

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106803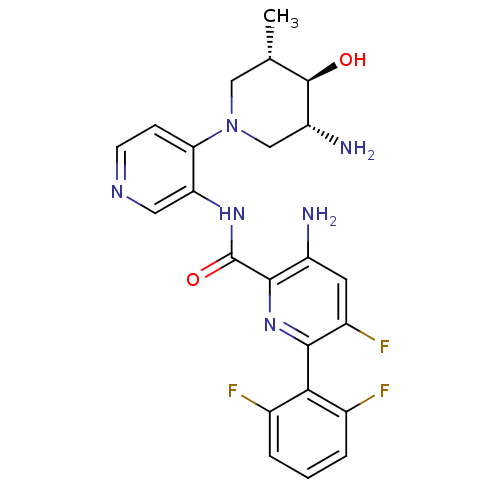 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445133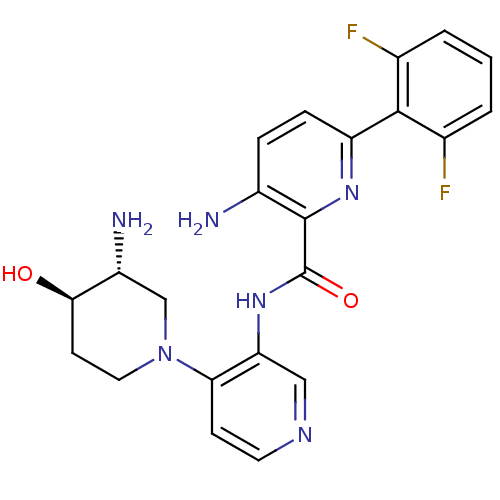 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50445124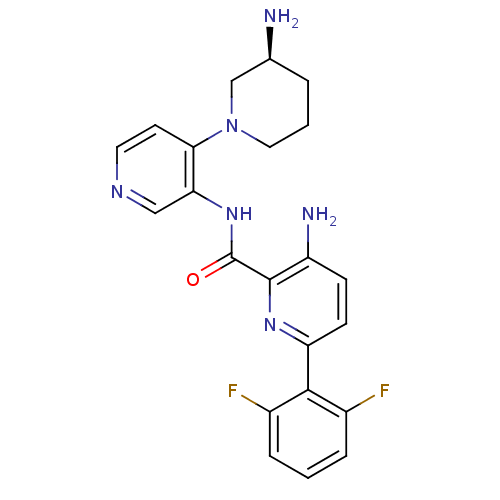 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106896 (US8592455, 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106803 (US8592455, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445124 (CHEMBL3103868) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by AlphaScreen assay | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50445133 (CHEMBL3103869) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337452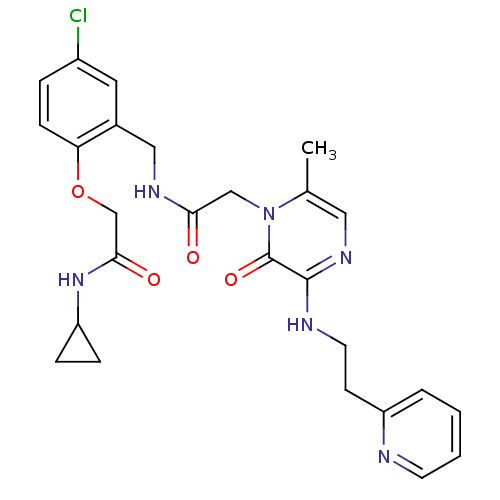 (2-(4-chloro-2-((2-(6-methyl-2-oxo-3-(2-(pyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1532-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.108 BindingDB Entry DOI: 10.7270/Q2ZS2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298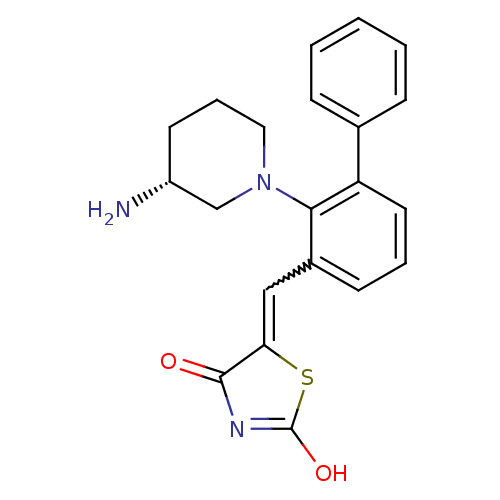 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM106870 (US8592455, 70) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337453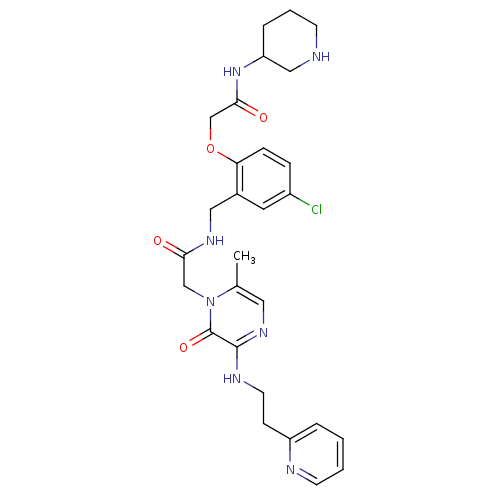 (2-(4-chloro-2-((2-(6-methyl-2-oxo-3-(2-(pyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1532-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.108 BindingDB Entry DOI: 10.7270/Q2ZS2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538323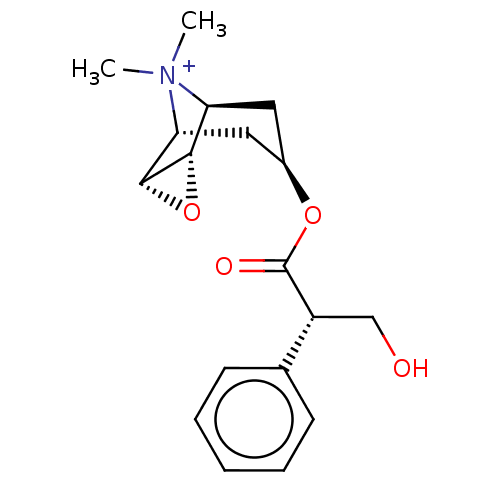 (CHEMBL3140030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 4-(2-((1E,3E)-5-((E)-3,3-Dimethyl-1-(6-oxo-6-((2-(3-(1-(4-(1-(2-oxo-2-(11-oxo-10,11-dihydro-5H-dibenzo[b,e][1,4]diazepin-5-yl)ethyl)p... | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM2 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538317 (CHEMBL4636083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538315 (CHEMBL4641328) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50213665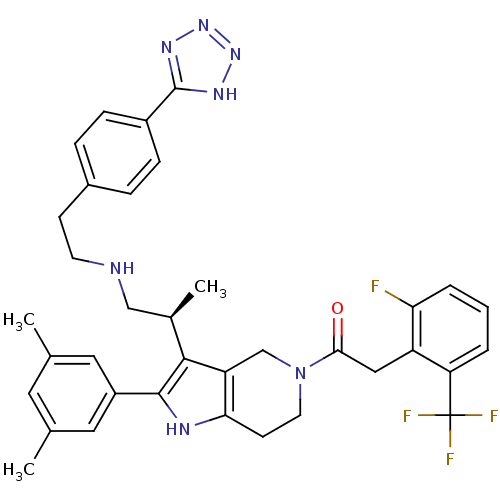 ((S)-1-(3-(1-(4-(1H-tetrazol-5-yl)phenethylamino)pr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR | Bioorg Med Chem Lett 17: 3845-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.009 BindingDB Entry DOI: 10.7270/Q2JD4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) | ACS Med Chem Lett 4: 1193-7 (2013) Article DOI: 10.1021/ml400307j BindingDB Entry DOI: 10.7270/Q23X8840 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50387298 (CHEMBL2048872) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of PIM3 kinase (unknown origin) using Biotin-AGAGRSRHSSYPAGT-OH as substrate after 2 hrs by alphascreen assay | J Med Chem 58: 8373-86 (2015) Article DOI: 10.1021/acs.jmedchem.5b01275 BindingDB Entry DOI: 10.7270/Q2H41VGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538316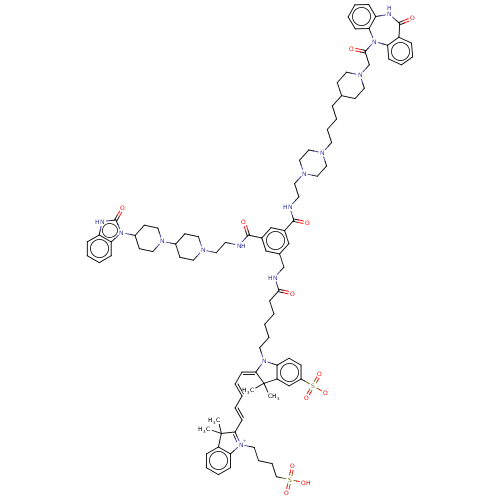 (CHEMBL4643273) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538321 (CHEMBL4643159) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337483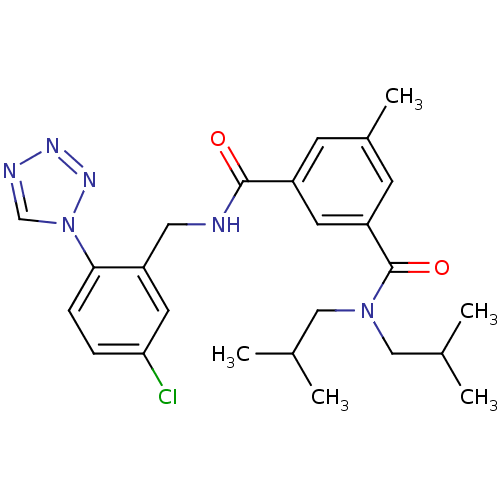 (CHEMBL1682777 | N1-(5-chloro-2-(1H-tetrazol-1-yl)b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1536-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.105 BindingDB Entry DOI: 10.7270/Q2V1252F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337479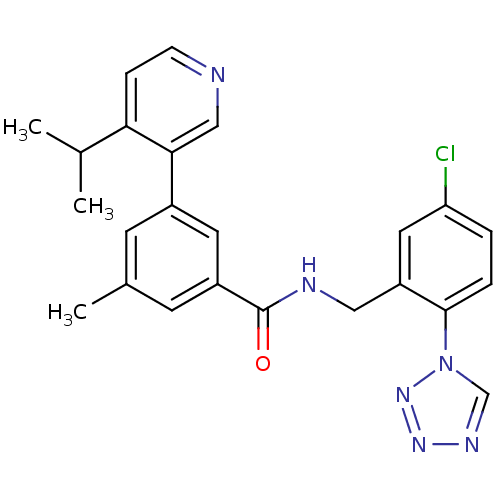 (CHEMBL1682781 | N-(5-chloro-2-(1H-tetrazol-1-yl)be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1536-40 (2011) Article DOI: 10.1016/j.bmcl.2010.12.105 BindingDB Entry DOI: 10.7270/Q2V1252F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349866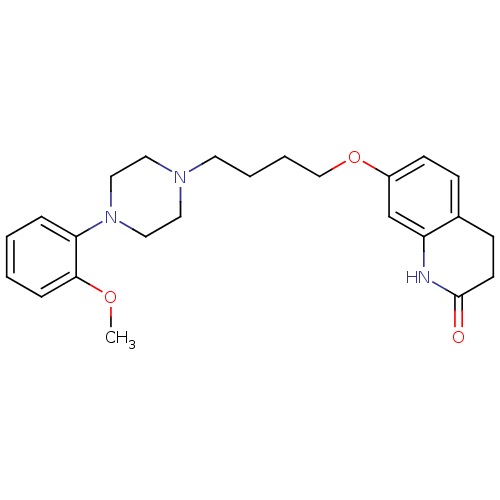 (CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in CHO cells after 1.5 hrs by microbeta counting method | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50213671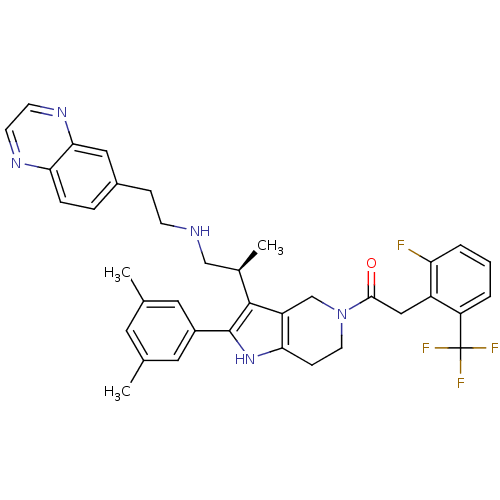 ((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(quinoxalin-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR | Bioorg Med Chem Lett 17: 3845-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.009 BindingDB Entry DOI: 10.7270/Q2JD4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50337470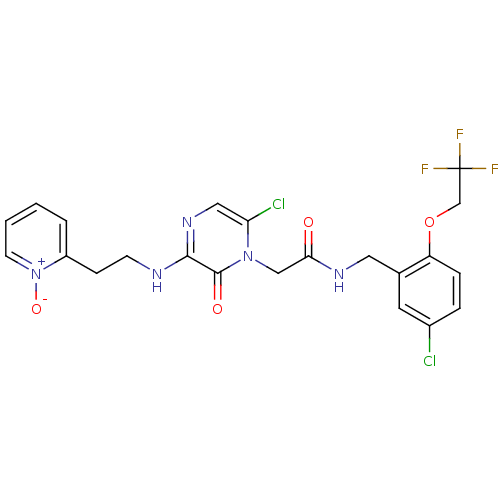 (2-(2-(5-chloro-4-(2-(5-chloro-2-(2,2,2-trifluoroet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 21: 1532-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.108 BindingDB Entry DOI: 10.7270/Q2ZS2WS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50039026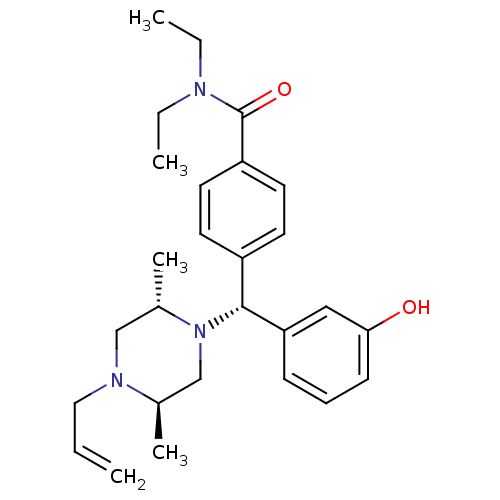 