 Found 1481 hits with Last Name = 'mark' and Initial = 'm'
Found 1481 hits with Last Name = 'mark' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156449
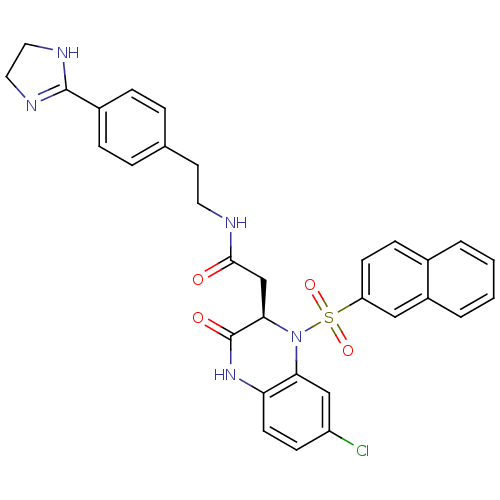
(2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...)Show SMILES Clc1ccc2NC(=O)[C@@H](CC(=O)NCCc3ccc(cc3)C3=NCCN3)N(c2c1)S(=O)(=O)c1ccc2ccccc2c1 |t:22| Show InChI InChI=1S/C31H28ClN5O4S/c32-24-10-12-26-27(18-24)37(42(40,41)25-11-9-21-3-1-2-4-23(21)17-25)28(31(39)36-26)19-29(38)33-14-13-20-5-7-22(8-6-20)30-34-15-16-35-30/h1-12,17-18,28H,13-16,19H2,(H,33,38)(H,34,35)(H,36,39)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156446
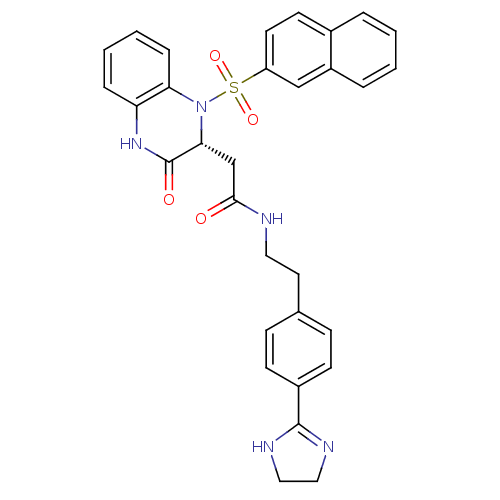
(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo rec... |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156446

(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to rhesus monkey Bradykinin receptor B1 |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156451
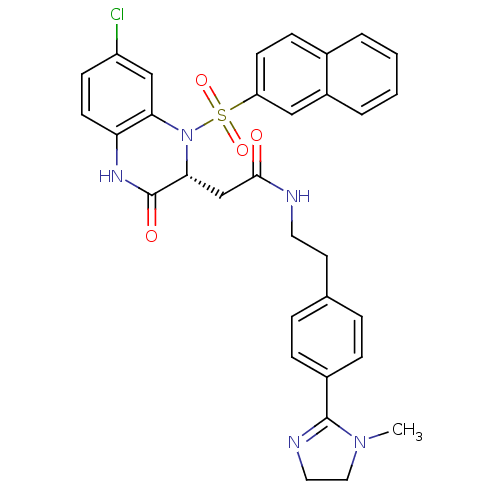
(2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...)Show SMILES CN1CCN=C1c1ccc(CCNC(=O)C[C@H]2N(c3cc(Cl)ccc3NC2=O)S(=O)(=O)c2ccc3ccccc3c2)cc1 |c:4| Show InChI InChI=1S/C32H30ClN5O4S/c1-37-17-16-35-31(37)23-8-6-21(7-9-23)14-15-34-30(39)20-29-32(40)36-27-13-11-25(33)19-28(27)38(29)43(41,42)26-12-10-22-4-2-3-5-24(22)18-26/h2-13,18-19,29H,14-17,20H2,1H3,(H,34,39)(H,36,40)/t29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156455
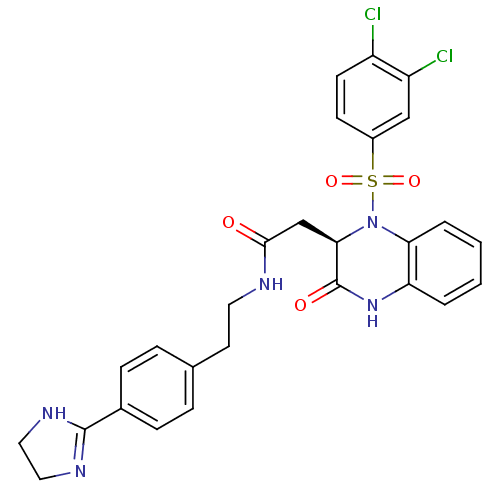
((R)-N-(4-(4,5-dihydro-1H-imidazol-2-yl)phenethyl)-...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)C2=NCCN2)C(=O)Nc2ccccc12 |r,t:27| Show InChI InChI=1S/C27H25Cl2N5O4S/c28-20-10-9-19(15-21(20)29)39(37,38)34-23-4-2-1-3-22(23)33-27(36)24(34)16-25(35)30-12-11-17-5-7-18(8-6-17)26-31-13-14-32-26/h1-10,15,24H,11-14,16H2,(H,30,35)(H,31,32)(H,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Oryctolagus cuniculus) | BDBM50156446

