

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340003 ((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339997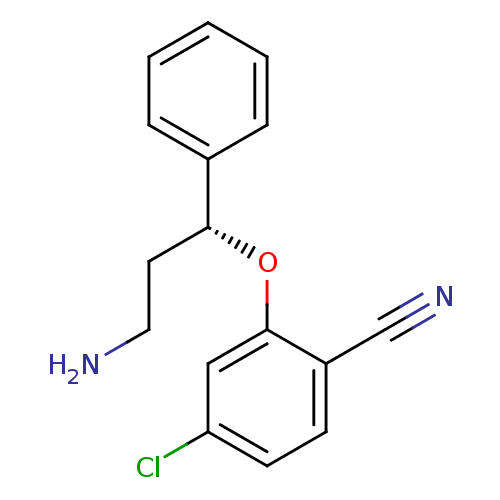 ((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339998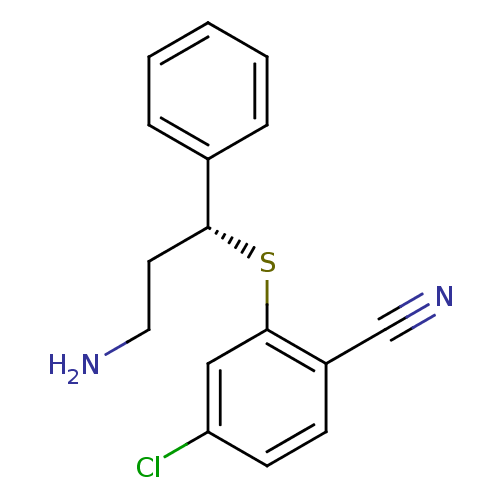 ((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340004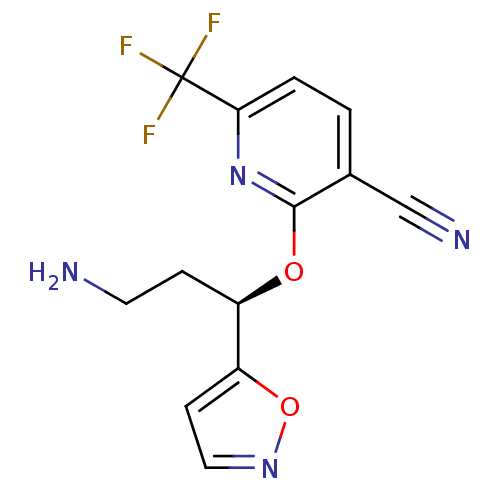 ((R)-2-(3-amino-1-(isoxazol-5-yl)propoxy)-6-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340002 (2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339996 ((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36395 (5-Fluoro-2-thiophen-2-yl-1,2-dihydroquinazolin-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091817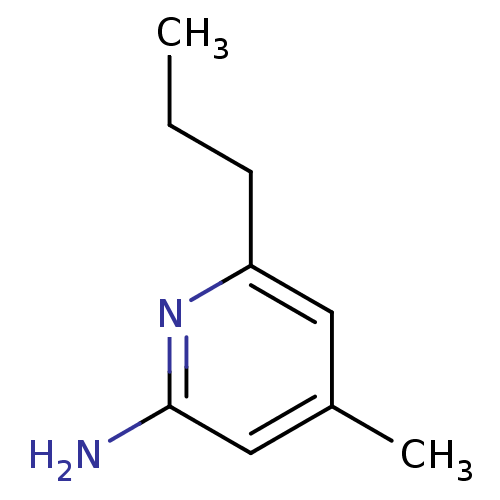 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040461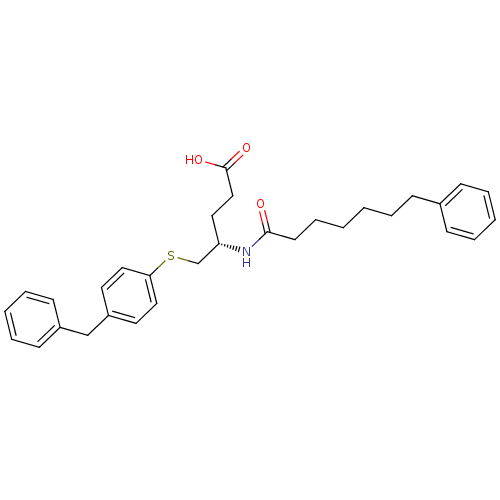 ((S)-5-(4-Benzyl-phenylsulfanyl)-4-((S)-7-phenyl-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040458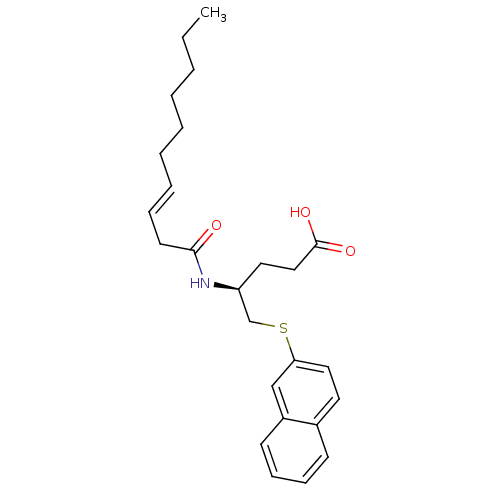 ((S)-4-((S)-(E)-Dec-3-enoylamino)-5-(naphthalen-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50040461 ((S)-5-(4-Benzyl-phenylsulfanyl)-4-((S)-7-phenyl-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description