

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805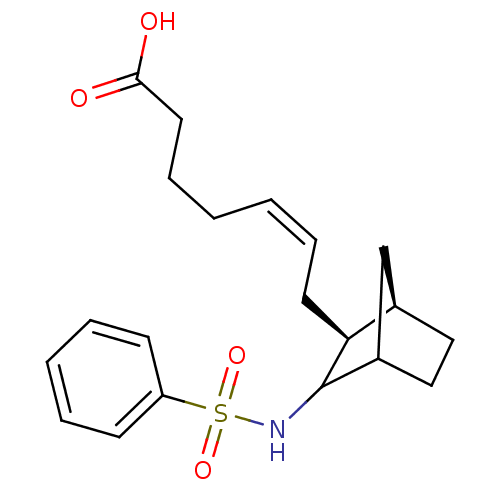 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462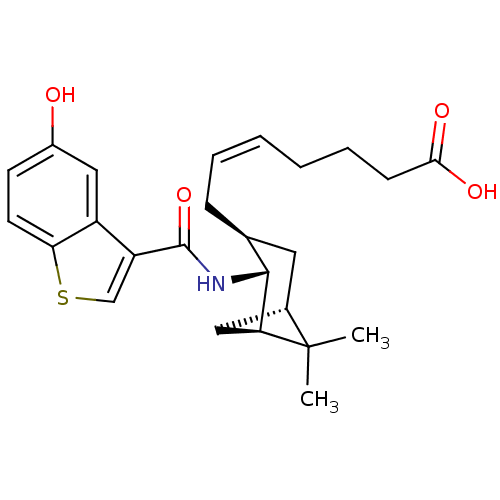 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (GUINEA PIG) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM85347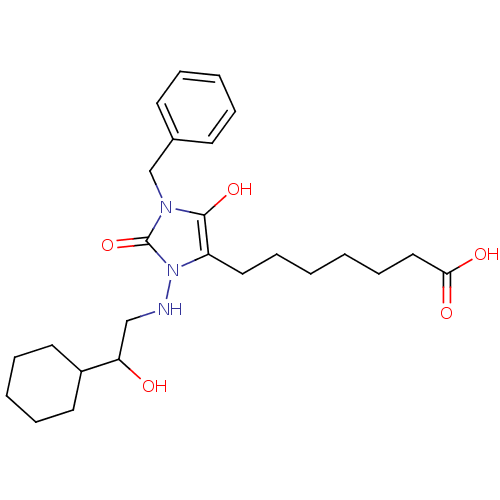 (BWA868C | CAS_122021 | NSC_122021) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin-H2 D-isomerase (Homo sapiens (Human)) | BDBM50008805 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co. Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 411-9 (2001) BindingDB Entry DOI: 10.7270/Q2028Q3C | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50060458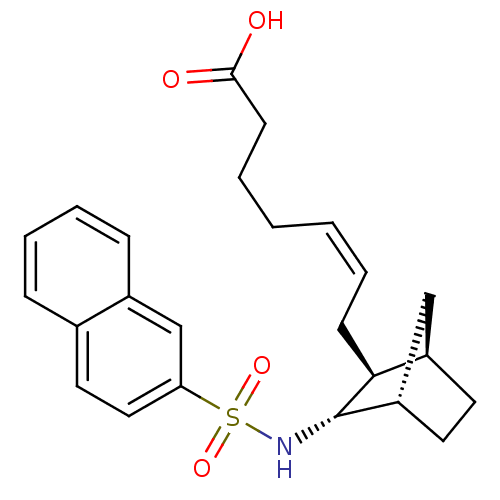 ((Z)-7-[(1R,2S,3S,4S)-3-(Naphthalene-2-sulfonylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]- (+)-S-145 specific binding to human platelet membranes in TXA2 receptor (TP) assay | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060454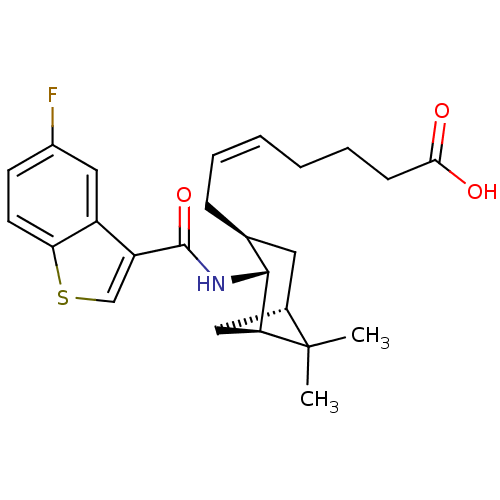 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Fluoro-benzo[b]thiophen...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128750 (7-{2-[(5-Fluoro-benzo[b]thiophene-3-carbonyl)-amin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077162 ((R)-3-(1H-Indol-3-yl)-2-[4-(4-methoxy-phenylethyny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128768 (7-{2-[(5-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060462 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063148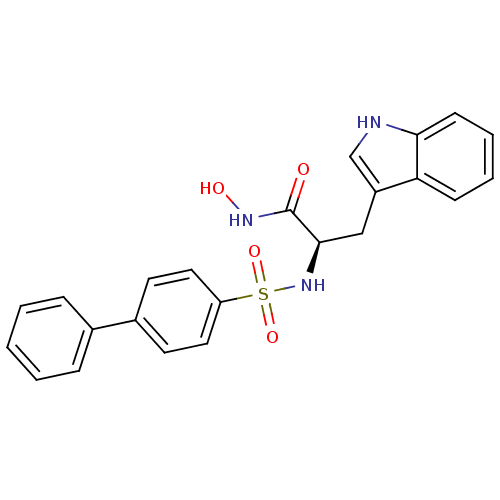 ((R)-2-(Biphenyl-4-sulfonylamino)-N-hydroxy-3-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053109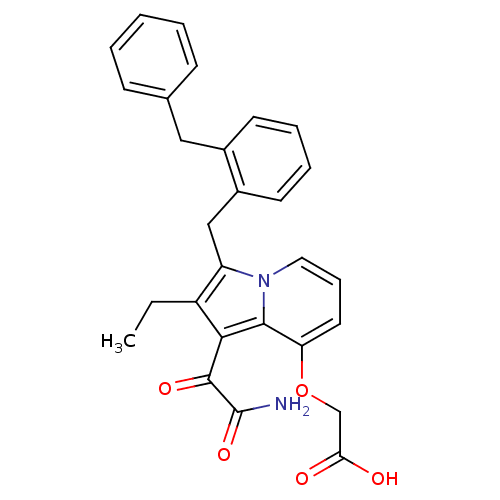 (CHEMBL331755 | [1-Aminooxalyl-3-(2-benzyl-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060453 ((Z)-7-{(1R,2R,3S,5S)-2-[(6-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGD-2 specific binding to Prostaglandin D2 receptor fromhuman platelet membranes | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053136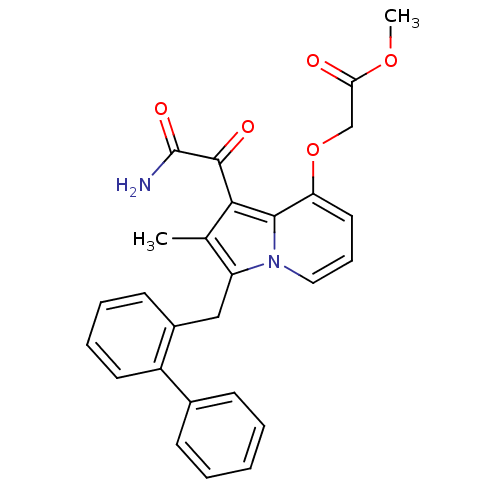 ((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-methyl-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077157 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063129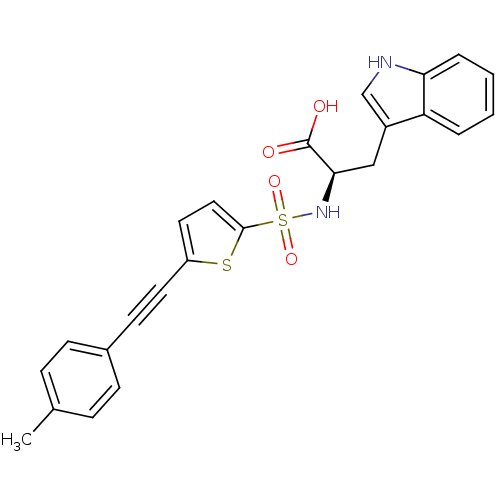 ((R)-3-(1H-Indol-3-yl)-2-(5-p-tolylethynyl-thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060454 ((Z)-7-{(1R,2R,3S,5S)-2-[(5-Fluoro-benzo[b]thiophen...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50085993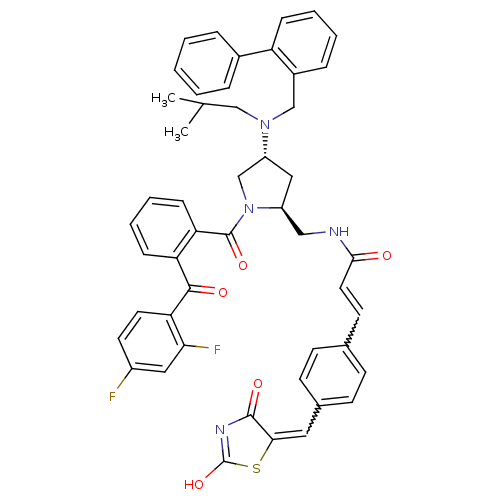 ((E)-N-(((2S,4R)-4-((biphenyl-2-ylmethyl)(isobutyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) | J Med Chem 43: 