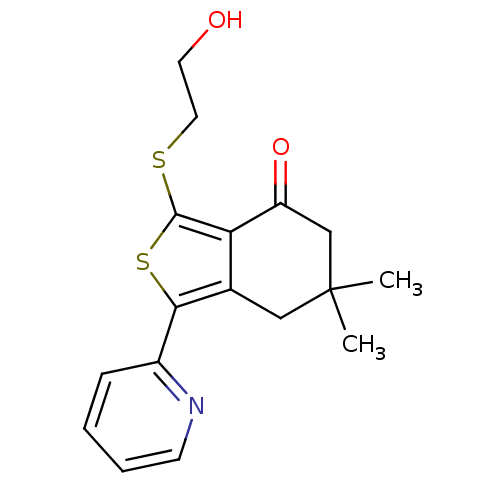 (3-(2-Hydroxy-ethylsulfanyl)-6,6-dimethyl-1-pyridin...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]- Ro 15-1788 binding from human GABA-A receptor alpha5-beta3-gamma2 subunits | J Med Chem 45: 1176-9 (2002) BindingDB Entry DOI: 10.7270/Q26Q1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50146441 (3-Methyl-5-pyridin-2-yl-8-(thiophen-2-ylsulfanyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | Bioorg Med Chem Lett 14: 2871-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.054 BindingDB Entry DOI: 10.7270/Q2KW5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50110705 (3-(2-Hydroxy-ethylsulfanyl)-6,6-dimethyl-1-pyridin...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human GABA A alpha5-beta3-gamma2 receptor,using a [3H]-Ro- 15-1788 radioligand | J Med Chem 46: 2227-40 (2003) Article DOI: 10.1021/jm020582q BindingDB Entry DOI: 10.7270/Q2BK1BP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553757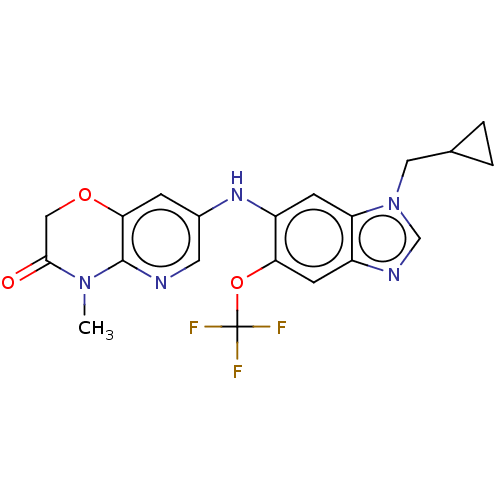 (CHEMBL4746471) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196754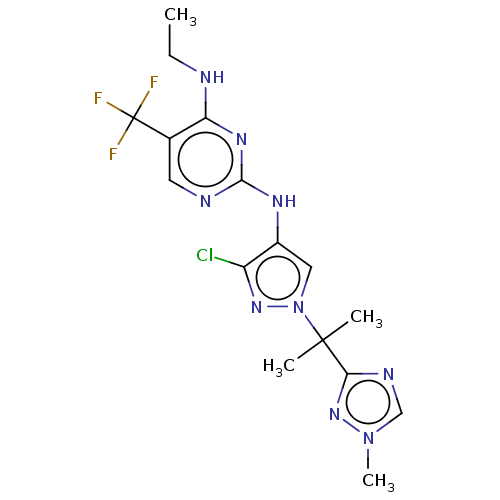 (US9212173, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50144858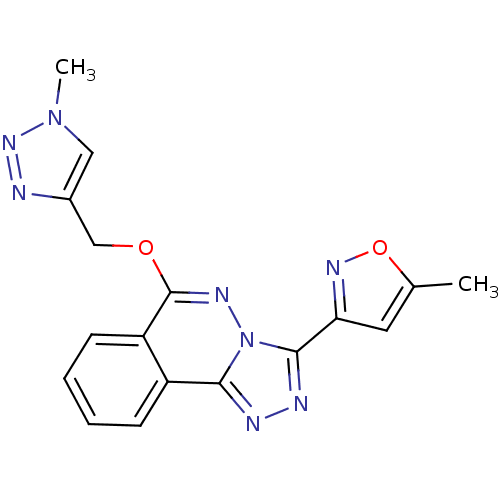 (3-(5-Methyl-isoxazol-3-yl)-6-(1-methyl-1H-[1,2,3]t...