

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469565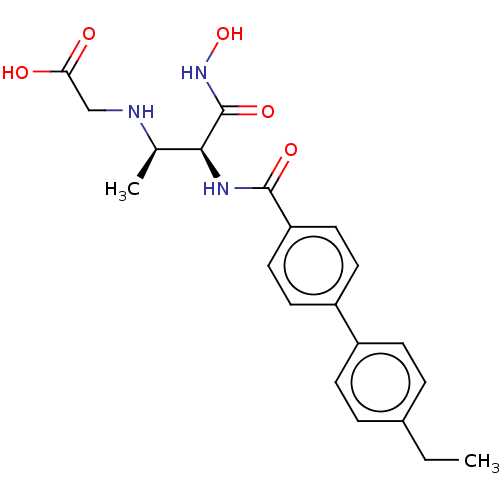 (CHEMBL4082918) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469558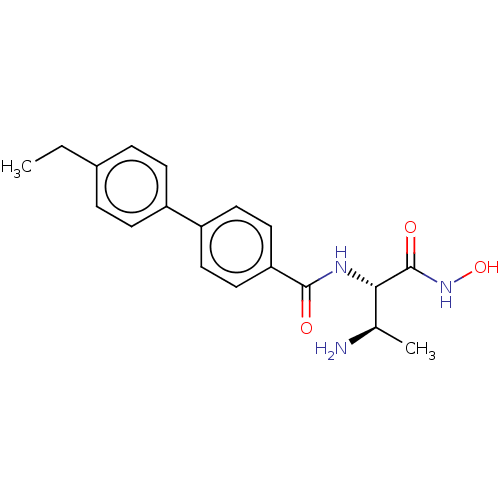 (CHEMBL4061041) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50249583 (CHEMBL4097399) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469562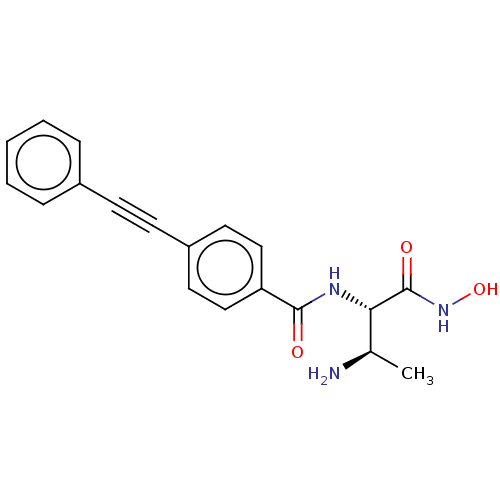 (CHEMBL4069725) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469559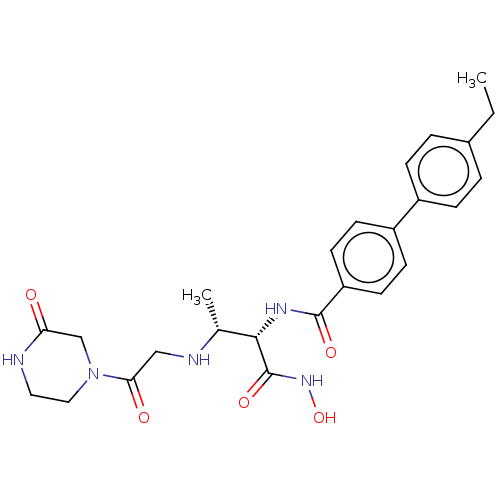 (CHEMBL4063087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469555 (CHEMBL4090716) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103282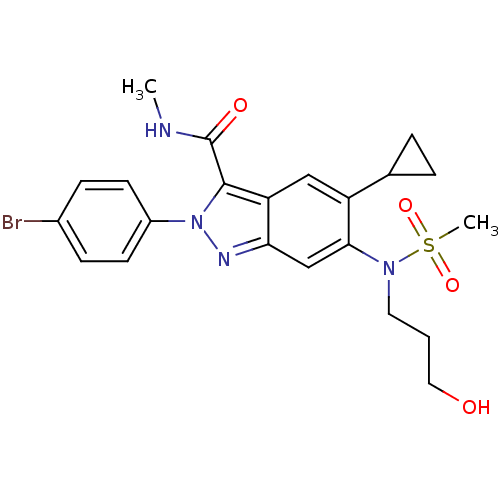 (US8546389, 4) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469560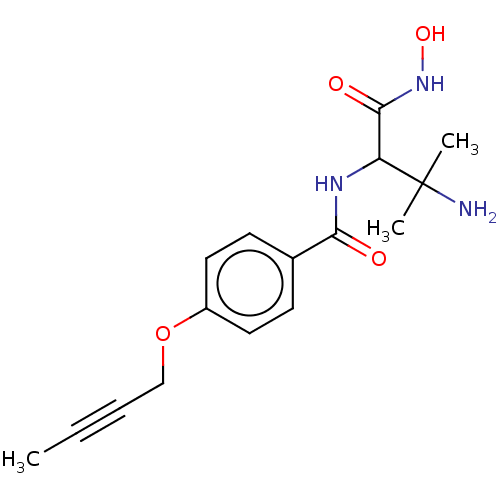 (CHEMBL4083624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469563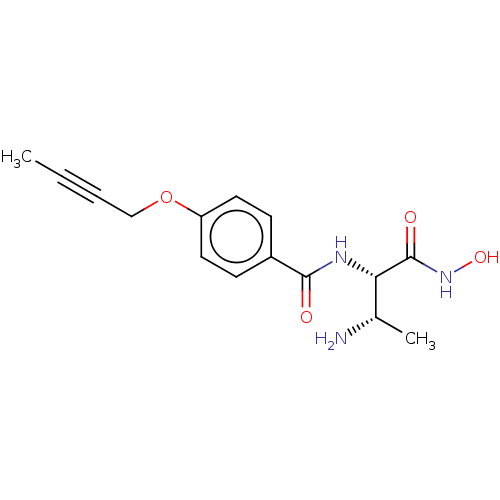 (CHEMBL4079368) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469550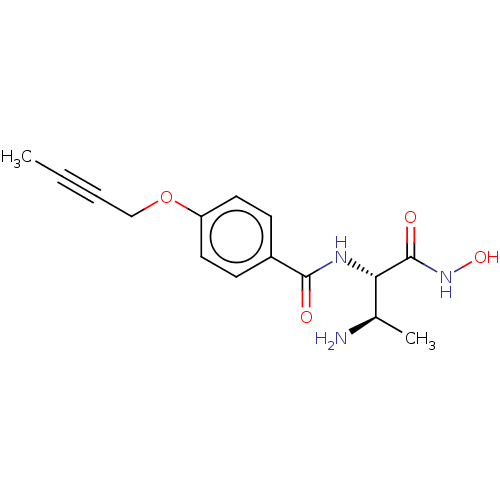 (CHEMBL4070478) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103288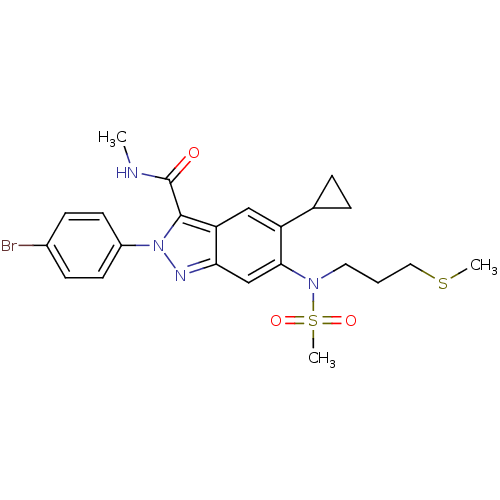 (US8546389, 99) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103278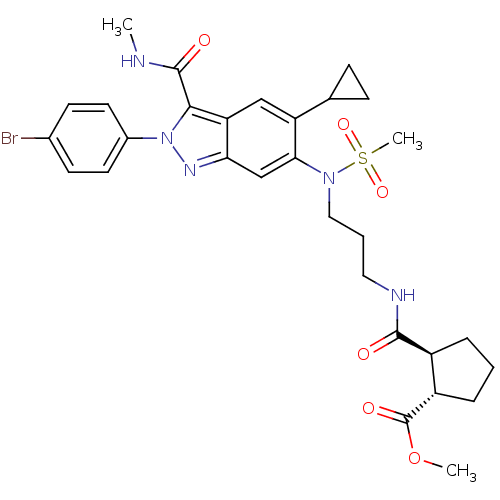 (US8546389, 216) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103276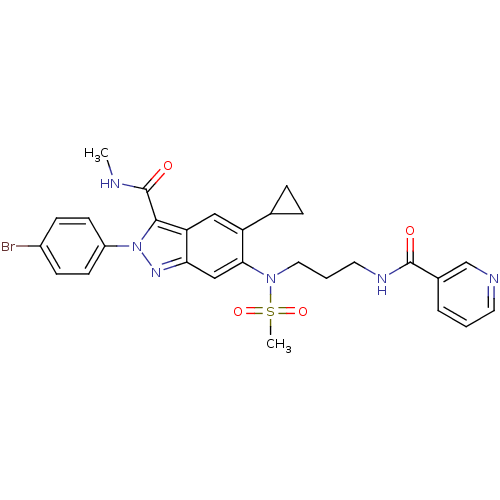 (US8546389, 191) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103255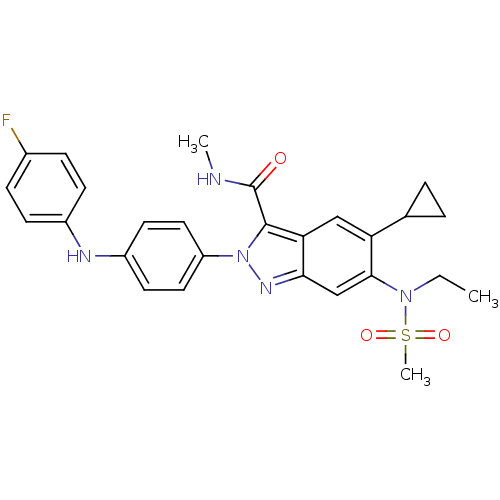 (US8546389, 22) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103256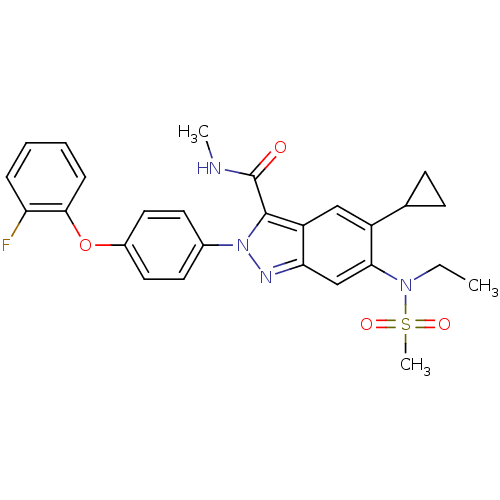 (US8546389, 28) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103284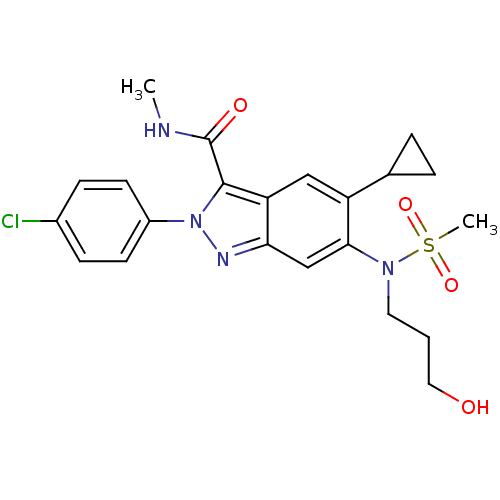 (US8546389, 54) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103279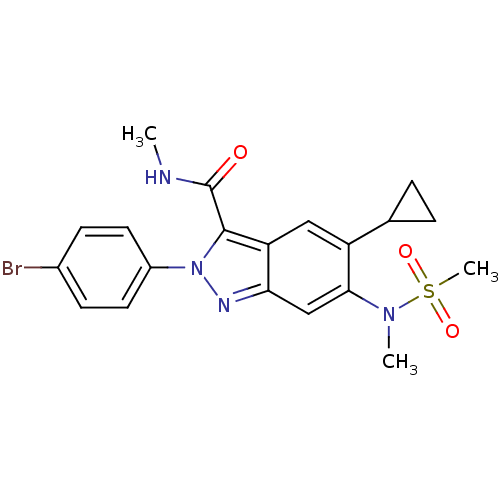 (US8546389, 234) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM32026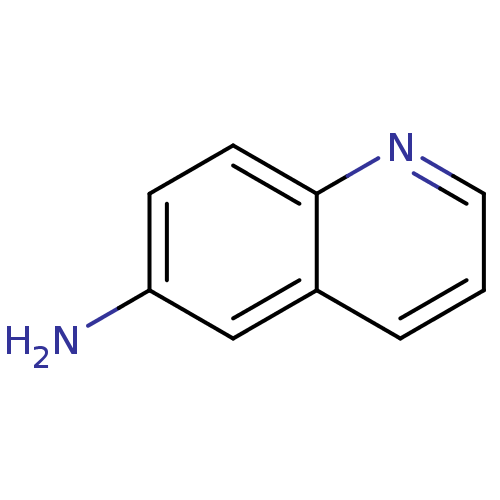 (6-quinolinamine | 6-quinolylamine | BDBM32208 | ML...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103280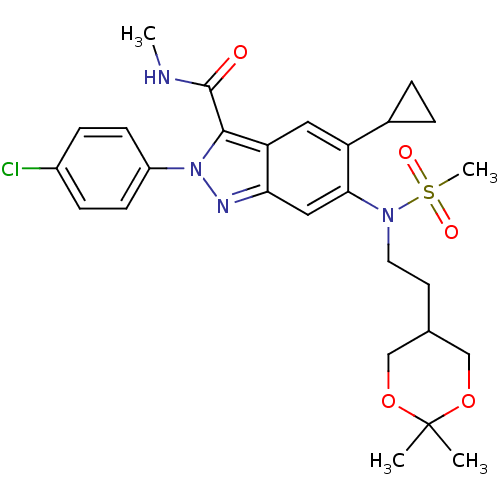 (US8546389, 235) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103272 (US8546389, 179) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103270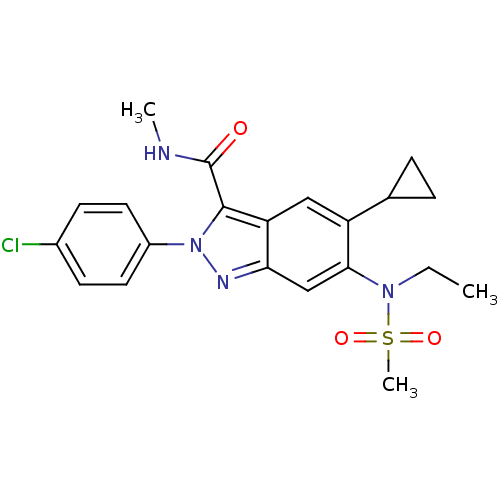 (US8546389, 175) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103269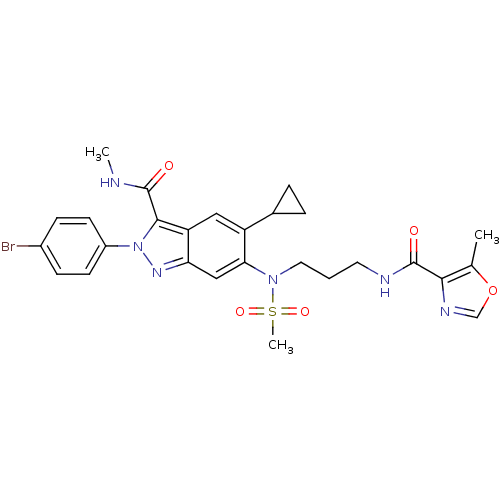 (US8546389, 174) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103266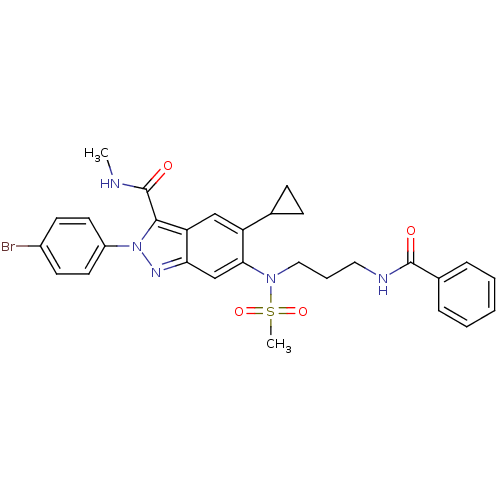 (US8546389, 166) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103261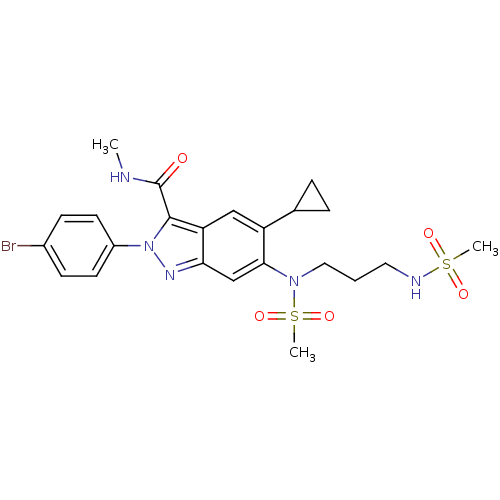 (US8546389, 134) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103260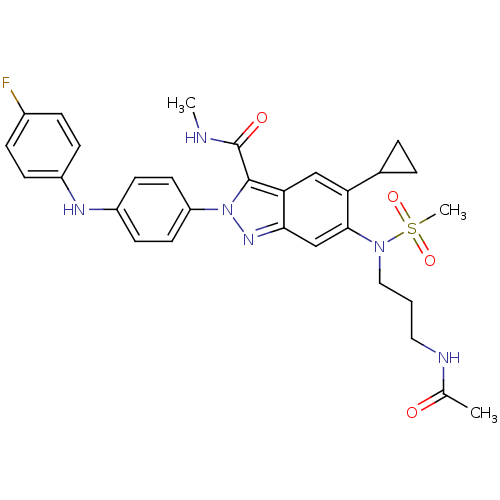 (US8546389, 119) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469557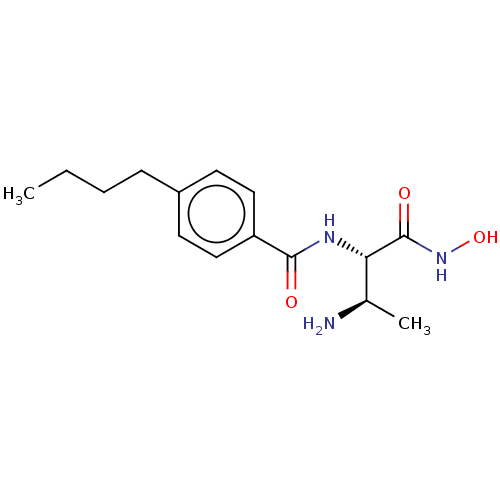 (CHEMBL4091408) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Pseudomonas aeruginosa) | BDBM50469554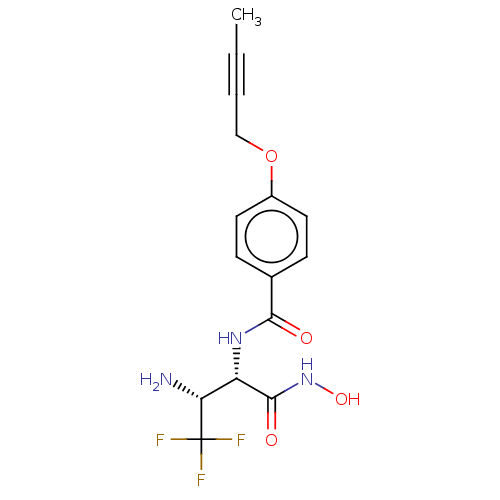 (CHEMBL4061854) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LpxC