

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496270 (CHEMBL3127679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496289 (CHEMBL3124968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496273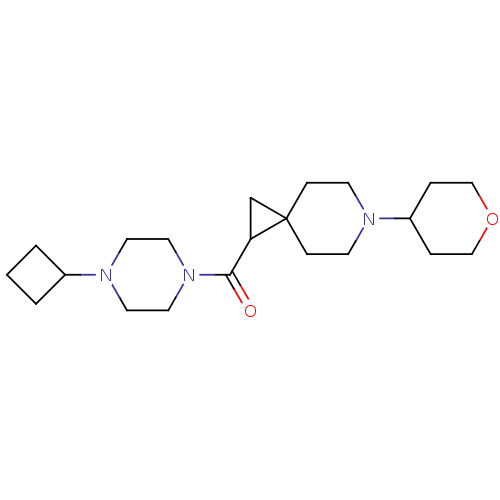 (CHEMBL3127700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496272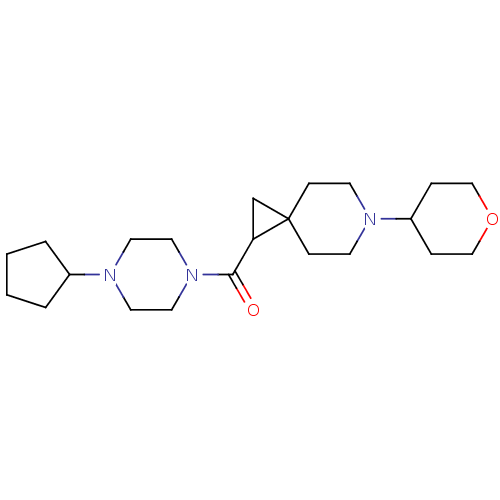 (CHEMBL3127701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496296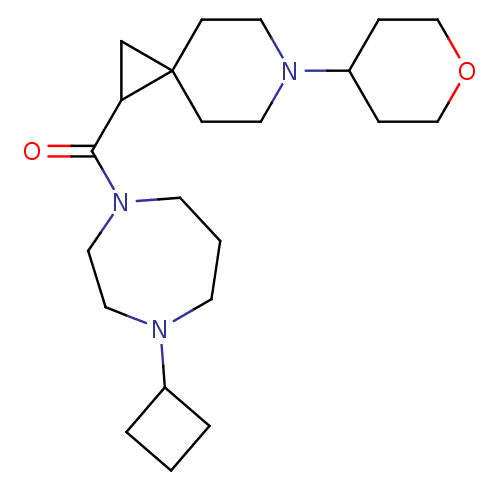 (CHEMBL3127704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496281 (CHEMBL3127698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50032651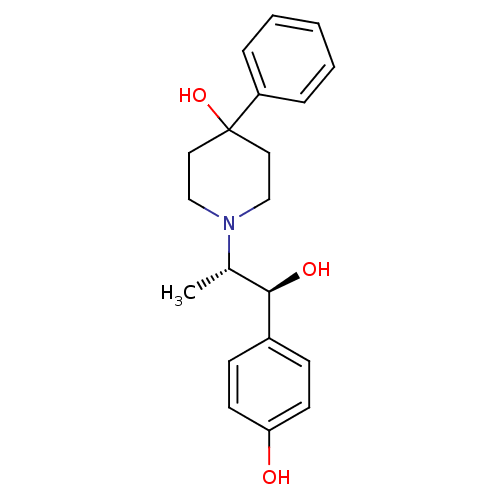 (1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496290 (CHEMBL3127705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496297 (CHEMBL3127699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344250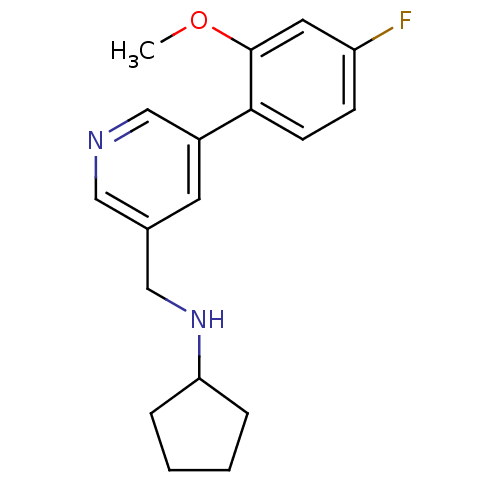 (CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496259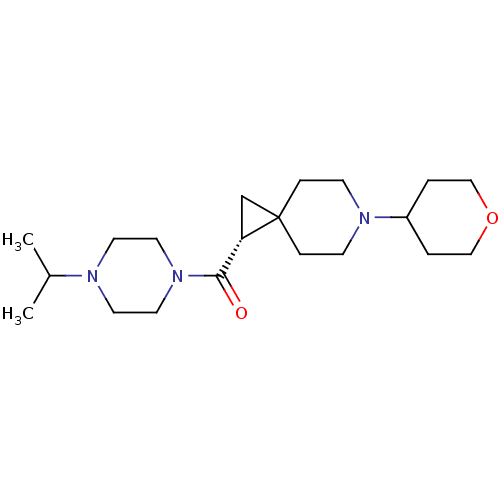 (CHEMBL3127672) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496295 (CHEMBL3127708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496276 (CHEMBL3127670) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496280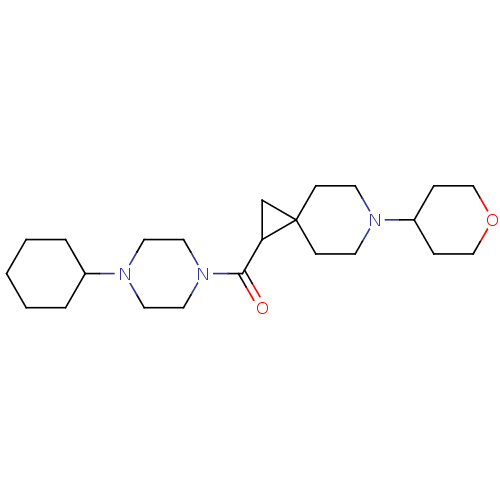 (CHEMBL3127702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344232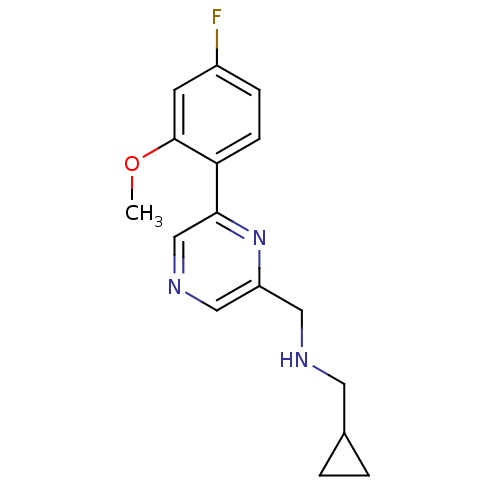 (1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496263 (CHEMBL3127709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344251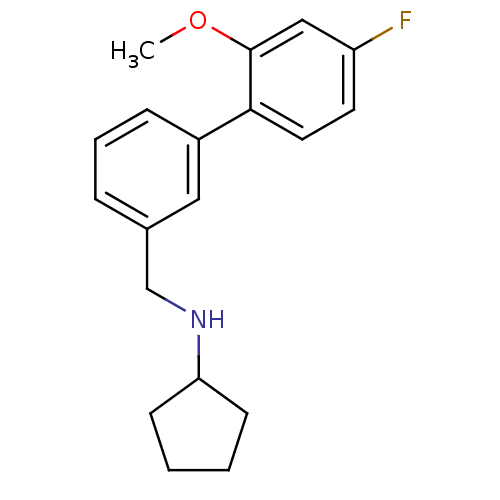 (CHEMBL1779004 | N-((4'-fluoro-2'-methoxybiphenyl-3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344233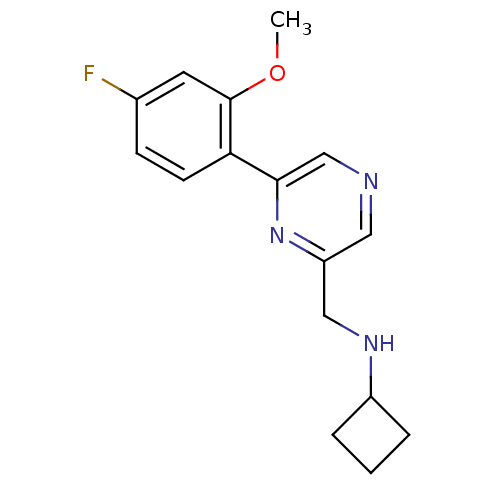 (CHEMBL1778866 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344234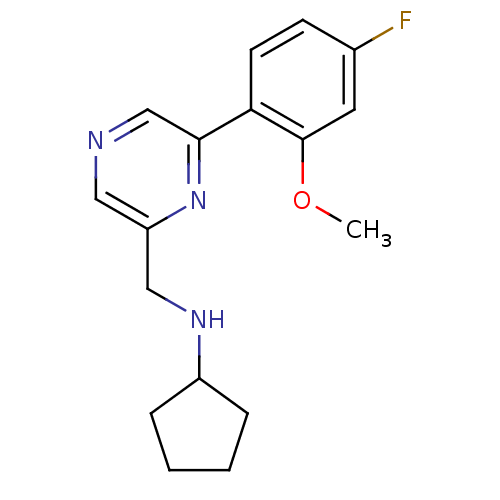 (CHEMBL1778867 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496275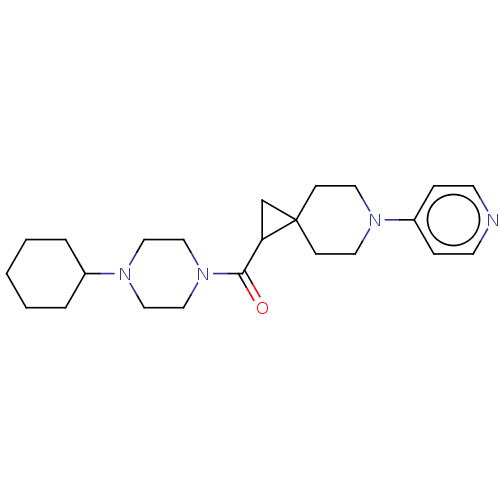 (CHEMBL3127671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496279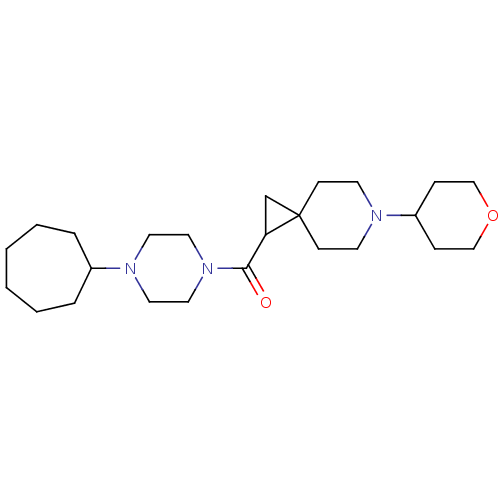 (CHEMBL3127703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344252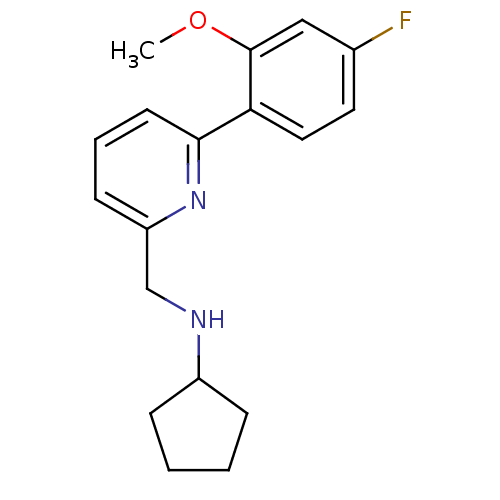 (CHEMBL1779005 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496278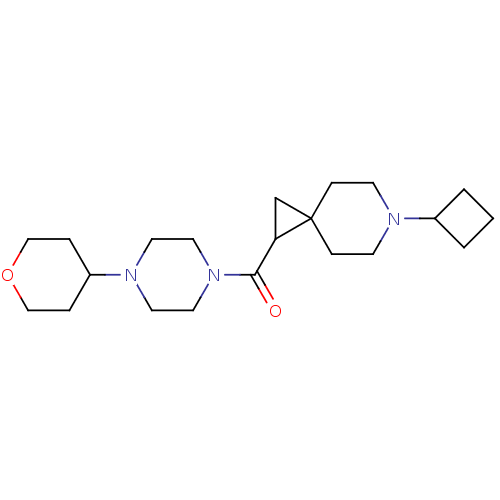 (CHEMBL3127706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344259 (CHEMBL1779012 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496261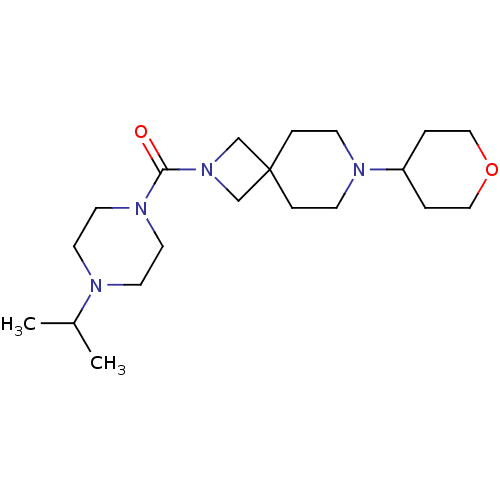 (CHEMBL3127680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496287 (CHEMBL3127669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344254 (CHEMBL1779007 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344260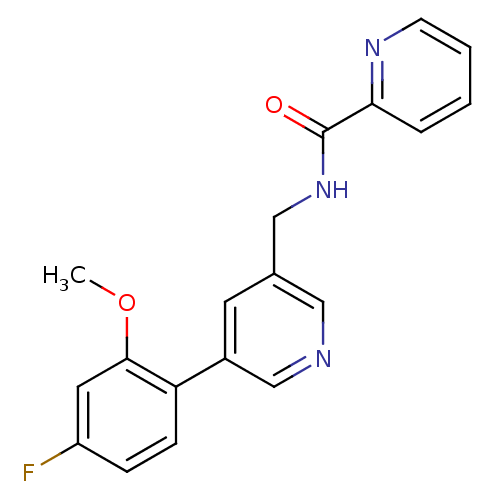 (CHEMBL1779013 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344241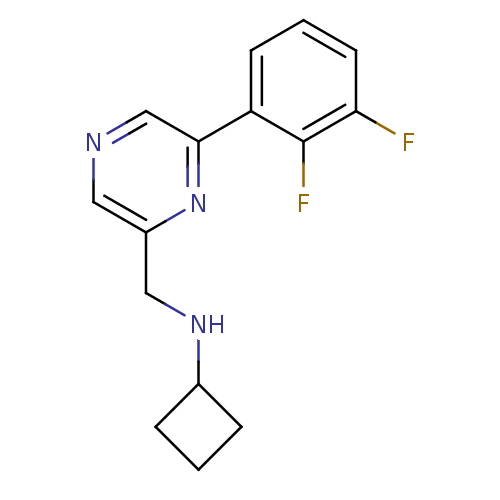 (CHEMBL1778994 | N-((6-(2,3-difluorophenyl)pyrazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344235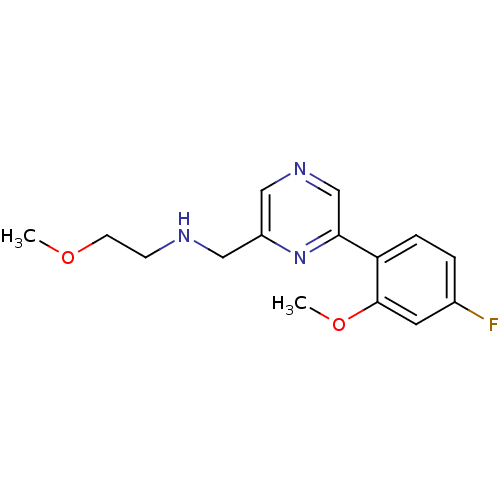 (CHEMBL1778868 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344236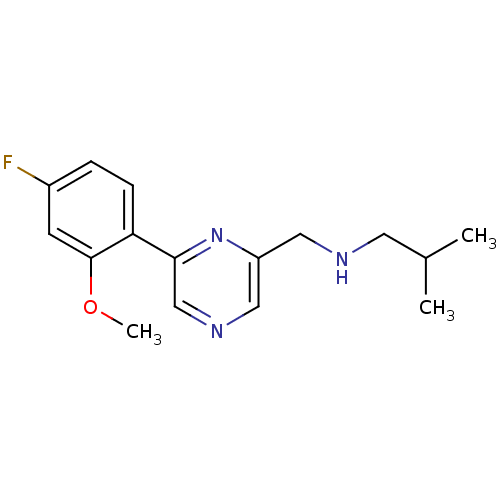 (CHEMBL1778869 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344237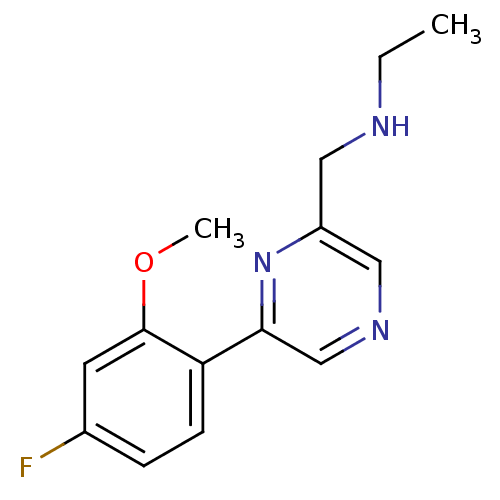 (CHEMBL1778870 