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha2-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196762 (US9212173, 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196759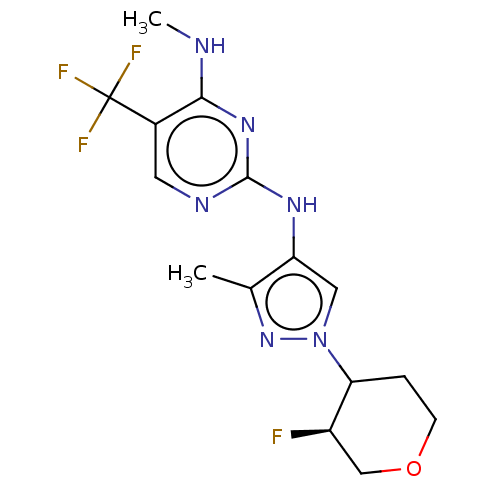 (US9212173, 37) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196752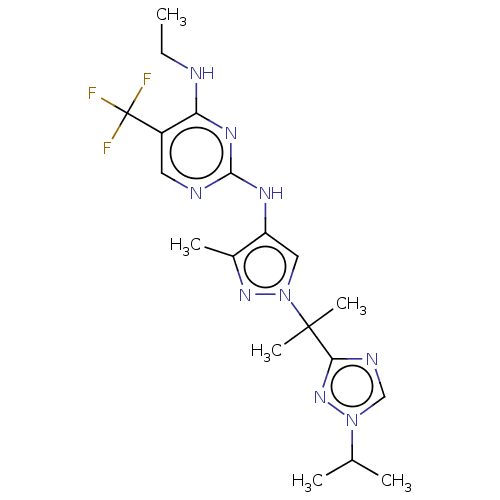 (US9212173, 30) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM129199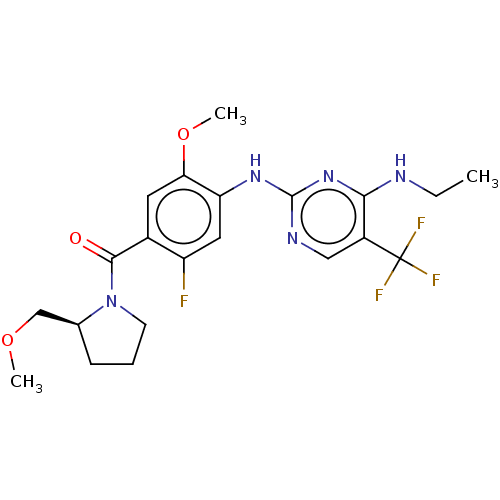 (US8802674, 306) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8802674 (2014) BindingDB Entry DOI: 10.7270/Q2GF0S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM129179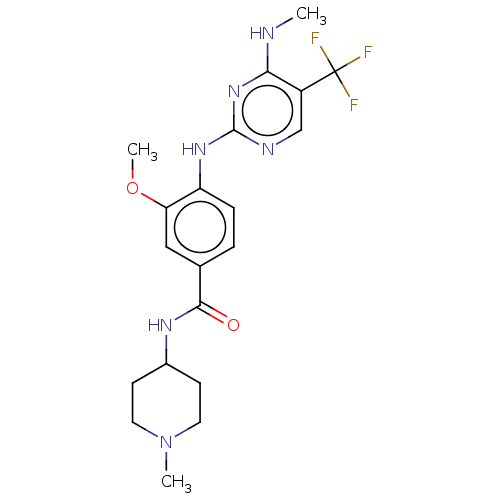 (US8802674, 282) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8802674 (2014) BindingDB Entry DOI: 10.7270/Q2GF0S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50144858 (3-(5-Methyl-isoxazol-3-yl)-6-(1-methyl-1H-[1,2,3]t...