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells | J Med Chem 51: 5893-6 (2008) Article DOI: 10.1021/jm8008986 BindingDB Entry DOI: 10.7270/Q26W9C0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818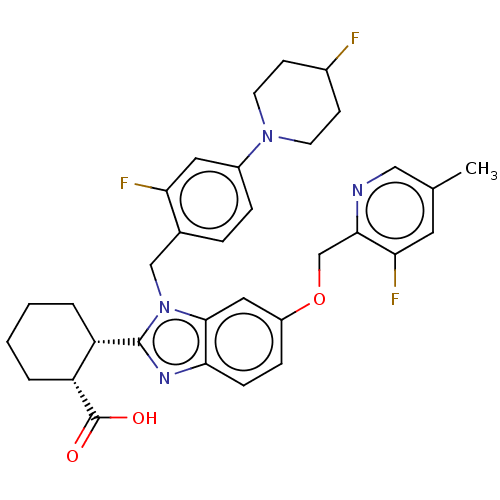 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50277608 (CHEMBL4173067) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145818 (US8952177, 174 | US9089569, 174 | US9695149, 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538324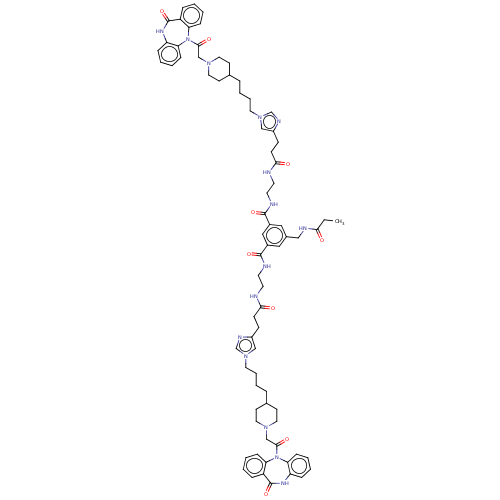 (CHEMBL4116470) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 4-(2-((1E,3E)-5-((E)-3,3-Dimethyl-1-(6-oxo-6-((2-(3-(1-(4-(1-(2-oxo-2-(11-oxo-10,11-dihydro-5H-dibenzo[b,e][1,4]diazepin-5-yl)ethyl)p... | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50213664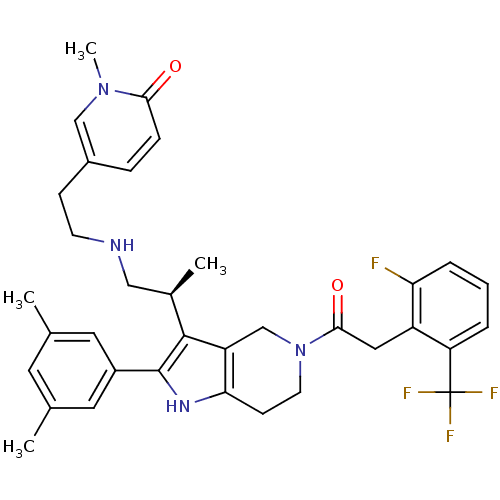 ((S)-5-(2-(2-(2-(3,5-dimethylphenyl)-5-(2-(2-fluoro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR | Bioorg Med Chem Lett 17: 3845-50 (2007) Article DOI: 10.1016/j.bmcl.2007.05.009 BindingDB Entry DOI: 10.7270/Q2JD4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820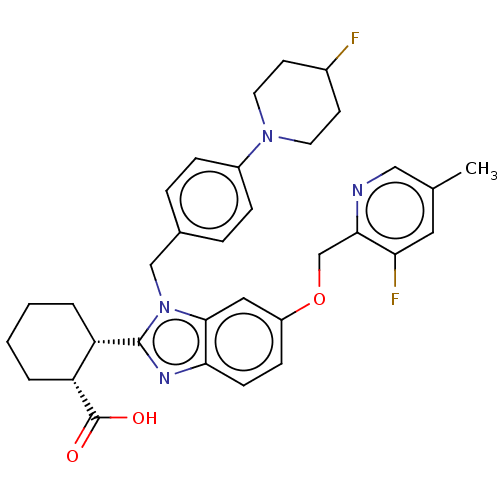 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145820 (US8952177, 176 | US9089569, 176 | US9695149, 176) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50252953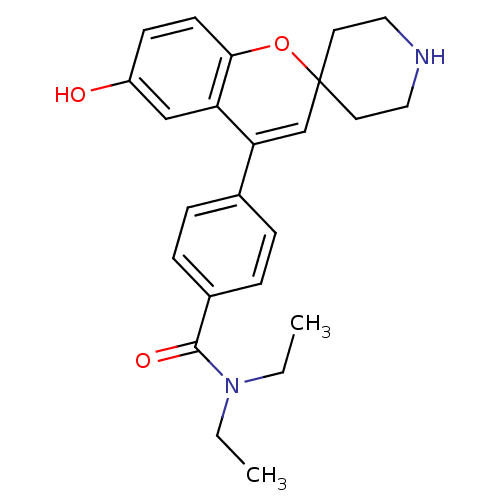 (CHEMBL494479 | N,N-Diethyl-4-(6-hydroxyspiro[chrom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human