1041-4 (2000) BindingDB Entry DOI: 10.7270/Q27P8XMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128768 (7-{2-[(5-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM8485 ((2R)-N-hydroxy-3-methyl-2-[(4-phenoxybenzene)sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50085984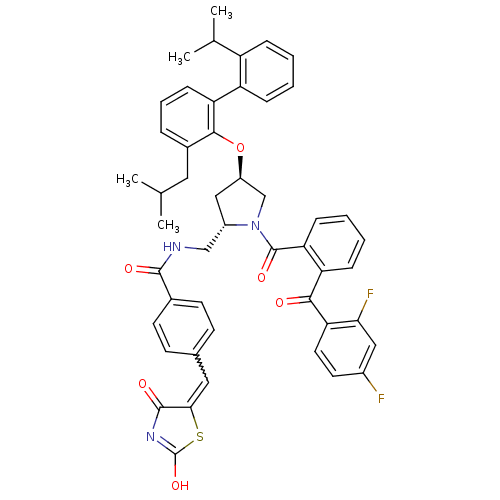 (CHEMBL9161 | N-[1-[2-(2,4-Difluoro-benzoyl)-benzoy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) | J Med Chem 43: 1041-4 (2000) BindingDB Entry DOI: 10.7270/Q27P8XMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128767 (7-{2-[(6-Hydroxy-benzo[b]thiophene-3-carbonyl)-ami...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Prostaglandin D2 receptor antagonist activity, evaluated by inhibition of [3H]-PGD-2 binding to human platelet membranes | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50020743 (CHEMBL53346 | Sodium; (+)-7-(3-benzenesulfonylamin...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of thromboxane A2 synthetase from human platelets by 1 uM of the compound | J Med Chem 31: 1847-54 (1988) BindingDB Entry DOI: 10.7270/Q2G44P95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50063139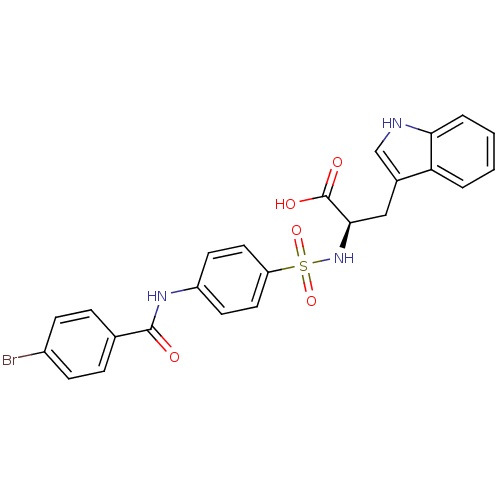 ((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase A (matrix metalloproteinase-2 MMP2) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063139 ((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50060453 ((Z)-7-{(1R,2R,3S,5S)-2-[(6-Hydroxy-benzo[b]thiophe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of cAMP formation evoked by the prostaglandin D2 receptor in human platelets | J Med Chem 40: 3504-7 (1997) Article DOI: 10.1021/jm970343g BindingDB Entry DOI: 10.7270/Q2959GP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053106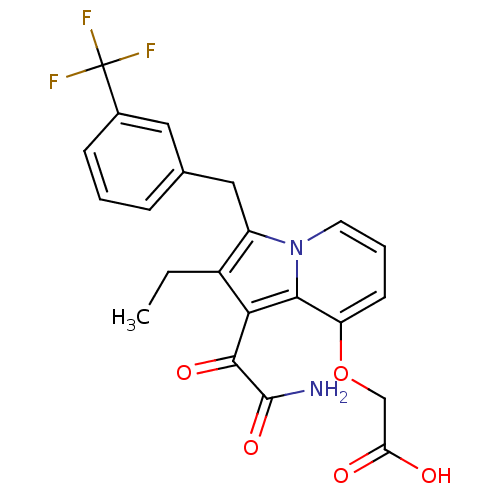 (CHEMBL120112 | [1-Aminooxalyl-2-ethyl-3-(3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053108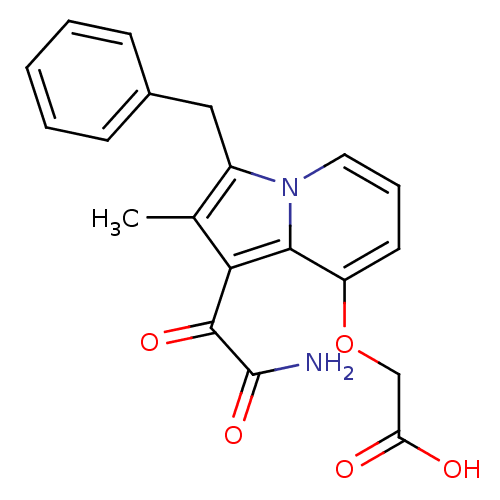 ((1-Aminooxalyl-3-benzyl-2-methyl-indolizin-8-yloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053137 ((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053102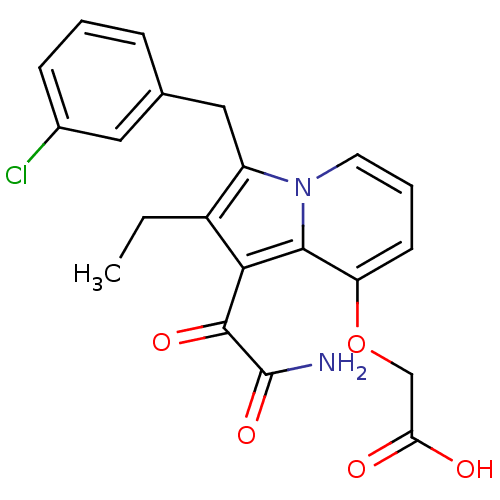 (CHEMBL121245 | [1-Aminooxalyl-3-(3-chloro-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50085986 (CHEMBL267258 | N-{4-(Biphenyl-2-ylmethyl-isobutyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) | J Med Chem 43: 1041-4 (2000) BindingDB Entry DOI: 10.7270/Q27P8XMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50128750 (7-{2-[(5-Fluoro-benzo[b]thiophene-3-carbonyl)-amin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Concentration required to inhibit the PGD-2 evoked cAMP formation in human platelets | J Med Chem 46: 2446-55 (2003) Article DOI: 10.1021/jm0205189 BindingDB Entry DOI: 10.7270/Q2WD3ZZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077161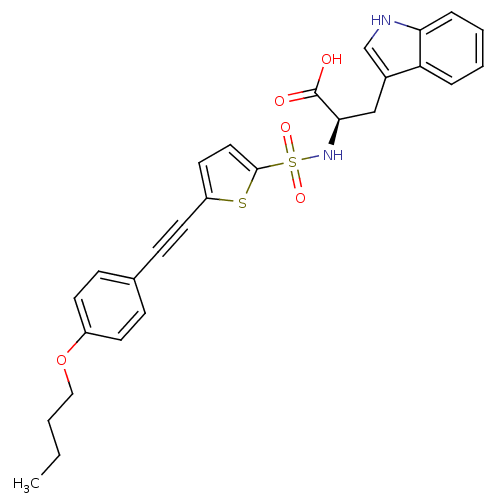 ((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063154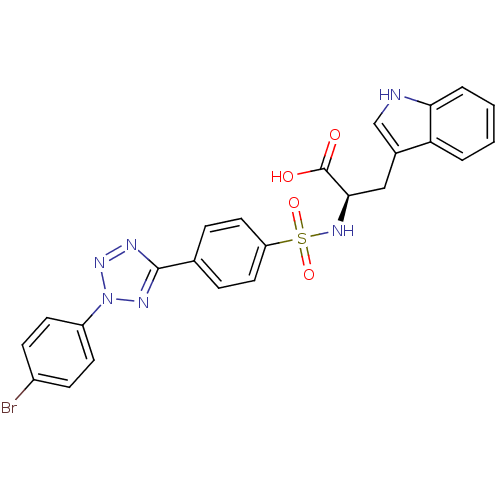 ((R)-2-{4-[2-(4-Bromo-phenyl)-2H-tetrazol-5-yl]-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053114 ((1-Aminooxalyl-3-biphenyl-3-ylmethyl-2-ethyl-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077152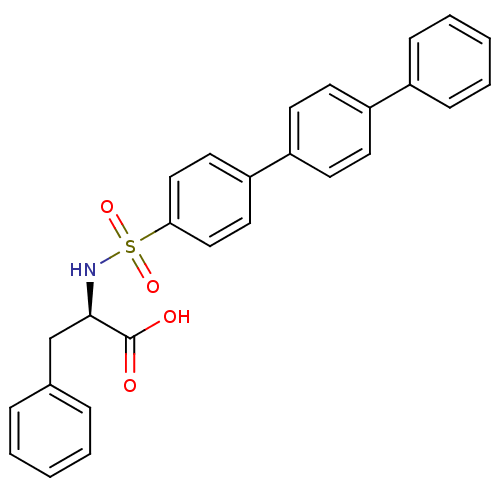 ((R)-3-Phenyl-2-([1,1';4',1'']terphenyl-4-sulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077155 ((R)-2-[5-(4-Butyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50077151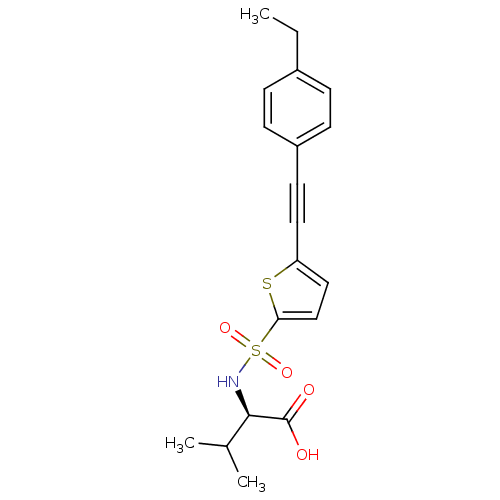 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-9 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077163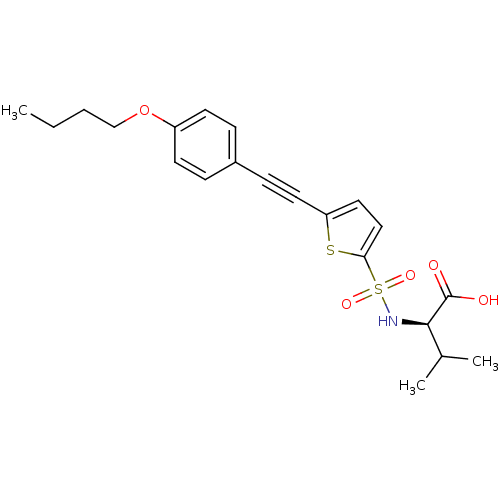 ((R)-2-[5-(4-Butoxy-phenylethynyl)-thiophene-2-sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50077151 ((R)-2-[5-(4-Ethyl-phenylethynyl)-thiophene-2-sulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against Matrix metalloproteinase-2 (concentration required for 50% inhibition of enzyme activity) | J Med Chem 42: 1723-38 (1999) Article DOI: 10.1021/jm980514x BindingDB Entry DOI: 