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha3-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50144858 (3-(5-Methyl-isoxazol-3-yl)-6-(1-methyl-1H-[1,2,3]t...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha5-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50448127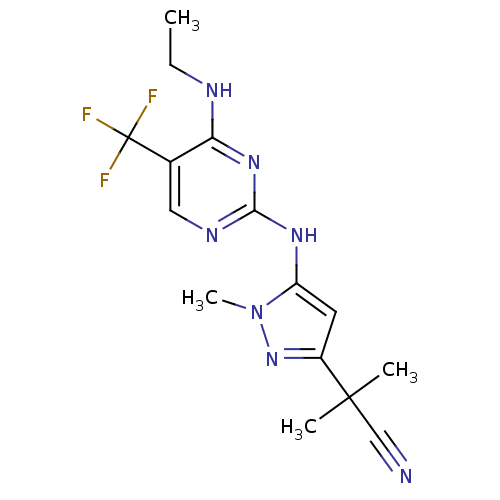 (CHEMBL3122119 | US9212173, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50448118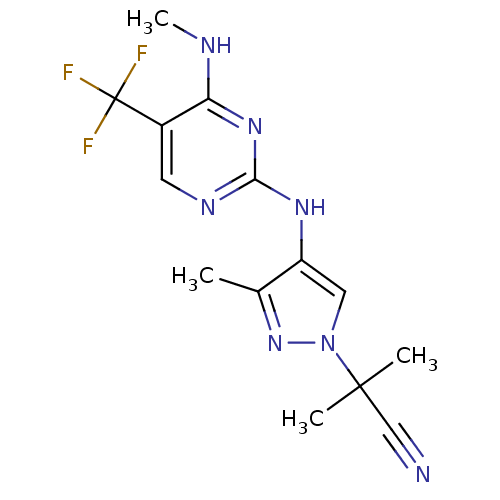 (CHEMBL3122113 | US10590114, No. 80 | US11111235, N...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 (unknown origin) | J Med Chem 57: 921-36 (2014) Article DOI: 10.1021/jm401654j BindingDB Entry DOI: 10.7270/Q2N0181N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50448127 (CHEMBL3122119 | US9212173, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 (unknown origin) | J Med Chem 57: 921-36 (2014) Article DOI: 10.1021/jm401654j BindingDB Entry DOI: 10.7270/Q2N0181N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50156083 (3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha3-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM129173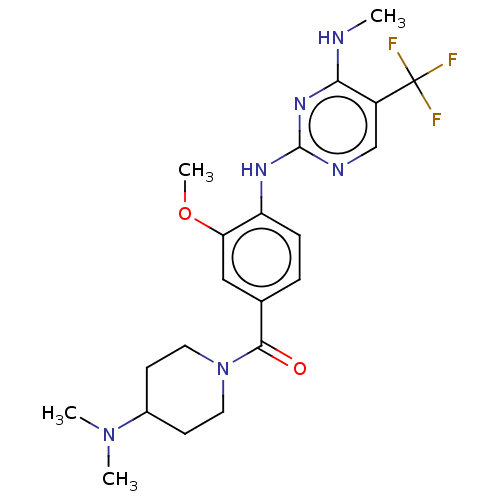 (US8802674, 276) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8802674 (2014) BindingDB Entry DOI: 10.7270/Q2GF0S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50156083 (3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...) | UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha1-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50156083 (3-tert-Butyl-7-(5-methyl-isoxazol-3-yl)-2-(2-methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha2-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50144858 (3-(5-Methyl-isoxazol-3-yl)-6-(1-methyl-1H-[1,2,3]t...) | UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro- 15-1788 from human gamma-aminobutyric-acid GABA-A receptor alpha1-beta3-gamma2 expressed in L(tk-) cells | J Med Chem 47: 5829-32 (2004) Article DOI: 10.1021/jm040863t BindingDB Entry DOI: 10.7270/Q2CZ36MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM403740 (6-Chloro-7-((3-(cyclopropylmethyl)-7-methyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50448126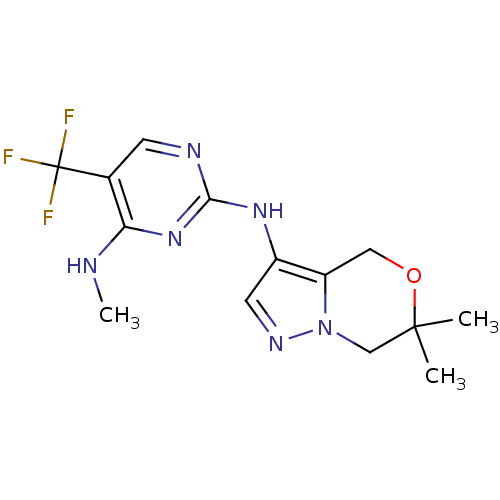 (CHEMBL3122105 | US9212186, 22) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 (unknown origin) | J Med Chem 57: 921-36 (2014) Article DOI: 