delta opioid receptor in CHO cells | J Med Chem 51: 5893-6 (2008) Article DOI: 10.1021/jm8008986 BindingDB Entry DOI: 10.7270/Q26W9C0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50538317 (CHEMBL4636083) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M4 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D2 from rat corpus striatum by using radioligand [3H]-sulpiride | J Med Chem 36: 1488-95 (1993) BindingDB Entry DOI: 10.7270/Q2KD1X0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785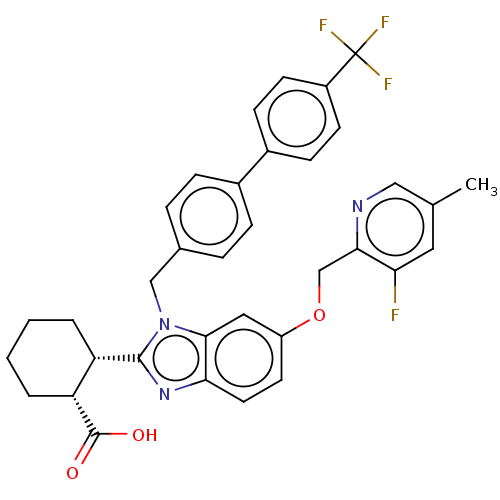 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9695149 (2017) BindingDB Entry DOI: 10.7270/Q2Q52MSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
JANSSEN PHARMACEUTICA NV US Patent | Assay Description The assay below is used to test the modulatory activity of compounds against FLAP. Human and mouse FLAP-encoding DNA was amplified by polymerase chai... | US Patent US9089569 (2015) BindingDB Entry DOI: 10.7270/Q23X85DB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM145785 (US8952177, 139 | US9089569, 139 | US9695149, 139) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV US Patent | Assay Description FLAP-containing membranes were prepared as was a FITC-labeled FLAP modulator (3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(quinolin-2-ylmethoxy)-1H-in... | US Patent US8952177 (2015) BindingDB Entry DOI: 10.7270/Q2G73CFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 16546 total ) | Next | Last >> |