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344255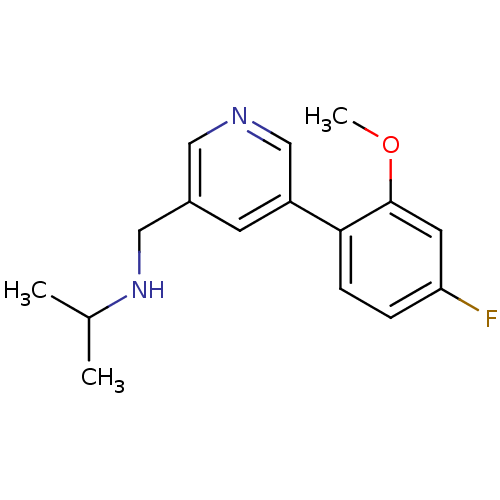 (CHEMBL1779008 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344262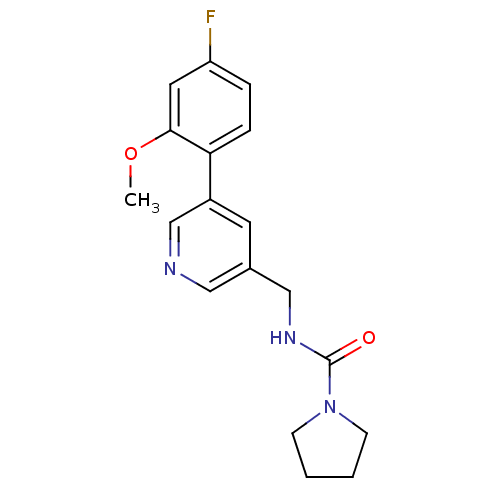 (CHEMBL1779015 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344264 (CHEMBL1779016 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344242 (1-cyclopropyl-N-((6-(2,3-difluorophenyl)pyrazin-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344265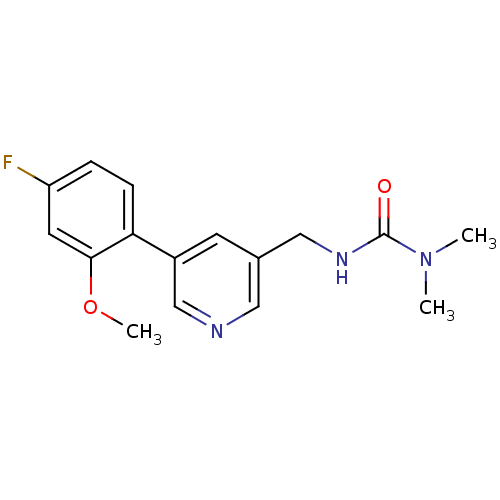 (3-((5-(4-fluoro-2-methoxyphenyl)pyridin-3-yl)methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344238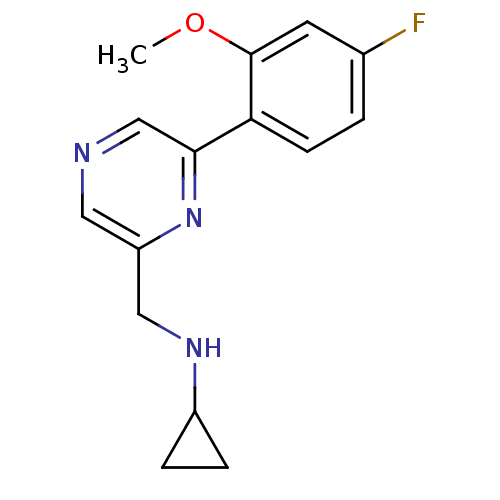 (CHEMBL1778991 | N-((6-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 429 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496277 (CHEMBL3127707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 439 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344256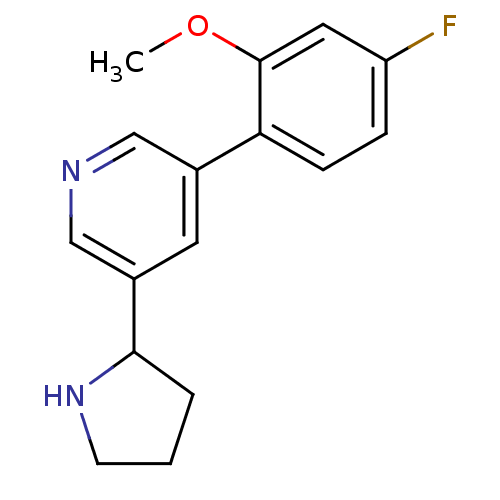 (3-(4-fluoro-2-methoxyphenyl)-5-(pyrrolidin-2-yl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344261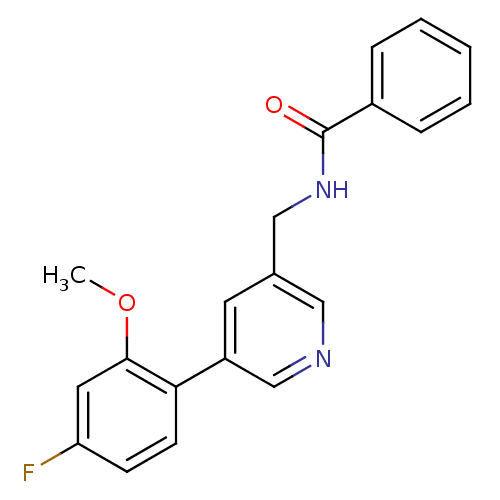 (CHEMBL1779014 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344257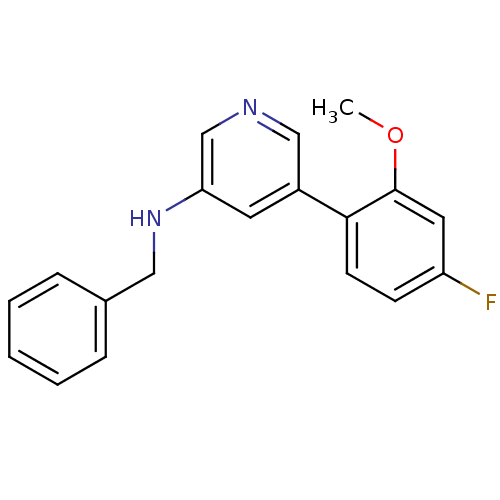 (CHEMBL1779010 | N-benzyl-5-(4-fluoro-2-methoxyphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344266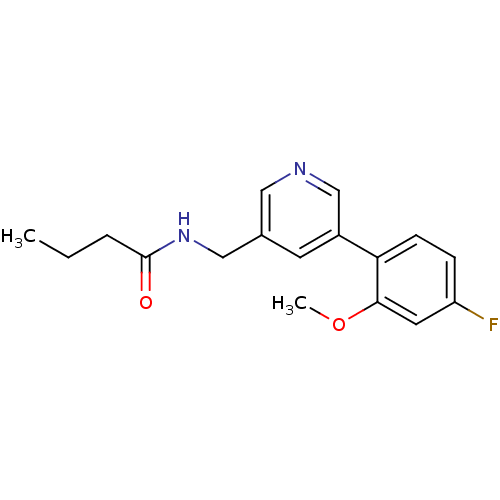 (CHEMBL1779018 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344267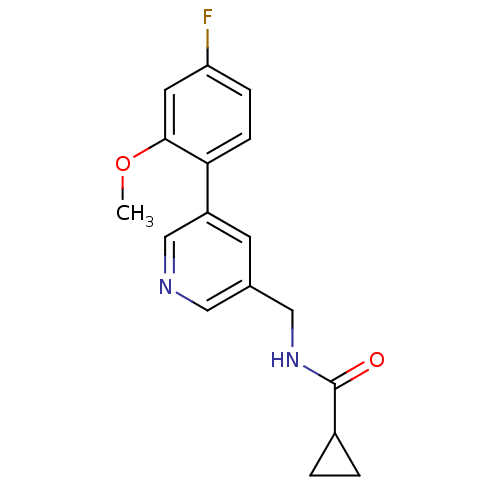 (CHEMBL1779019 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344244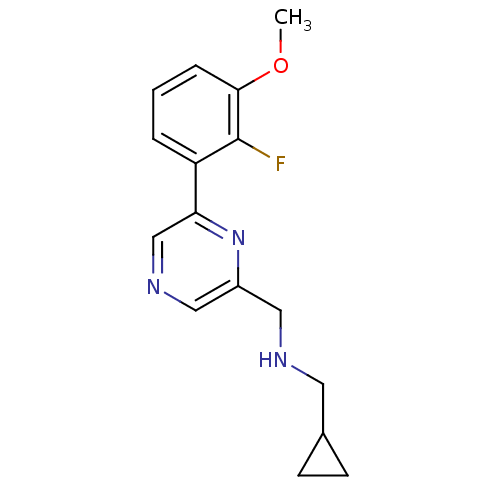 (1-cyclopropyl-N-((6-(2-fluoro-3-methoxyphenyl)pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344239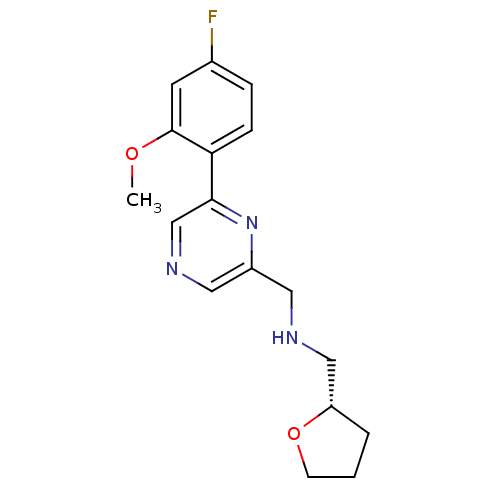 ((S)-1-(6-(4-fluoro-2-methoxyphenyl)pyrazin-2-yl)-N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344258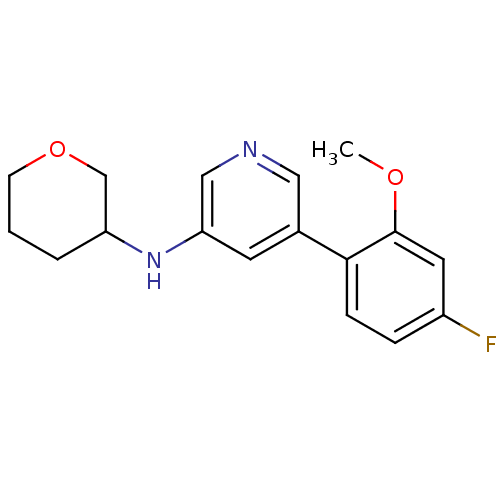 (5-(4-fluoro-2-methoxyphenyl)-N-(tetrahydro-2H-pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 705 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344268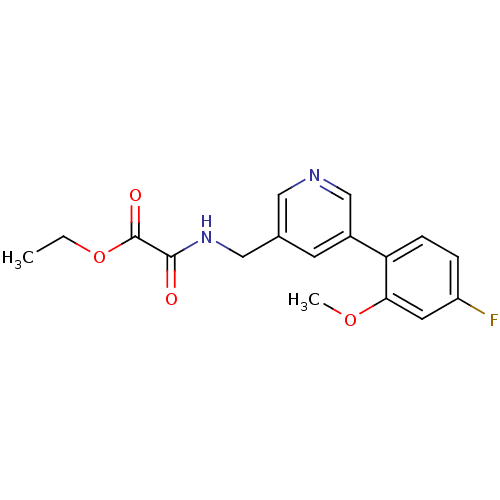 (CHEMBL1779020 | ethyl 2-((5-(4-fluoro-2-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496291 (CHEMBL3127697) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344246 (3-(6-((cyclopentylamino)methyl)pyrazin-2-yl)benzon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 279 total ) | Next | Last >> |