10.1021/jm401654j BindingDB Entry DOI: 10.7270/Q2N0181N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196742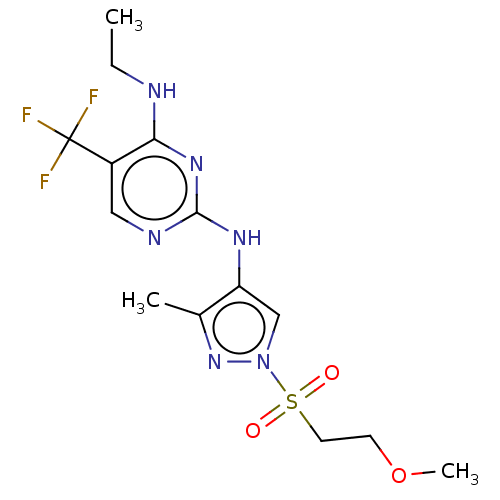 (US9212173, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM196741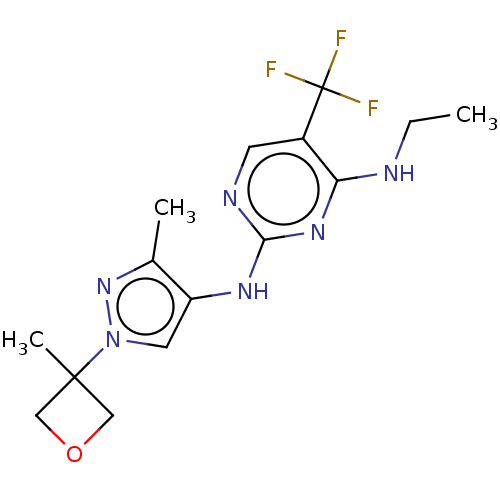 (US9212173, 19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.975 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... | US Patent US9212173 (2015) BindingDB Entry DOI: 10.7270/Q2222SKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553762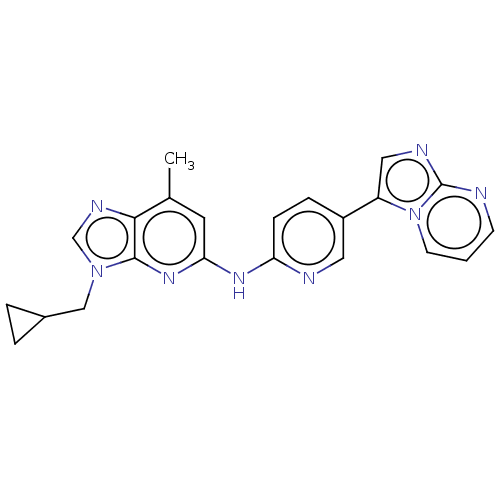 (CHEMBL4758608) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128256 (US8796296, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50448117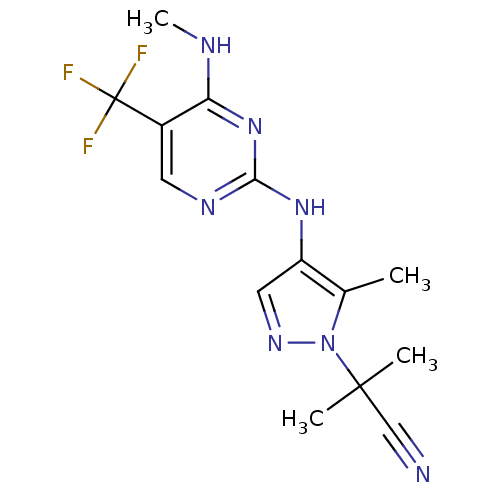 (CHEMBL3122114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 (unknown origin) | J Med Chem 57: 921-36 (2014) Article DOI: 10.1021/jm401654j BindingDB Entry DOI: 10.7270/Q2N0181N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128254 (US8796296, 9) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128248 (US8796296, 3) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128247 (US8796296, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM127916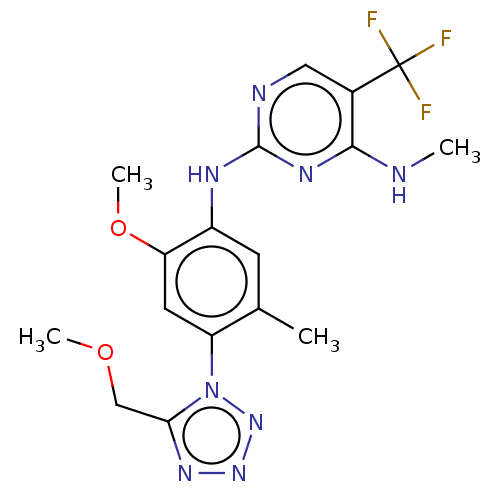 (US8791130, 46) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8791130 (2014) BindingDB Entry DOI: 10.7270/Q2K9367R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM127915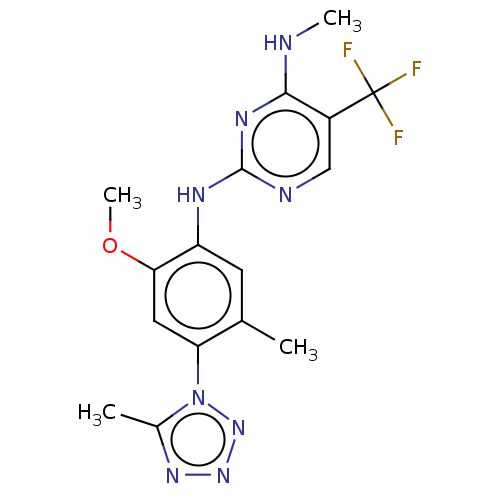 (US8791130, 45) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8791130 (2014) BindingDB Entry DOI: 10.7270/Q2K9367R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM127911 (US8791130, 41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8791130 (2014) BindingDB Entry DOI: 10.7270/Q2K9367R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM127896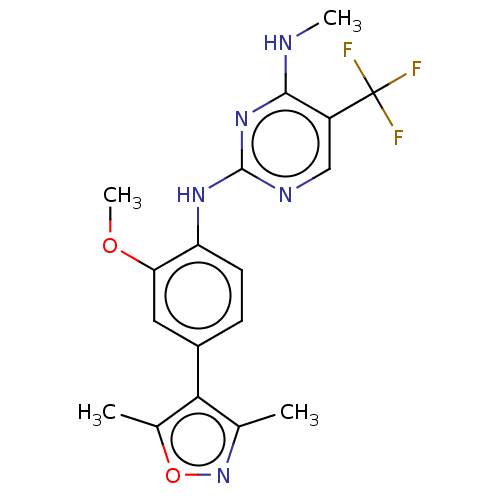 (US8791130, 26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8791130 (2014) BindingDB Entry DOI: 10.7270/Q2K9367R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM127895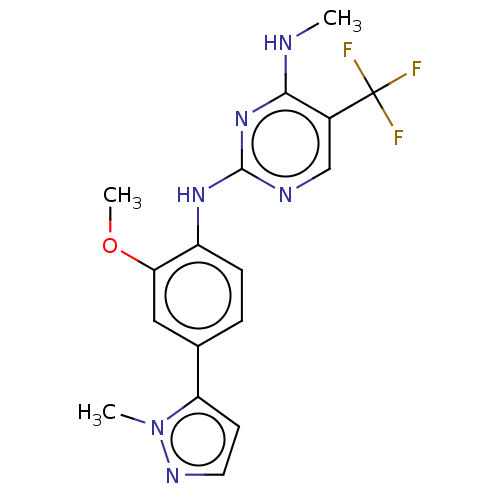 (US8791130, 25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8791130 (2014) BindingDB Entry DOI: 10.7270/Q2K9367R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128276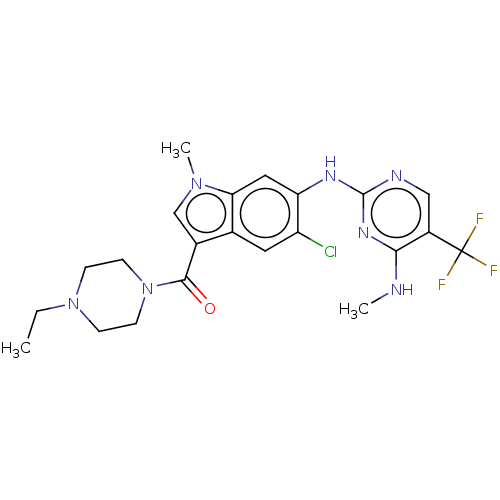 (US8796296, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128275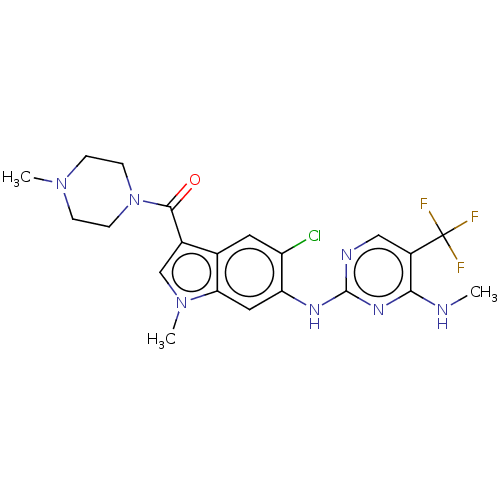 (US8796296, 31) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128274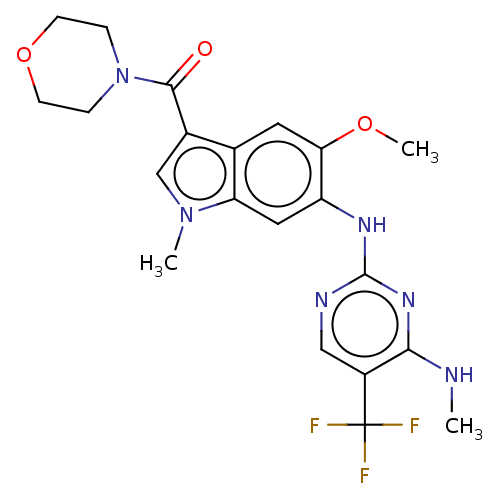 (US8796296, 30) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM128273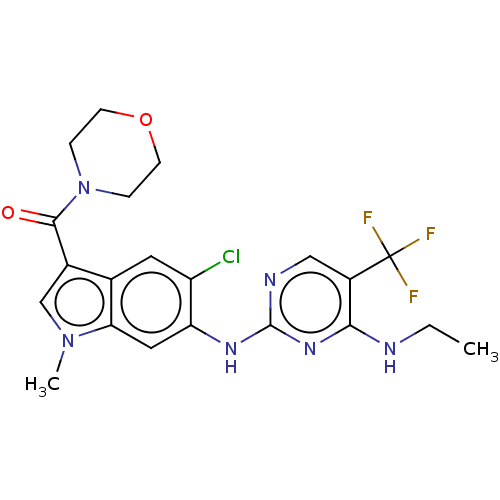 (US8796296, 29) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US8796296 (2014) BindingDB Entry DOI: 10.7270/Q28W3C0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM182713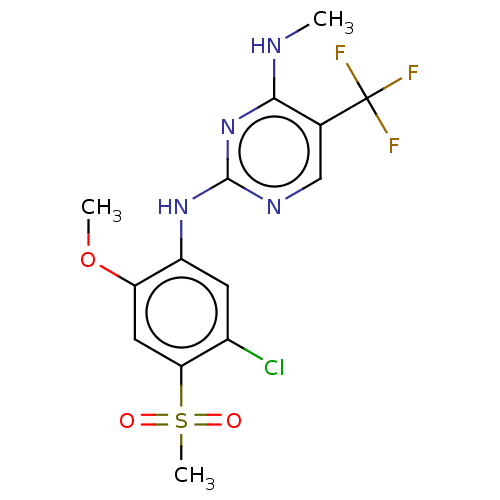 (US9145402, 20) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... | US Patent US9145402 (2015) BindingDB Entry DOI: 10.7270/Q2J67FQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50398676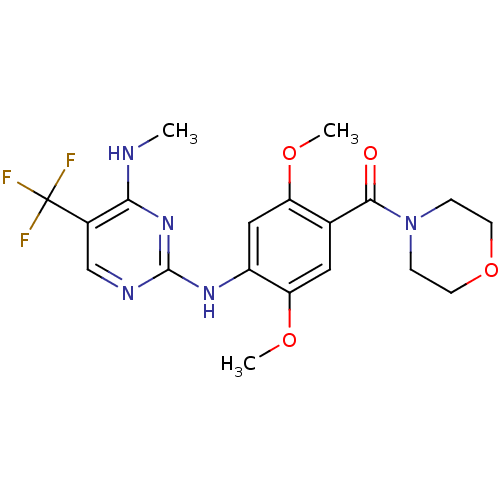 (CHEMBL2178125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50398668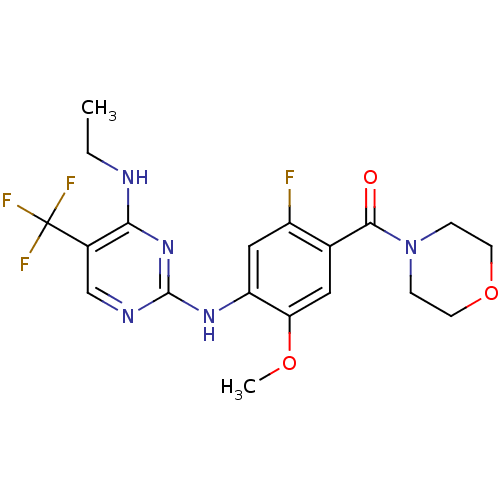 (CHEMBL2178134 | US8802674, 256) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50398667 (CHEMBL2178135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50398662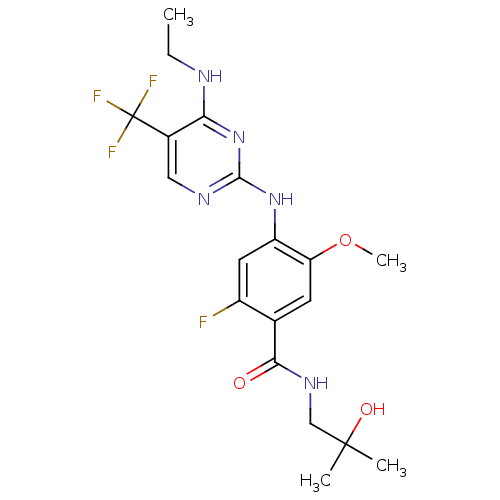 (CHEMBL2178140) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assay | J Med Chem 55: 9416-33 (2012) Article DOI: 10.1021/jm301020q BindingDB Entry DOI: 10.7270/Q2P55PN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50146443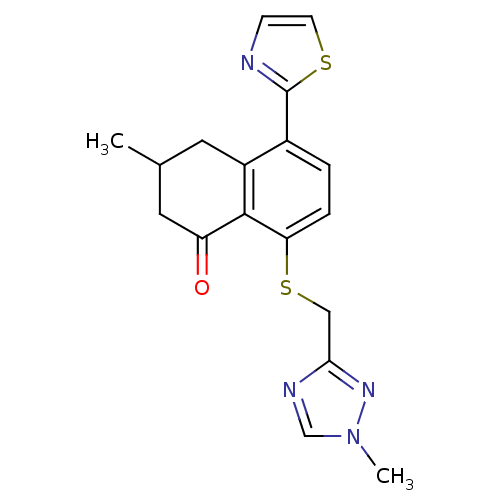 (3-Methyl-8-(1-methyl-1H-[1,2,4]triazol-3-ylmethyls...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | Bioorg Med Chem Lett 14: 2871-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.054 BindingDB Entry DOI: 10.7270/Q2KW5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50146433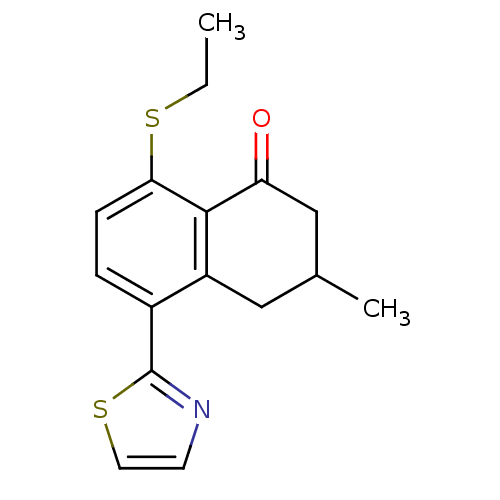 (8-Ethylsulfanyl-3-methyl-5-thiazol-2-yl-3,4-dihydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits | Bioorg Med Chem Lett 14: 2871-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.054 BindingDB Entry DOI: 10.7270/Q2KW5GK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM129180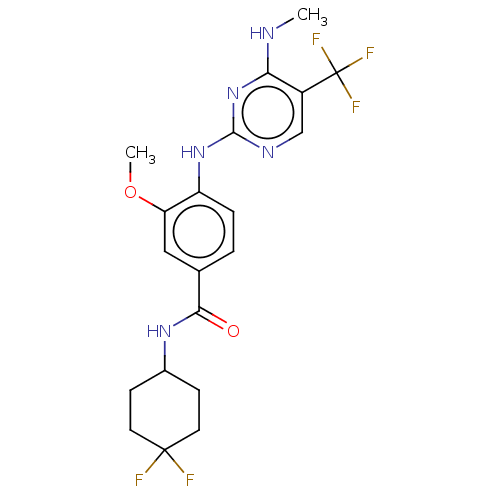 (US8802674, 283) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. ... | US Patent US8802674 (2014) BindingDB Entry DOI: 10.7270/Q2GF0S6N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2177 total ) | Next | Last >> |