10.7270/Q2KP81BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053133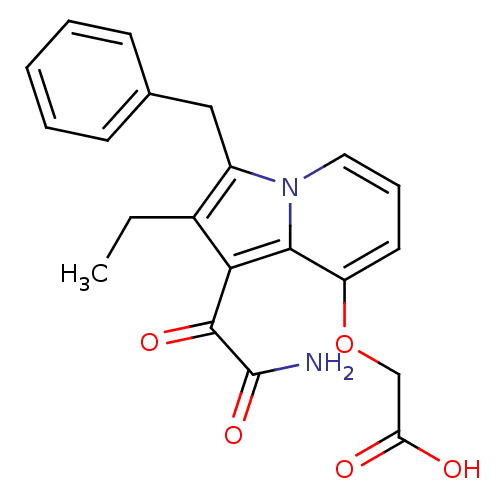 ((1-Aminooxalyl-3-benzyl-2-ethyl-indolizin-8-yloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053123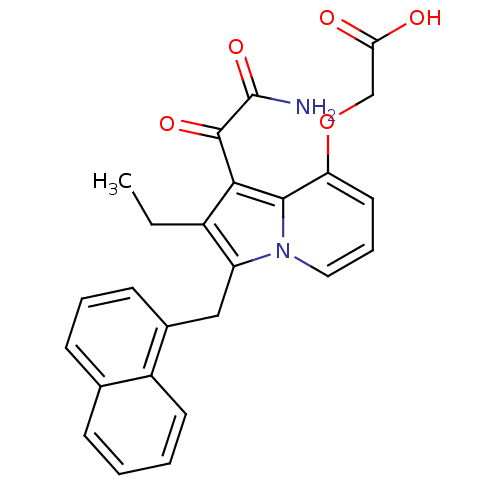 ((1-Aminooxalyl-2-ethyl-3-naphthalen-1-ylmethyl-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human secretory phospholipase A2 (sPLA2), chromogenic screening assay. | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50063148 ((R)-2-(Biphenyl-4-sulfonylamino)-N-hydroxy-3-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human gelatinase B (Matrix metalloproteinase-9) | J Med Chem 41: 640-9 (1998) Article DOI: 10.1021/jm9707582 BindingDB Entry DOI: 10.7270/Q2HD7TS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Homo sapiens (Human)) | BDBM50053096 (CHEMBL123774 | [1-Aminooxalyl-3-(4-butyl-benzyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). | J Med Chem 39: 3636-58 (1996) Article DOI: 10.1021/jm960395q BindingDB Entry DOI: 10.7270/Q2NC609M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic phospholipase A2 (Homo sapiens (Human)) | BDBM50085987 (4-(2,4-Dioxo-thiazolidin-5-ylidenemethyl)-N-[1-[2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) | J Med Chem 43: 1041-4 (2000) BindingDB Entry DOI: 10.7270/Q27P8XMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 661 total ) | Next | Last >> |