Compound was tested for in vitro activity against s-phospholipase A2 (s-PLA2) isolated from human platelets | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339995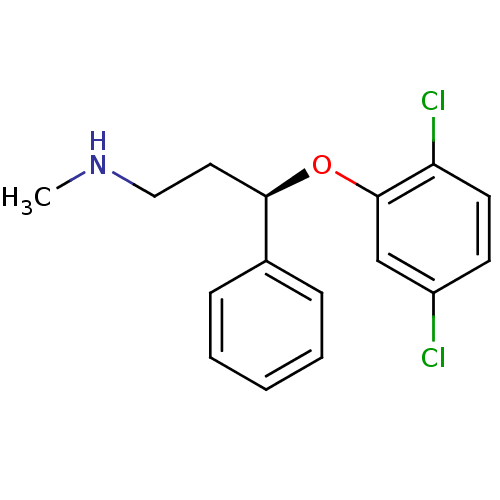 ((R)-3-(2,5-dichlorophenoxy)-N-methyl-3-phenylpropa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040464 ((S)-3-((S)-(E)-Dec-3-enoylamino)-4-(naphthalen-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004502 (CHEMBL101972 | Phosphoric acid 2-hexadecanoylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro inhibition of porcine pancreatic phospholipase A2 evaluated by monomeric substrate assay | J Med Chem 35: 2939-51 (1992) BindingDB Entry DOI: 10.7270/Q2RF5T0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040455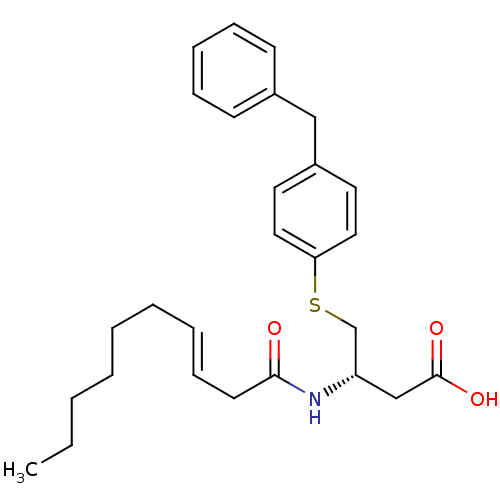 ((S)-4-(4-Benzyl-phenylsulfanyl)-3-((S)-(E)-dec-3-e...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040461 ((S)-5-(4-Benzyl-phenylsulfanyl)-4-((S)-7-phenyl-he...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description Compound was tested for in vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and micellar substrate with deoxycholate (DOC) | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004495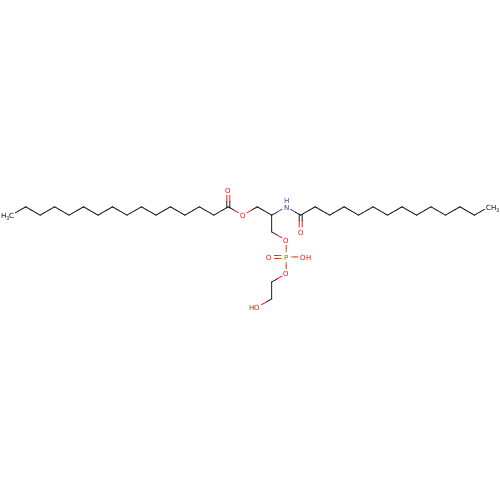 (CHEMBL101015 | Hexadecanoic acid 3-[hydroxy-(2-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro inhibition of porcine pancreatic phospholipase A2 evaluated by monomeric substrate assay | J Med Chem 35: 2939-51 (1992) BindingDB Entry DOI: 10.7270/Q2RF5T0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36396 (CD24894148 | N-[2-(4-Amino-5,8-difluoro-1,2-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091817 (4-Methyl-6-propyl-pyridin-2-ylamine | 4-Methyl-6-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50124535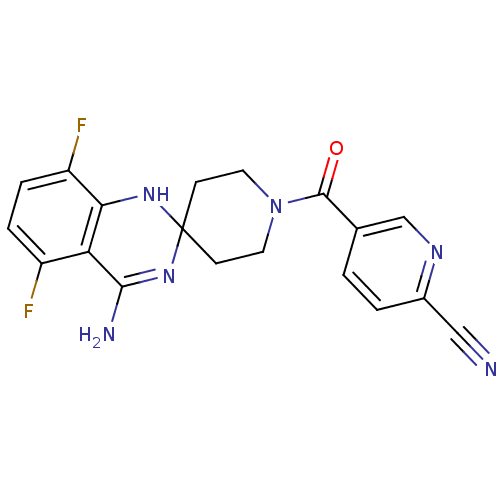 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36401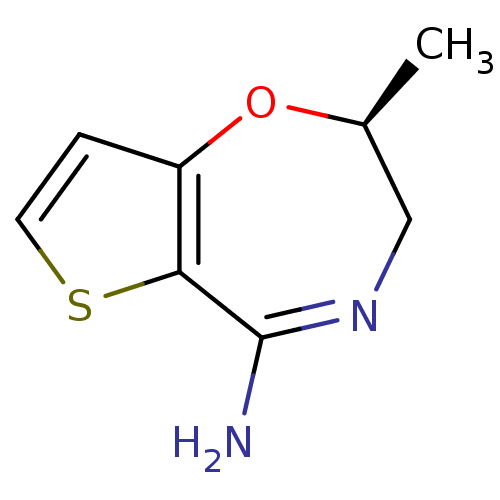 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50124535 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340001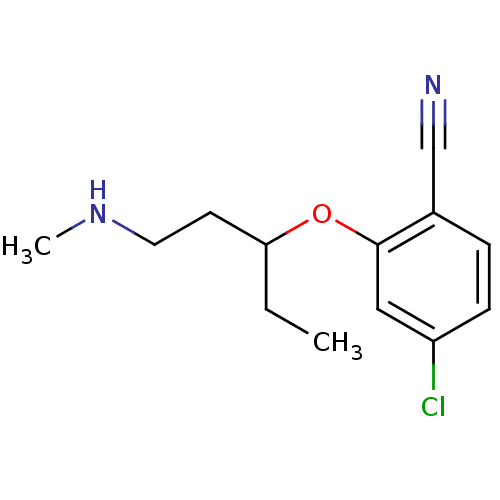 (CHEMBL1762478 | rac-4-chloro-2-(1-(methylamino)pen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50040466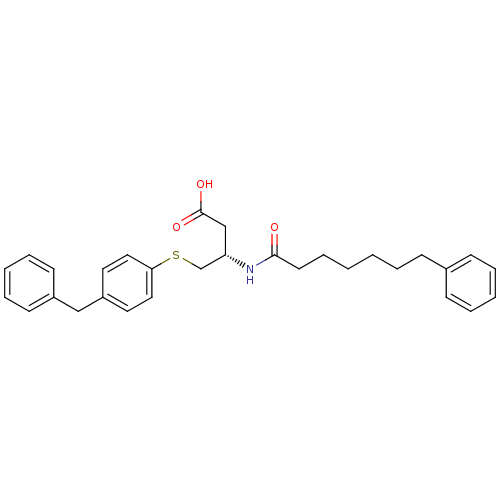 ((S)-4-(4-Benzyl-phenylsulfanyl)-3-((S)-7-phenyl-he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description