(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to rabbit Bradykinin receptor B1 |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156450
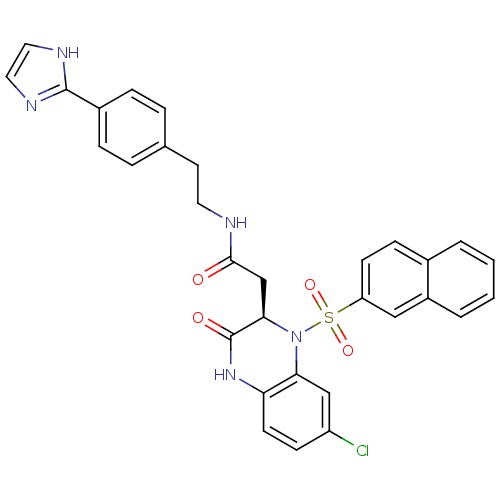
(2-[(R)-7-Chloro-1-(naphthalene-2-sulfonyl)-3-oxo-1...)Show SMILES Clc1ccc2NC(=O)[C@@H](CC(=O)NCCc3ccc(cc3)-c3ncc[nH]3)N(c2c1)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C31H26ClN5O4S/c32-24-10-12-26-27(18-24)37(42(40,41)25-11-9-21-3-1-2-4-23(21)17-25)28(31(39)36-26)19-29(38)33-14-13-20-5-7-22(8-6-20)30-34-15-16-35-30/h1-12,15-18,28H,13-14,19H2,(H,33,38)(H,34,35)(H,36,39)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156448
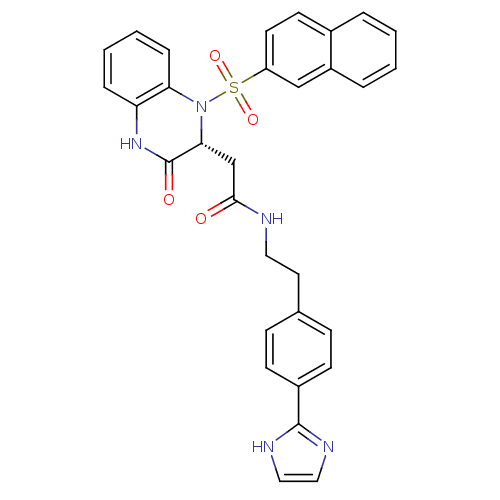
(CHEMBL185811 | N-{2-[4-(1H-Imidazol-2-yl)-phenyl]-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)-c1ncc[nH]1 Show InChI InChI=1S/C31H27N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,17-19,28H,15-16,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50020217
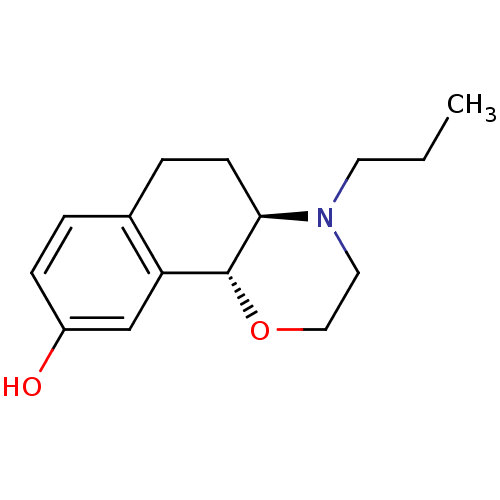
((4aR,10bR)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-na...)Show InChI InChI=1S/C15H21NO2/c1-2-7-16-8-9-18-15-13-10-12(17)5-3-11(13)4-6-14(15)16/h3,5,10,14-15,17H,2,4,6-9H2,1H3/t14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM85827