using UDP-3-O-(R-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 30 mins followed by sub... | J Med Chem 60: 5002-5014 (2017) Article DOI: 10.1021/acs.jmedchem.7b00377 BindingDB Entry DOI: 10.7270/Q2FN18MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103268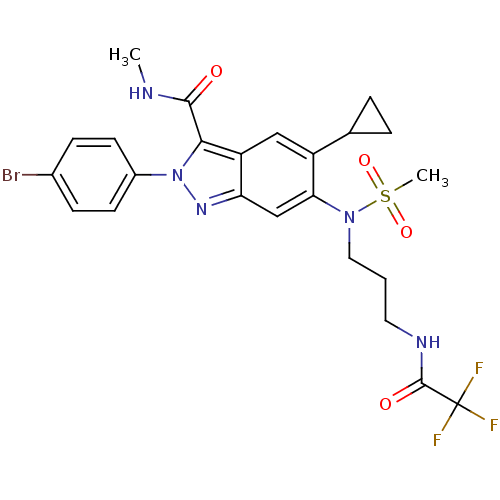 (US8546389, 172) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103267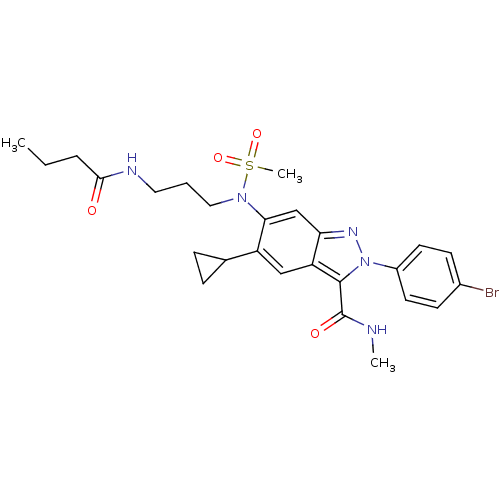 (US8546389, 171) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103264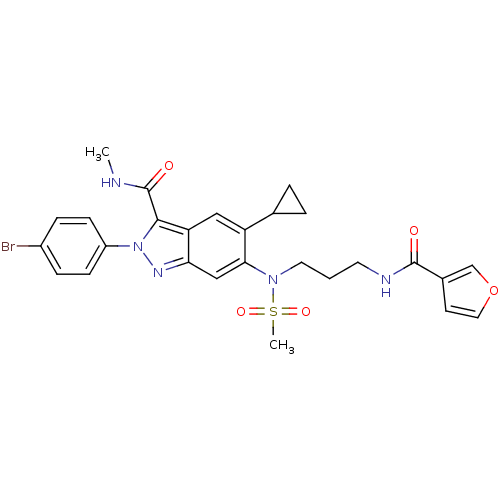 (US8546389, 160) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103265 (US8546389, 164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103287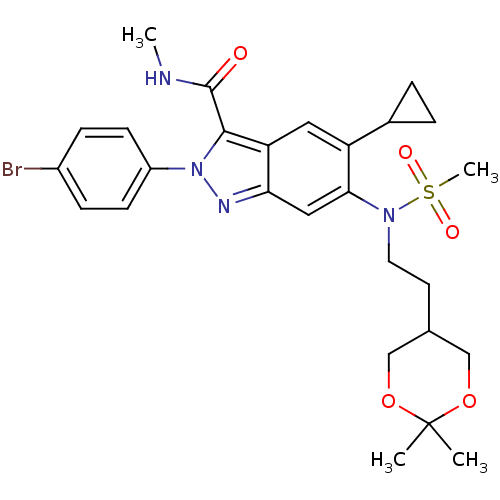 (US8546389, 95) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103258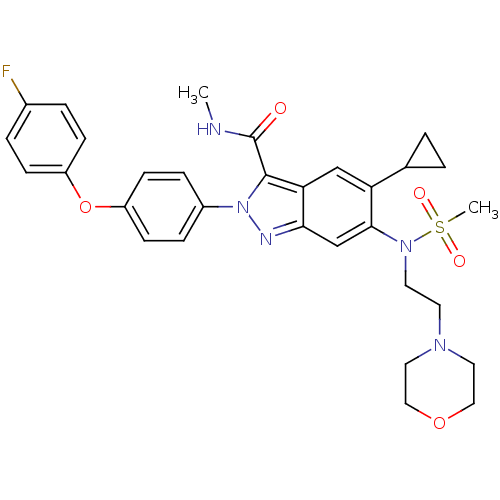 (US8546389, 112) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM103254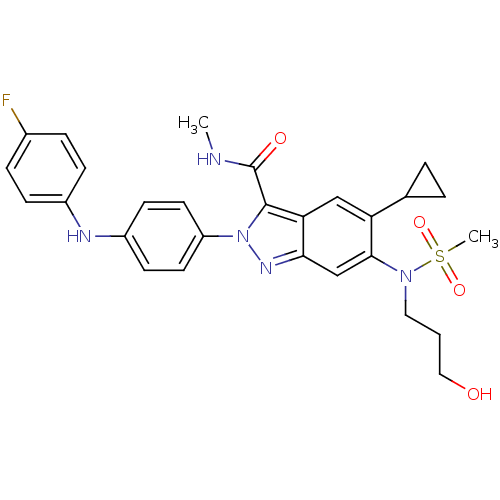 (US8546389, 21) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103283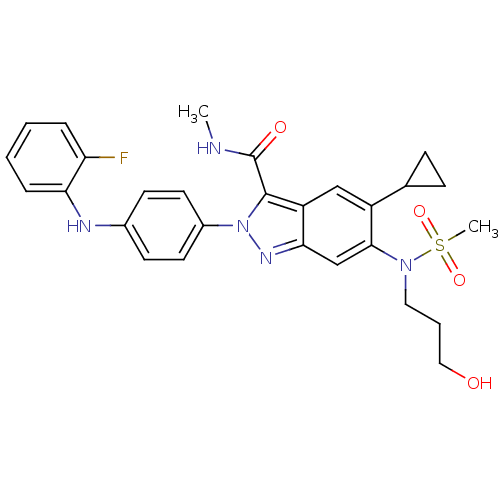 (US8546389, 5) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103277 (US8546389, 200) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103254 (US8546389, 21) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103275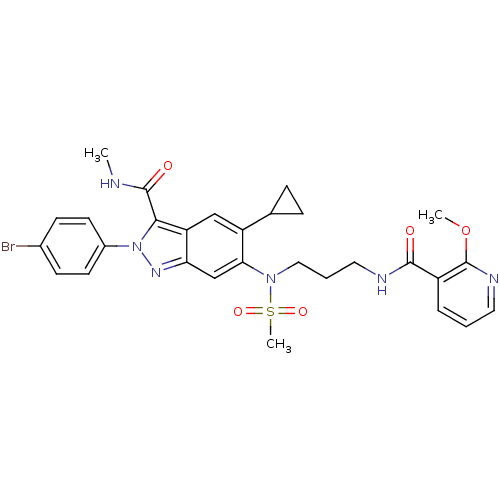 (US8546389, 190) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103285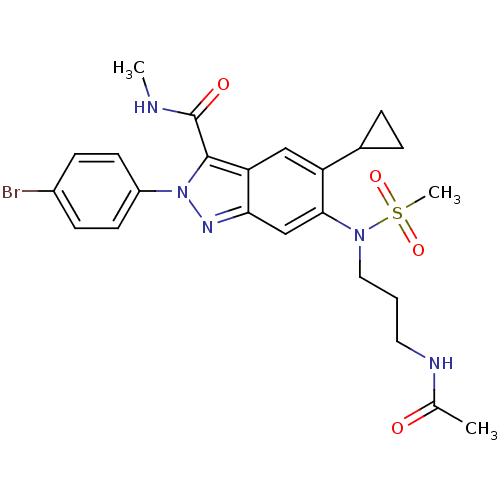 (US8546389, 59) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103273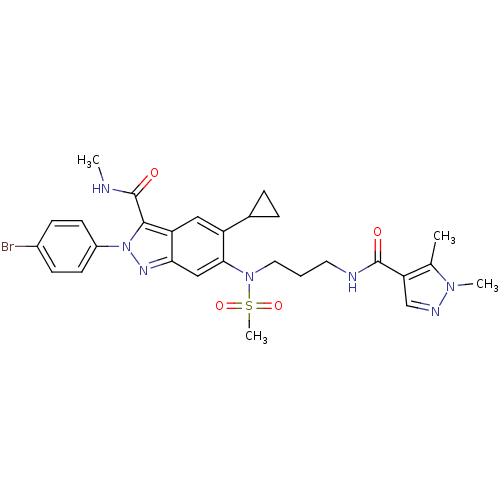 (US8546389, 180) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103274 (US8546389, 189) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103286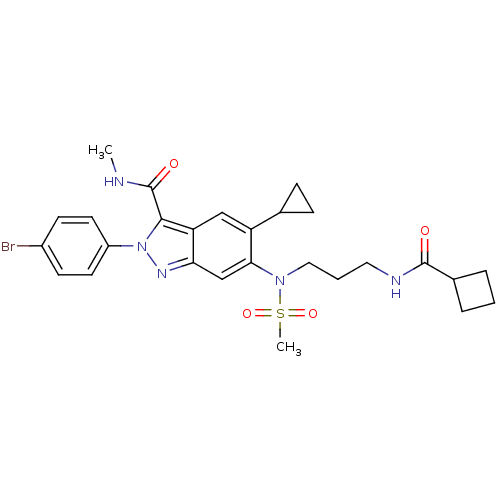 (US8546389, 62) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103271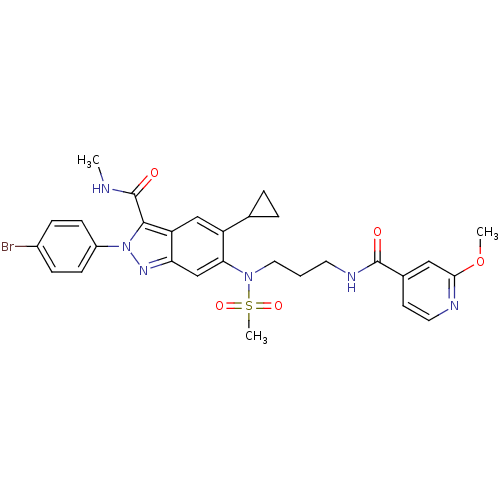 (US8546389, 177) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM103264 (US8546389, 160) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM103283 (US8546389, 5) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103259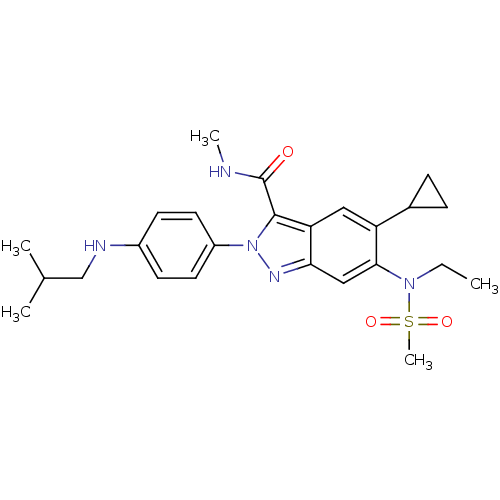 (US8546389, 117) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM103263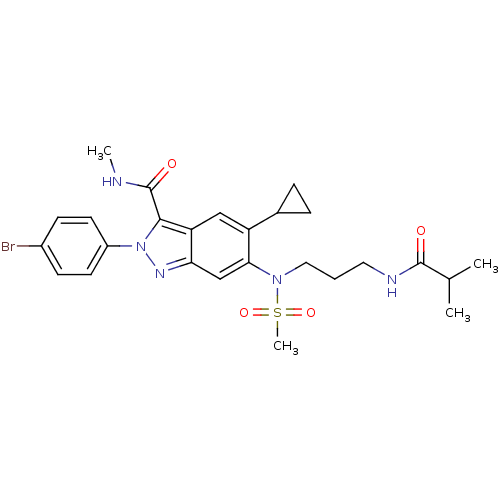 (US8546389, 156) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 1b (isolate BK) (HCV)) | BDBM103287 (US8546389, 95) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM32206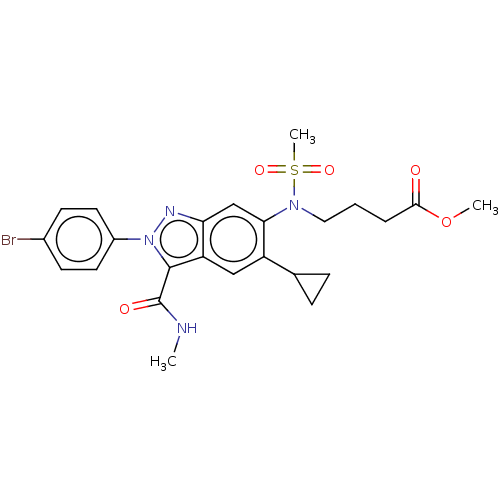 (US8546389, 66) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate NZL1) (HCV)) | BDBM32222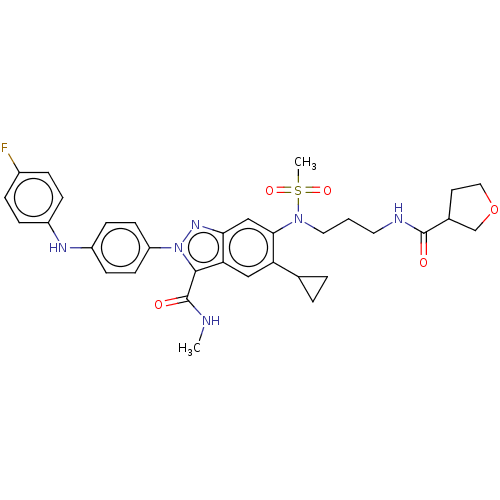 (US8546389, 120) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Biota Scientific Management Pty Ltd. US Patent | Assay Description HCV polymerase inhibition assay: HCV polymerase reactions were carried out using a modified mothod of Howe et al., Antimicrobial Agents and Chemothe... | US Patent US8546389 (2013) BindingDB Entry DOI: 10.7270/Q2DZ06XK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 151 total ) | Next | Last >> |