Compound was tested for in vitro activity against s-phospholipase A2 (s-PLA2) isolated from human platelets | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339999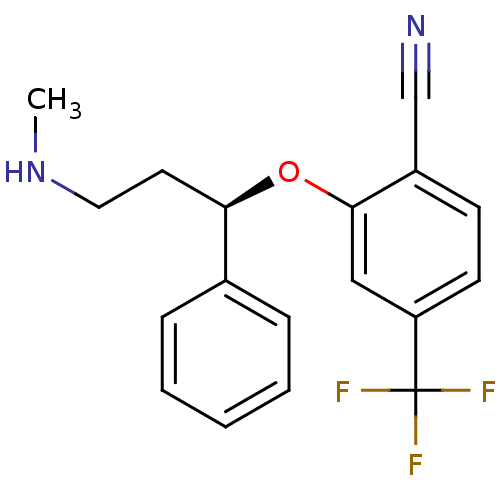 ((R)-2-(3-(methylamino)-1-phenylpropoxy)-4-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148167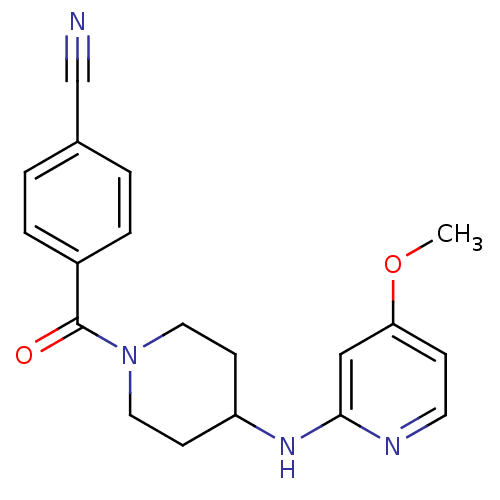 (4-(4-(4-methoxypyridin-2-ylamino)piperidine-1-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36401 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM36401 ((2S)-2-Methyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50148164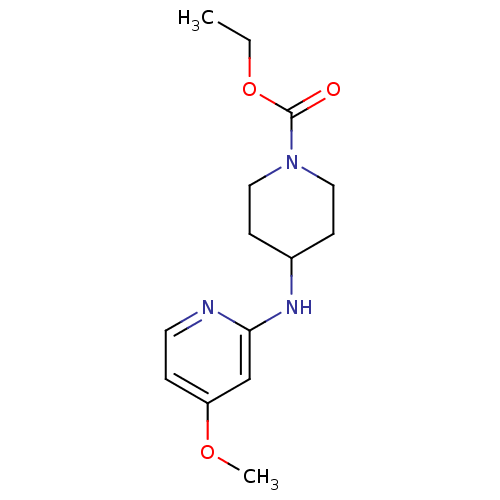 (4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040465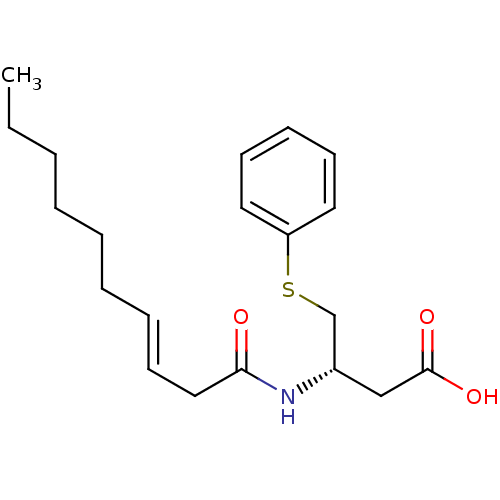 ((S)-3-((S)-(E)-Dec-3-enoylamino)-4-phenylsulfanyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50124535 (1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340000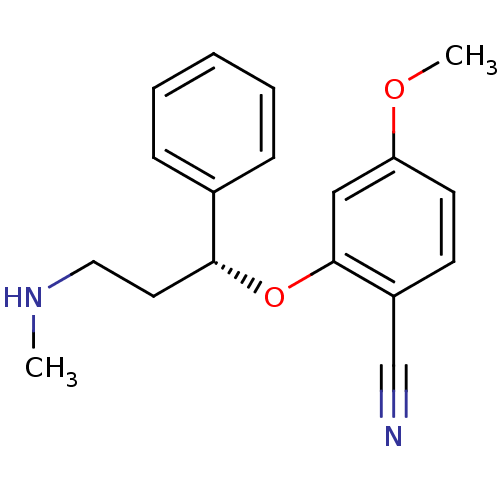 ((R)-4-methoxy-2-(3-(methylamino)-1-phenylpropoxy)b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50040455 ((S)-4-(4-Benzyl-phenylsulfanyl)-3-((S)-(E)-dec-3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and micellar substrate