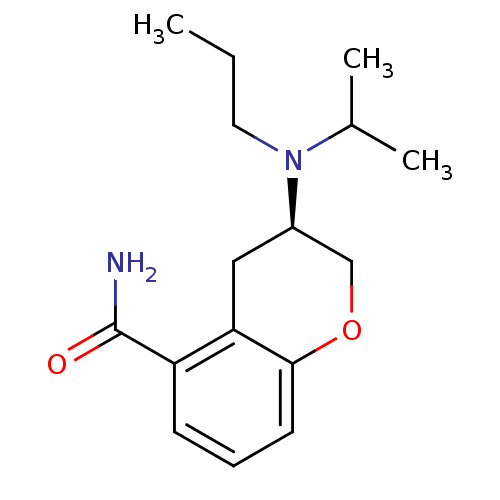
((R)-3-[Isopropyl(propyl)amino]-3,4-dihydro-2H-1-be...)Show InChI InChI=1S/C16H24N2O2/c1-4-8-18(11(2)3)12-9-14-13(16(17)19)6-5-7-15(14)20-10-12/h5-7,11-12H,4,8-10H2,1-3H3,(H2,17,19)/t12-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 299: 883-93 (2001)
BindingDB Entry DOI: 10.7270/Q2PK0DQ2 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210360
(CHEMBL234096 | N-(2-((4-cyano-4-(2-(trifluoromethy...)Show SMILES FC(F)(F)CC(=O)Nc1cccnc1NCC1CCC(CC1)(C#N)c1ccccc1C(F)(F)F |(-8.05,-29.89,;-6.71,-30.66,;-5.95,-29.33,;-7.48,-31.99,;-5.38,-31.43,;-5.38,-32.96,;-6.71,-33.73,;-4.06,-33.75,;-4.05,-35.29,;-5.39,-36.06,;-5.39,-37.6,;-4.05,-38.36,;-2.73,-37.6,;-2.73,-36.06,;-1.41,-35.27,;-.08,-34.5,;1.27,-35.25,;1.28,-36.8,;2.61,-37.57,;3.96,-36.77,;3.94,-35.22,;2.59,-34.47,;5.36,-36.1,;6.75,-35.44,;4.51,-38.19,;6.05,-38.2,;6.81,-39.54,;6.03,-40.87,;4.48,-40.86,;3.73,-39.52,;2.19,-39.5,;.65,-39.51,;2.13,-37.97,;2.26,-41.04,)| Show InChI InChI=1S/C23H22F6N4O/c24-22(25,26)12-19(34)33-18-6-3-11-31-20(18)32-13-15-7-9-21(14-30,10-8-15)16-4-1-2-5-17(16)23(27,28)29/h1-6,11,15H,7-10,12-13H2,(H,31,32)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Canis familiaris) | BDBM50156446

(CHEMBL359553 | N-{2-[4-(4,5-Dihydro-1H-imidazol-2-...)Show SMILES O=C(C[C@H]1N(c2ccccc2NC1=O)S(=O)(=O)c1ccc2ccccc2c1)NCCc1ccc(cc1)C1=NCCN1 |t:41| Show InChI InChI=1S/C31H29N5O4S/c37-29(32-16-15-21-9-11-23(12-10-21)30-33-17-18-34-30)20-28-31(38)35-26-7-3-4-8-27(26)36(28)41(39,40)25-14-13-22-5-1-2-6-24(22)19-25/h1-14,19,28H,15-18,20H2,(H,32,37)(H,33,34)(H,35,38)/t28-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the [35S]- radiolabelled compound to dog Bradykinin receptor B1 |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210361

(CHEMBL234097 | N-(2-((4-cyano-4-(2-(trifluoromethy...)Show SMILES FC(F)(F)c1ccccc1C1(CCC(CNc2ncccc2NC(=O)c2ccno2)CC1)C#N |(22.56,-44.3,;24.1,-44.29,;24.04,-42.76,;24.17,-45.83,;25.64,-44.31,;26.4,-45.64,;27.94,-45.66,;28.72,-44.32,;27.96,-42.99,;26.42,-42.98,;25.87,-41.55,;24.52,-42.35,;23.19,-41.58,;23.18,-40.04,;21.83,-39.28,;20.5,-40.06,;19.18,-40.84,;19.18,-42.39,;17.86,-43.15,;16.52,-42.38,;16.52,-40.84,;17.86,-40.07,;17.85,-38.53,;16.52,-37.75,;15.19,-38.51,;16.52,-36.21,;17.76,-35.31,;17.29,-33.85,;15.75,-33.85,;15.28,-35.31,;24.5,-39.26,;25.85,-40.01,;27.27,-40.88,;28.66,-40.23,)| Show InChI InChI=1S/C24H22F3N5O2/c25-24(26,27)18-5-2-1-4-17(18)23(15-28)10-7-16(8-11-23)14-30-21-19(6-3-12-29-21)32-22(33)20-9-13-31-34-20/h1-6,9,12-13,16H,7-8,10-11,14H2,(H,29,30)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026044
(3-Bromo-5-ethyl-N-(1-ethyl-pyrrolidin-2-ylmethyl)-...)Show InChI InChI=1S/C17H25BrN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50002338
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156452

(2-[(R)-1-(3,4-Dichloro-benzenesulfonyl)-3-oxo-1,2,...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)-n2ccnc2)C(=O)Nc2ccccc12 Show InChI InChI=1S/C27H23Cl2N5O4S/c28-21-10-9-20(15-22(21)29)39(37,38)34-24-4-2-1-3-23(24)32-27(36)25(34)16-26(35)31-12-11-18-5-7-19(8-6-18)33-14-13-30-17-33/h1-10,13-15,17,25H,11-12,16H2,(H,31,35)(H,32,36)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50090828

((-)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9-...)Show InChI InChI=1S/C14H19NO2S/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for agonist binding affinity by measuring displacement of [3H]NPA from Human Dopamine receptor D2L expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50010586
((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...)Show InChI InChI=1S/C14H19NO3/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50010586

((+)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4,9-diox...)Show InChI InChI=1S/C14H19NO3/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026051
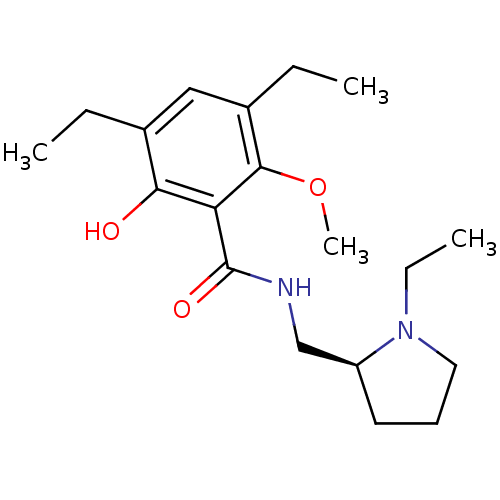
(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-h...)Show InChI InChI=1S/C19H30N2O3/c1-5-13-11-14(6-2)18(24-4)16(17(13)22)19(23)20-12-15-9-8-10-21(15)7-3/h11,15,22H,5-10,12H2,1-4H3,(H,20,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50090829
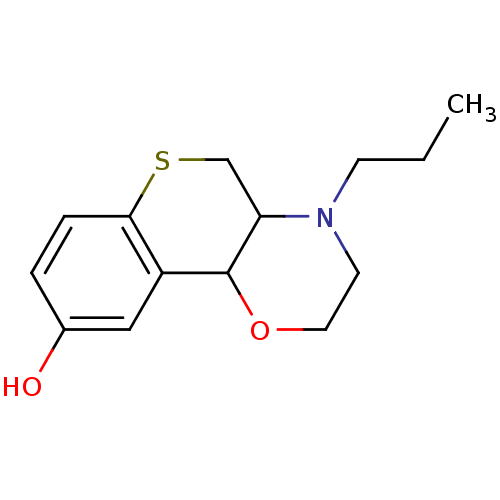
((trans)1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa...)Show InChI InChI=1S/C14H19NO2S/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026059

(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-h...)Show InChI InChI=1S/C17H26N2O3/c1-4-12-8-9-14(22-3)15(16(12)20)17(21)18-11-13-7-6-10-19(13)5-2/h8-9,13,20H,4-7,10-11H2,1-3H3,(H,18,21)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026051

(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-h...)Show InChI InChI=1S/C19H30N2O3/c1-5-13-11-14(6-2)18(24-4)16(17(13)22)19(23)20-12-15-9-8-10-21(15)7-3/h11,15,22H,5-10,12H2,1-4H3,(H,20,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210370

(CHEMBL388031 | N-(2-((4-cyano-4-(2,3-difluoropheny...)Show SMILES Fc1cccc(c1F)C1(CCC(CNc2ncccc2NC(=O)CC(F)(F)F)CC1)C#N |(1.61,-8.98,;2.34,-7.63,;3.88,-7.58,;4.61,-6.23,;3.79,-4.92,;2.26,-4.96,;1.52,-6.32,;-.02,-6.37,;1.45,-3.66,;.09,-4.47,;-1.23,-3.7,;-1.25,-2.15,;-2.6,-1.39,;-3.92,-2.17,;-5.25,-2.95,;-5.25,-4.5,;-6.58,-5.27,;-7.92,-4.5,;-7.92,-2.95,;-6.58,-2.18,;-6.59,-.64,;-7.92,.14,;-9.25,-.63,;-7.92,1.68,;-9.25,2.44,;-10.59,3.21,;-8.49,3.78,;-10.02,1.11,;.08,-1.37,;1.42,-2.12,;2.72,-2.79,;3.98,-1.92,)| Show InChI InChI=1S/C22H21F5N4O/c23-16-4-1-3-15(19(16)24)21(13-28)8-6-14(7-9-21)12-30-20-17(5-2-10-29-20)31-18(32)11-22(25,26)27/h1-5,10,14H,6-9,11-12H2,(H,29,30)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026044

(3-Bromo-5-ethyl-N-(1-ethyl-pyrrolidin-2-ylmethyl)-...)Show InChI InChI=1S/C17H25BrN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50090828

((-)-1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9-...)Show InChI InChI=1S/C14H19NO2S/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026059

(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-h...)Show InChI InChI=1S/C17H26N2O3/c1-4-12-8-9-14(22-3)15(16(12)20)17(21)18-11-13-7-6-10-19(13)5-2/h8-9,13,20H,4-7,10-11H2,1-3H3,(H,18,21)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(MOUSE) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 377-86 (1998)
BindingDB Entry DOI: 10.7270/Q20000MP |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50026059

(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2-h...)Show InChI InChI=1S/C17H26N2O3/c1-4-12-8-9-14(22-3)15(16(12)20)17(21)18-11-13-7-6-10-19(13)5-2/h8-9,13,20H,4-7,10-11H2,1-3H3,(H,18,21)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Läkemedel AB
Curated by PDSP Ki Database
| |
Acta Pharmacol Toxicol (Copenh) 58: 61-70 (1986)
Article DOI: 10.1111/j.1600-0773.1986.tb00071.x
BindingDB Entry DOI: 10.7270/Q2PG1Q7V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50090832

((cis)1-Propyl-2,3,10,10a-tetrahydro-1H,4aH-4-oxa-9...)Show InChI InChI=1S/C14H19NO2S/c1-2-5-15-6-7-17-14-11-8-10(16)3-4-13(11)18-9-12(14)15/h3-4,8,12,14,16H,2,5-7,9H2,1H3/t12-,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy
Curated by ChEMBL
| Assay Description
Tested for antagonist binding affinity by measuring displacement of [3H]spiperone from Human Dopamine receptor D3 expressed in CHO K-1 cells |
J Med Chem 43: 2871-82 (2000)
BindingDB Entry DOI: 10.7270/Q2X067R1 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210364
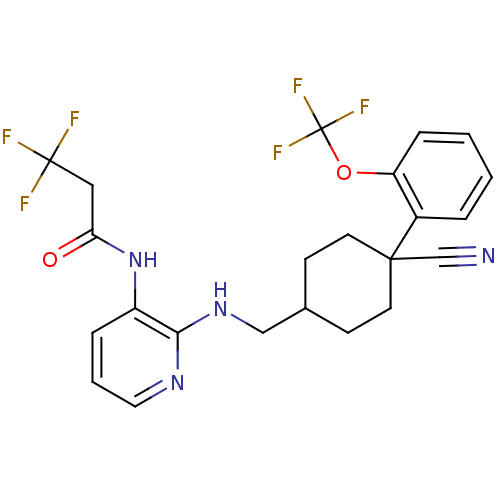
(CHEMBL233887 | N-(2-((4-cyano-4-(2-(trifluorometho...)Show SMILES FC(F)(F)CC(=O)Nc1cccnc1NCC1CCC(CC1)(C#N)c1ccccc1OC(F)(F)F |(20.88,-14.74,;22.21,-15.51,;22.98,-14.17,;21.45,-16.84,;23.55,-16.28,;23.55,-17.82,;22.21,-18.59,;24.87,-18.6,;24.89,-20.14,;23.55,-20.91,;23.55,-22.46,;24.89,-23.23,;26.22,-22.46,;26.22,-20.91,;27.54,-20.13,;28.87,-19.35,;30.22,-20.11,;30.24,-21.66,;31.56,-22.43,;32.92,-21.62,;32.9,-20.08,;31.55,-19.33,;34.21,-20.78,;35.49,-19.92,;33.7,-22.94,;35.23,-22.95,;36.01,-24.27,;35.24,-25.61,;33.7,-25.61,;32.92,-24.28,;31.38,-24.28,;30.69,-25.67,;29.91,-26.99,;32.05,-26.4,;29.34,-24.94,)| Show InChI InChI=1S/C23H22F6N4O2/c24-22(25,26)12-19(34)33-17-5-3-11-31-20(17)32-13-15-7-9-21(14-30,10-8-15)16-4-1-2-6-18(16)35-23(27,28)29/h1-6,11,15H,7-10,12-13H2,(H,31,32)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210363
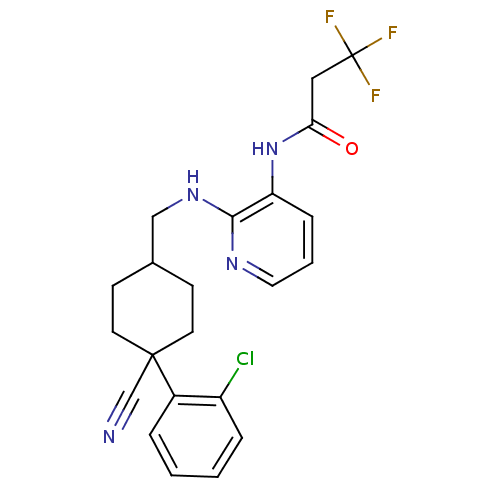
(CHEMBL233886 | N-(2-((4-(2-chlorophenyl)-4-cyanocy...)Show SMILES FC(F)(F)CC(=O)Nc1cccnc1NCC1CCC(CC1)(C#N)c1ccccc1Cl |(4.04,-13.65,;5.38,-14.42,;6.15,-13.09,;4.62,-15.75,;6.71,-15.19,;6.71,-16.73,;5.38,-17.49,;8.04,-17.51,;8.05,-19.05,;6.71,-19.81,;6.71,-21.36,;8.05,-22.13,;9.38,-21.36,;9.38,-19.81,;10.71,-19.03,;12.04,-18.25,;13.38,-19.01,;13.4,-20.56,;14.72,-21.33,;16.08,-20.52,;16.06,-18.98,;14.71,-18.23,;17.34,-19.63,;18.59,-18.74,;16.15,-22.05,;17.5,-22.77,;17.57,-24.31,;16.27,-25.12,;14.91,-24.41,;14.84,-22.87,;13.47,-22.16,)| Show InChI InChI=1S/C22H22ClF3N4O/c23-17-5-2-1-4-16(17)21(14-27)9-7-15(8-10-21)13-29-20-18(6-3-11-28-20)30-19(31)12-22(24,25)26/h1-6,11,15H,7-10,12-13H2,(H,28,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50210371
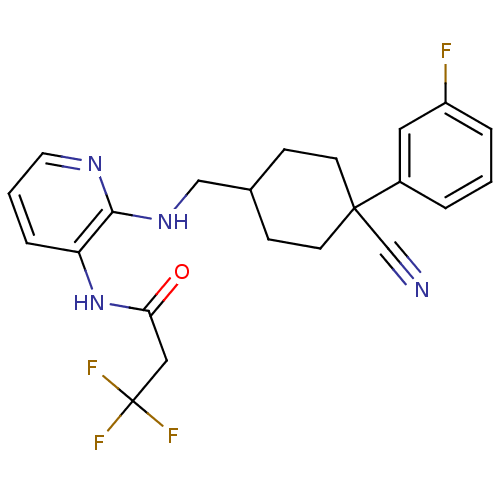
(CHEMBL388030 | N-(2-((4-cyano-4-(3-fluorophenyl)cy...)Show SMILES Fc1cccc(c1)C1(CCC(CNc2ncccc2NC(=O)CC(F)(F)F)CC1)C#N |(20.86,-36.92,;21.64,-35.6,;23.18,-35.6,;23.96,-34.29,;23.2,-32.94,;21.66,-32.93,;20.87,-34.25,;20.9,-31.59,;19.54,-32.41,;18.22,-31.64,;18.21,-30.09,;16.86,-29.33,;15.53,-30.11,;14.2,-30.89,;14.2,-32.44,;12.87,-33.21,;11.53,-32.44,;11.53,-30.89,;12.88,-30.12,;12.87,-28.58,;11.54,-27.8,;10.21,-28.57,;11.54,-26.26,;10.21,-25.49,;8.87,-24.72,;10.98,-24.16,;9.44,-26.83,;19.53,-29.3,;20.88,-30.06,;22.27,-30.89,;23.63,-30.18,)| Show InChI InChI=1S/C22H22F4N4O/c23-17-4-1-3-16(11-17)21(14-27)8-6-15(7-9-21)13-29-20-18(5-2-10-28-20)30-19(31)12-22(24,25)26/h1-5,10-11,15H,6-9,12-13H2,(H,28,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity at human bradykinin B1 receptor |
Bioorg Med Chem Lett 17: 3006-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.059
BindingDB Entry DOI: 10.7270/Q29C6X4F |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50156453
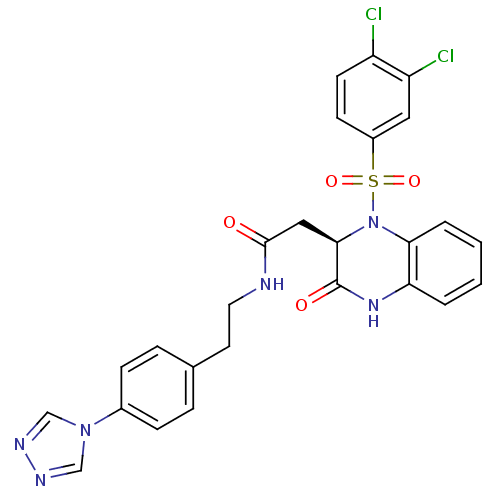
(2-[(R)-1-(3,4-Dichloro-benzenesulfonyl)-3-oxo-1,2,...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1[C@H](CC(=O)NCCc2ccc(cc2)-n2cnnc2)C(=O)Nc2ccccc12 Show InChI InChI=1S/C26H22Cl2N6O4S/c27-20-10-9-19(13-21(20)28)39(37,38)34-23-4-2-1-3-22(23)32-26(36)24(34)14-25(35)29-12-11-17-5-7-18(8-6-17)33-15-30-31-16-33/h1-10,13,15-16,24H,11-12,14H2,(H,29,35)(H,32,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay |
Bioorg Med Chem Lett 14: 6045-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.074
BindingDB Entry DOI: 10.7270/Q2V1248Q |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50107746

(CHEMBL3600828)Show SMILES Clc1ccc(cc1)-c1ccc(nc1)C#Cc1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C25H23ClN2O/c26-23-9-6-21(7-10-23)22-8-12-24(27-19-22)11-3-20-4-13-25(14-5-20)29-18-17-28-15-1-2-16-28/h4-10,12-14,19H,1-2,15-18H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Antagonist activity against rat MCHR1 |
Bioorg Med Chem Lett 25: 3264-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.077
BindingDB Entry DOI: 10.7270/Q25D8TMB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data