with deoxycholate (DOC) | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36399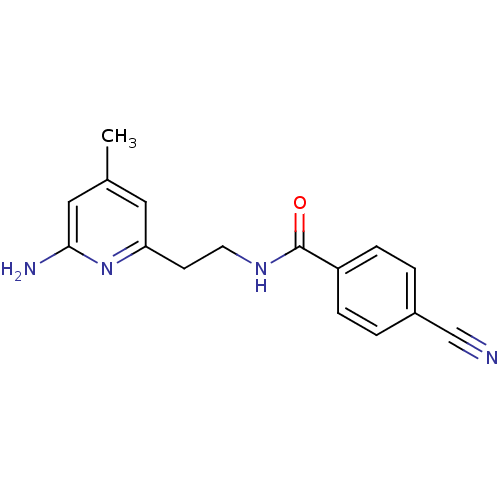 (CID10221335 | N-[2-(6-Amino-4-methylpyridin-2-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091800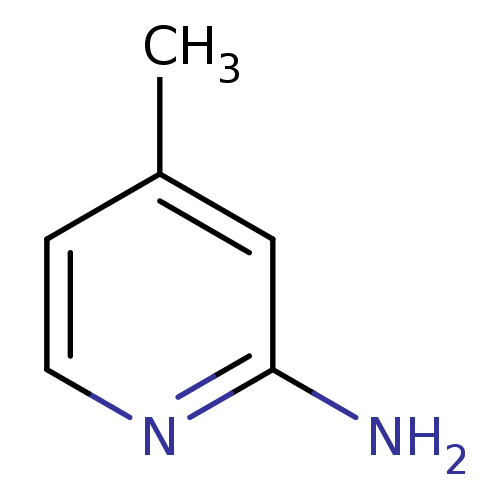 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM36397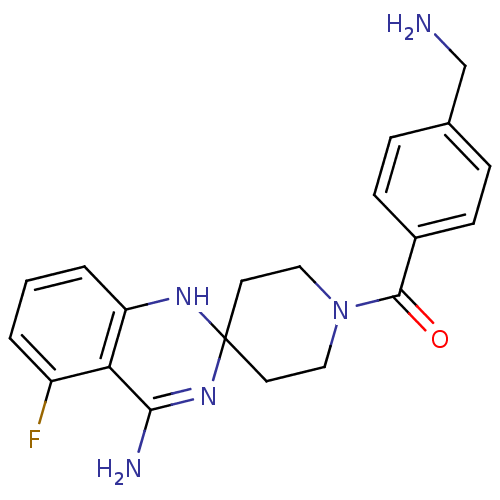 ((4-Amino-5-fluorospiro[1H-quinazoline-2,4'-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040456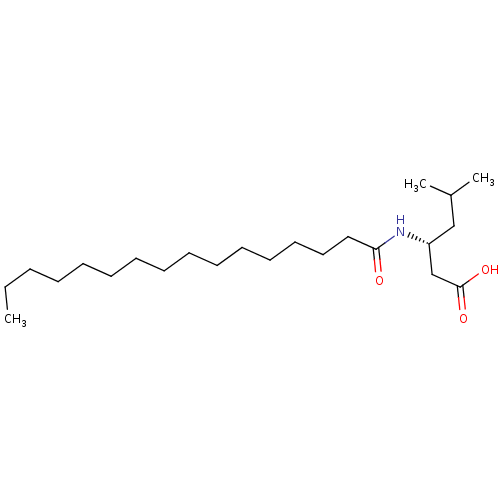 ((R)-3-((S)-Hexadecanoylamino)-5-methyl-hexanoic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339998 ((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of iNOS in intact human DLD1 cells assessed as nitric oxide production | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040463 ((S)-4-((S)-Hexadecanoylamino)-6-methyl-heptanoic a...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM36402 ((3R)-3-Propyl-2,3-dihydrothieno[2,3-f][1,4]oxazepi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50004499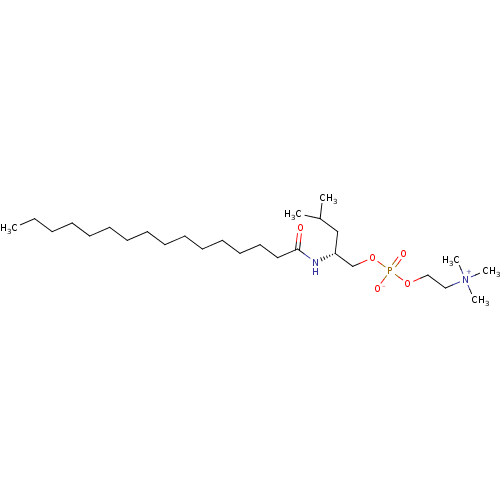 ((R)-O-(2-Hexadecanamidoisohexyl) phosphocholine | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro inhibition of porcine pancreatic phospholipase A2 evaluated by Deoxycholate-phospholipid mixed micelle assay | J Med Chem 35: 2939-51 (1992) BindingDB Entry DOI: 10.7270/Q2RF5T0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50040462 ((R)-3-((S)-(E)-Dec-3-enoylamino)-5-methyl-hexanoic...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase [136-227] (Sus scrofa) | BDBM50004499 ((R)-O-(2-Hexadecanamidoisohexyl) phosphocholine | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Fisons plc Curated by ChEMBL | Assay Description In vitro activity against porcine pancreatic phospholipase-A2 (PLA2) and monomerically dispersed substrate | J Med Chem 37: 557-9 (1994) BindingDB Entry DOI: 10.7270/Q28W3CB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50340000 ((R)-4-methoxy-2-(3-(methylamino)-1-phenylpropoxy)b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.0 | 37 |
The Scripps Research Institute | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | Nat Chem Biol 4: 700-7 (2008) Article DOI: 10.1038/nchembio.115 BindingDB Entry DOI: 10.7270/Q2SB443Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50340002 (2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |